- *Corresponding Author:
- Dongjing Shi
Department of Anesthesia, Beijing Tongren Hospital, Capital Medical University, Xicheng, Beijing 100000, China
E-mail: sdjtjmu@126.com
| This article was originally published in a special issue,“New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “128-132” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the significance attributed to the utilization of sintilimab in conjunction with bevacizumab for patients suffering from advanced hepatocellular carcinoma. During the period spanning from March 2020 to March 2023, our center enrolled a 104 individuals with advanced hepatocellular carcinoma, who were then allocated into either the observation group (treated with sintilimab and bevacizumab) or the control group (treated with sintilimab). Safety was the primary outcome of this study, evaluated by monitoring and recording adverse events. The QLQ-C30 questionnaire was utilized to evaluate healthrelated life quality and hepatocellular carcinoma-specific symptoms, and overall survival rate was the secondary outcome. The two groups exhibited similar baseline characteristics, without any significant differences (p>0.05). The two groups did not significantly differ in terms of the incidence of each adverse event; however, the observation group had a significantly higher overall incidence rate of adverse events than the control group (p<0.05). The QLQ-C30 scores of both groups showed significant improvement compared to baseline, with more significant improvements observed in the observation group in terms of overall health status, physical functioning and emotional functioning (p<0.05). In terms of overall survival rate, the observation group outperformed the control group significantly. In conclusion, when treating individuals with advanced hepatocellular carcinoma, the joined administration of sintilimab and bevacizumab may result in a higher frequency of adverse events. Nevertheless, this treatment approach offers the benefits of improved overall survival rate and enhanced quality of life, underscoring its considerable clinical value and suitability for promotion.
Keywords
Immunotherapy, hepatocellular carcinoma, quality of life, sintilimab, bevacizumab
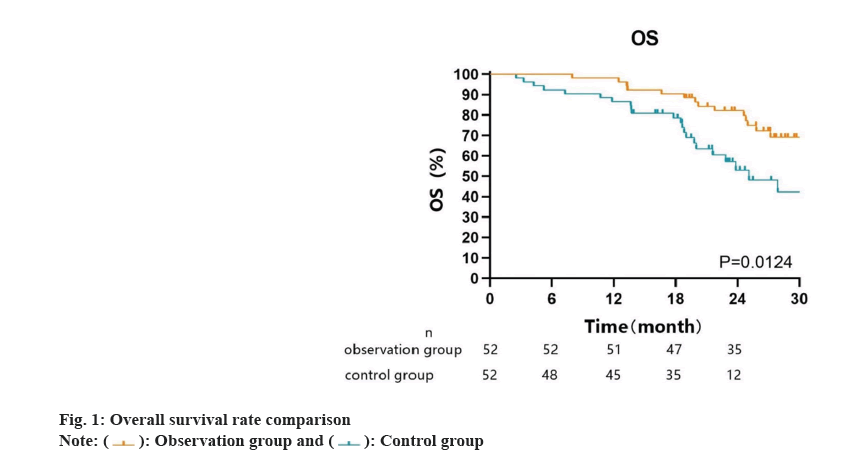
Considered as a common malignancy, Hepatocellular Carcinoma (HCC) has a significant global presence, especially in Asian countries[1]. Due to its insidious course and highly invasive nature, HCC is often diagnosed at an advanced stage, limiting the chances for effective treatment and posing great challenges[2]. Patients with advanced HCC often experience limited effectiveness when undergoing traditional treatment approaches like surgical resection, radiotherapy or chemotherapy. Surgical resection is typically suitable only for early-stage cases, while advanced HCC has already spread to other sites, making it unsuitable for surgical resection[3]. Chemotherapy and radiotherapy can alleviate symptoms in some cases, but they often fail to significantly prolong patient’s survival. In recent years, immunotherapy has emerged as a promising treatment strategy and has attracted widespread attention. Immunotherapy is designed to activate the patient's own immune system against cancer cells, offering potential efficacy and tolerability[4]. Active exploration is underway in immunotherapy research for advanced HCC, with the objective of understanding the immune system’s potential in restraining the progression and spread of tumors. Sintilimab and bevacizumab are drugs approved in recent years for the treatment of certain malignancies. They specifically inhibit the immune checkpoint proteins Programmed cell Death-1 (PD-1) and Programmed cell Death-Ligand 1 (PD-L1), disrupting the mechanisms through which tumor cells escape immune system detection[5,6]. These drugs have shown significant therapeutic effects in other cancer types, sparking interest in their use in managing advanced HCC[7]. To evaluate the efficacy and safety of this novel treatment strategy, this research intends to explore the clinical significance of sintilimab in conjunction with bevacizumab in patients with advanced HCC, providing new insights for clinical treatment. Between March 2020 and March 2023, a group of 104 individuals with advanced HCC underwent treatment at our center. The purpose of the research was to assess the clinical implications attributed to the utilization of sintilimab in conjunction with bevacizumab for patients suffering from advanced HCC. The individuals were assigned to an observation group (receiving the combination of sintilimab and bevacizumab) and a control group (receiving conventional sintilimab treatment), based on the different treatment methods. Prior to their involvement in this study, written informed consent was obtained from each patient, and the study protocol received approval from our institutional review board, adhering to the principles outlined in the Helsinki declaration. Inclusion criteria individuals aged 18 y or above with no gender restrictions, diagnosed with advanced HCC confirmed through histology or cytology, or exhibiting one or more measurable target lesions as per the Chinese Tumor Political Guidelines or modified Response Evaluation Criteria In Solid Tumors (RECIST) criteria[8]. Additionally, patients should have a Child-Pugh a grade, an Eastern Cooperative Oncology Group (ECOG) performance status ≤1, and normal functioning of all organs. Furthermore, it is essential for both patients and their family members to be fully aware of and comprehend the specific details of this study, and provide informed consent by signing the appropriate documents. Exclusion criteria individuals with advanced HCC where the extent of liver involvement is <50 %; patients with evident invasion of the main portal vein by clear bile duct or portal vein; patients who have previously undergone systemic therapy for unrespectable HCC; and individuals with mental illness that may hinder their ability to comply with the research plan. Patients in the observation group received sintilimab combined with bevacizumab (sintilimab 200 mg per dose, bevacizumab 200 mg per dose, once every 3 w), while the patients in the control group received sintilimab alone (sintilimab 200 mg per dose, once every 3 w). Safety is the primary outcome of this study and will be assessed based on the monitoring and recording of adverse events. The Common Terminology Criteria for Adverse Events (CTCAE) will be used to evaluate and grade specific adverse events. QLQ-C30 will be used to evaluate health-related life-quality and liver cancer-specific symptoms as secondary outcomes[9]. In clinical trials, the QLQ-C30 is utilized as a self-reported measurement tool, comprising 30 items, to assess the health status, functioning and symptoms of cancer patients[10]. The questionnaire consists of five functional scales (physical, role, emotional, cognitive and social functioning), three symptom scales (fatigue, nausea and vomiting, and pain), six single items (dyspnea, insomnia, appetite loss, constipation, diarrhea and financial difficulties), and a global health status scale. For our purposes, we will concentrate on analyzing the results of the functional scales and the global health status scale in the questionnaire. Scores on these scales range from 0 to 100, with higher scores indicating a better health-related quality of life in terms of overall functioning and health status. Conversely, higher scores on the symptom scales reflect a greater burden of symptoms. QLQ-HCC18 will be used to assess liver cancer-specific symptoms, which comprises 18 self-reported indicators of functional and symptom burden related to liver cancer. QLQ-HCC18 comprises five symptom scales, namely fatigue, jaundice, nutrition, pain, and fever. Additionally, it includes two single-item scales for assessing sexual function and abdominal distension, as well as a functional scale dedicated to body image evaluation. The severity or problem level is indicated by higher scores on the unweight scales, which range from 0 to 100. The study also analyzed the overall survival rate as a secondary outcome in both groups of patients. The last follow-up will be conducted in July 2023 through outpatient visits and telephone follow-ups, with the surgical time as the start point and patient death as the end point. Follow-ups will be conducted every 3 mo post-operatively to record the survival status of the patients. Statistical Package for the Social Sciences (SPSS) 22.0 will be utilized for analyzing the data. GraphPad Prism 9.3 will be used to plot the patient survival curve and disease-free survival curve. The count units will be presented as counts and percentages (n, %). The Chi-square (χ2) test will be used for categorical data, and the mean±standard deviation (x̄±s) will be used for continuous data that is normally distributed. The Mann-Whitney U test will be utilized for analyzing continuous data that does not conform to a normal distribution. In our analysis, statistical significance will be defined by a significance level of p<0.05. The baseline characteristics of the two patient groups did not exhibit any noteworthy disparities as shown in Table 1. The occurrence of various adverse events did not significantly differ between the groups. Nonetheless, a notably greater number of individuals in the observation group experienced such events when compared to the control group as shown in Table 2. Prior to treatment, there were no noteworthy variations in QLQ-C30 scores observed between the two patient cohorts. Following 6 cycles of treatment, significant enhancements were noted in various functional scores for both groups. Notably, patients who received the combination of sintilimab and bevacizumab displayed more pronounced improvements in overall health status, physical function, and emotional function when compared to those who received sintilimab alone as shown in Table 3. Two patients (1.92 %) were lost to follow-up. The observation group had a median follow-up time of 26.7 mo (range was 8-37 mo), whereas for the control group, it was 19.8 mo (range was 3-37 mo). The overall survival rate was significantly higher in the observation group compared to the control group as shown in fig. 1. Advanced liver cancer treatment remains a prominent and controversial topic in the medical field. New treatment methods and drugs continue to emerge, aiming to improve the survival rate and relieve symptoms in advanced liver cancer patients[11,12]. As a cutting-edge treatment strategy, immunotherapy presents an optimistic outlook for individuals facing the challenges of advanced liver cancer. Sintilimab combined with bevacizumab, as representative immunotherapy drugs, has significant clinical and research value in terms of its effectiveness and safety in advanced liver cancer patients. The simultaneous usage of sintilimab and bevacizumab exhibited a noteworthy therapeutic efficacy in patients with advanced liver cancer, as evidenced by the results of this study. Firstly, in terms of the safety of treatment, we used adverse reactions as an evaluation criterion. The results revealed that the combination therapy did not result in a higher occurrence of adverse reactions; instead, it was reduced in the occurrence of rash[13]. The main reason may be that as targeted immunotherapy drugs, they primarily act on the immune system to regulate the interaction between T cells and tumor cells. This may result in over activation of the immune system, but usually manifests as more specific side effects. However, the combination therapy has different but complementary effects in negative regulation of immune activity. Additionally, immunotherapy drugs may induce a sustained immune response in patients, leading to long-term immune tolerance. These findings suggest that the patient's immune system might continue to recognize and combat tumor cells even after discontinuing the drugs, thus preserving the treatment's impact and minimizing the risk of sustained adverse events. Studies have also found that the combination therapy has good clinical efficacy when employed as a first-line treatment for inoperable liver cancer[14]. Conversely, the QLQ-C30 is a life-quality questionnaire used for general cancer patients. This study showed significant changes in QLQ-C30 scores in both groups of patients compared to admission, and the observed changes were notably higher in the observation group[15]. This indicates that the therapeutic effect of sintilimab combined with bevacizumab brings a better experience for patients. With the improvement in treatment outcomes, various aspects of physical function and symptoms of the patients were significantly alleviated. As an immunotherapy drug, sintilimab combined with bevacizumab can enhance the patient's immune system's ability to attack tumors, as demonstrated in the follow-up after treatment, where the observation group showed a statistically higher overall survival rate compared to the control group. This may result in tumor shrinkage, disease stability, or even remission, reducing the negative impact of the disease on the patient's physical and psychological health and potentially improving the quality of life. Advanced liver cancer patients often experience symptoms such as pain, fatigue and loss of appetite, and sintilimab combined with bevacizumab treatment may help alleviate these symptoms, improve patient comfort, and thereby improve life quality. For cancer patients, mental health is equally important[16]. Some patients undergoing immunotherapy may experience an increase in hope and positive emotions during the treatment process, which can help improve the patient's mental state and psychological well-being. In summary, for advanced liver cancer, the use of sintilimab combined with bevacizumab may lead to an increased incidence of adverse reactions but also brings certain improvements in quality of life, while also extending the overall survival rate of patients. This treatment approach has high clinical value and is worthy of promotion and application.
| Group | n | Age (n) | Gender (n) | BMI (kg/m2) | ECOG score (n) | Complications (n) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≥60 | <60 | M | F | 0 | 1 | No | Yes | |||
| Observation | 52 | 33 | 19 | 37 | 15 | 23.06±2.80 | 29 | 23 | 22 | 30 |
| Control | 52 | 30 | 22 | 35 | 17 | 22.83±2.73 | 25 | 27 | 26 | 26 |
| χ2/t | 0.36 | 0.18 | 1.21 | 0.62 | 0.62 | |||||
| p | 0.547 | 0.671 | 0.227 | 0.432 | 0.431 | |||||
Table 1: Baseline Characteristics
| Group | n | Adverse events (n %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hypertension | Diarrhea | Fatigue | Anorexia | Dysphonia | Albuminuria | Nausea and vomiting | Rash | Number of adverse events | ||
| Observation | 52 | 6 (11.54 %) | 11 (21.15 %) | 4 (7.69 %) | 8 (15.38 %) | 4 (7.69 %) | 3 (5.77 %) | 4 (7.69 %) | 4 (7.69 %) | 38 (73.08) |
| Control | 52 | 4 (7.69 %) | 13 (25.00 %) | 1 (1.92 %) | 6 (11.54 %) | 2 (3.85 %) | 1 (1.92 %) | 2 (3.85 %) | 2 (3.85 %) | 28 (53.85) |
| χ2/t | 0.44 | 0.22 | 1.89 | 0.33 | 0.71 | 1.04 | 0.71 | 0.71 | 4.15 | |
| p | 0.506 | 0.642 | 0.169 | 0.566 | 0.400 | 0.308 | 0.400 | 0.400 | 0.042 | |
Table 2: Adverse Events in the Two Groups (n, %)
| Item | Observation | Control | t | p | ||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| Overall health status | 49.86±10.57 | 66.08±14.47* | 50.06±9.66 | 57.16±16.47* | 7.051 | <0.0001 |
| Physical function | 65.46±11.18 | 84.80±16.29* | 62.23±10.79 | 76.22±15.47* | 6.665 | <0.0001 |
| Role function | 59.18±12.12 | 85.41±21.23* | 57.36±11.76 | 84.70±19.29* | 1.024 | 0.645 |
| Emotional function | 63.28±13.13 | 83.69±16.94* | 59.36±12.85 | 76.70±19.30* | 5.964 | <0.0001 |
| Cognitive function | 68.40±11.65 | 89.48±14.26* | 65.52±11.23 | 88.91±15.04* | 0.826 | 0.826 |
| Social function | 64.18±12.06 | 80.65±23.06* | 66.06±12.54 | 78.02±21.42* | 1.663 | 0.136 |
Table 3: QLQ-C30 Scores before and after Treatment (x̄±s, POINT)
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
[Crossref] [Google Scholar] [PubMed]
- Ouyang Li, Xu Li. Key research on liver cancer in 2022. Chin J Pract Surg 2019;43(1):66-70.
- Wang T, Wang W. Current status and development of immunotherapy for hepatocellular carcinoma. J Sichuan Univ 2019;54(3):692-8.
- Pan Xi. Current status and research progress of immunotherapy for advanced primary liver cancer. Chin J Med 2019;29(12):37-9.
- Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, et al. Use of immunotherapy with programmed cell death 1 vs. programmed cell death ligand 1 inhibitors in patients with cancer: A systematic review and meta-analysis. JAMA Oncol 2020;6(3):375-84.
[Crossref] [Google Scholar] [PubMed]
- Ning Y, Lin J, Liang J. Research progress of PD-1/PD-L1 inhibitors in immunotherapy of liver cancer. J Pract Med Clin 2019;26(6):566-71.
- Zhang X, Guo HS, Wu CX. Clinical progress and prospect of immunotherapy for liver cancer. Chin Chronic Dis Prev Control 2019;31(2):152-5.
- Chinese Guidelines for Integrated Diagnosis and Treatment of Cancer (CACA)-part of liver cancer. J Integ Cancer Ther Electronic 2022;8(3):31-63.
- Wang Y, Zhu L, Chen P. Evaluation of QOL EORTC QLQ-C30 for cancer patients. Chin J Health Statistics 2015;32(3):512-3.
- Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The check mate 040 randomized clinical trial. JAMA Oncol 2020;6(11):e204564.
[Crossref] [Google Scholar] [PubMed]
- Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: The check mate 016 study. J Clin Oncol 2017;35(34):3851.
[Crossref] [Google Scholar] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17(7):883-95.
[Crossref] [Google Scholar] [PubMed]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369(2):122-33.
[Crossref] [Google Scholar] [PubMed]
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382(20):1894-905.
[Crossref] [Google Scholar] [PubMed]
- Zhou L, Yu C, Miao T. Correlation between adjuvant immunotherapy and quality of life and prognosis in patients with hepatocellular carcinoma. Clin Med Res Pract 2018;3(19):14-5.
- Wu Z, Shi S, Wang L. Effect of cellular immunotherapy on advanced hepatocellular carcinoma. Chin J Med 2018;30(2):9-12.