- *Corresponding Author:
- T. Zeng
Department of Dermatology, Jingzhou Hospital Affiliated to Yangtze University, Jingzhou, Hubei 434100, China
E-mail: 13339737387@163.com
| This article was originally published in a special issue, “Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “203-209” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To determine the efficacy of betamethasone injection combined with famciclovir on herpes zoster and its effects on cytokines. The clinical data of 89 patients with herpes zoster treated in our hospital between October 2020 and June 2023 were analyzed retrospectively. Among them, 42 patients treated with famciclovir (0.25 g) were included in the control group, and the other 47 patients treated with both famciclovir (0.25 g) and betamethasone injection (1 ml) were included in the study group. The levels of serum inflammatory factors (interleukin-6 and interleukin-10) in the two groups were analyzed and compared before and after treatment. The visual analogue scale was adopted for evaluating and comparing the pain degree of the two groups before and after treatment. The overall response rate and incidence of adverse reactions in the two groups were analyzed and compared, and the risk factors impacting the prognosis of patients were analyzed through logistics regression. Before treatment, the two groups were not greatly different in the levels of interleukin-6 and interleukin-10 (p>0.05), while after treatment, the interleukin-6 level in both groups decreased notably (p<0.0001), and the interleukin-10 level in them increased notably (p<0.0001). In addition, after treatment, the study group presented a notably lower interleukin-6 level and a notably higher interleukin-10 level than the control group (p<0.0001). Before treatment, the two groups were not greatly different in visual analogue scale score (p>0.05), while after treatment, visual analogue scale scores of both groups decreased notably (p<0.0001), with a more notable decrease in the study group than that in the control group (p<0.0001). A notably lower overall response rate was found in the control group than that in the study group (p=0.020), but no notable difference was found between the two groups in the total incidence of adverse reactions (p=0.834). According to univariate analysis, age, course of disease, underlying diseases, history of alcoholism and medication regimen were risk factors affecting the prognosis of patients. According to multivariate analysis, underlying diseases and medication regimen were independent risk factors affecting the prognosis of patients. Compared with famciclovir alone, betamethasone injection combined with famciclovir is more effective in treating patients with herpes zoster, which can effectively improve the inflammatory factors and relieve the pain of patients, without increasing adverse reactions. In addition, underlying diseases and medication regimens are independent risk factors for the prognosis of patients with herpes zoster.
Keywords
Betamethasone injection, famciclovir, herpes zoster, efficacy, inflammatory factors
Herpes Zoster (HZ) is an acute infectious skin disease, mainly caused by varicella-zoster virus infection, which affects nerves and skin and usually appears in clusters of blisters on one side of the body, with obvious pain[1,2]. HZ is self-healing, but some patients may have residual neuralgia[3]. The incidence of HZ increases with age, and an older patient face more obvious nerve pain[4]. Long-term severe pain will irritate patients and seriously compromise their quality of life.
At the current stage, the specific pathogenesis of HZ-related pain has not been completely clarified, and it is generally believed to be bound up with the plasticity and sensitivity of peripheral and central neurons[5]. The principles for the treatment of HZ are antivirus, pain relief, nerve nutrition, anti-inflammation and prevention and treatment of complications[6]. Famciclovir is a nucleoside antiviral drug, which can selectively and effectively inhibit the synthesis and replication of herpesvirus deoxyribonucleic acid, so it is often adopted in the treatment of HZ and primary genital herpes[7]. Betamethasone injection is a kind of injection to inhibit corticoid inflammatory response and residual nerve pain, which can effectively control corticosteroid sensitive diseases[8]. However, there are few studies on the combination of the two drugs for HZ.
Therefore, this study explored the efficacy of betamethasone injection combined with famciclovir on HZ and its influences on inflammatory cytokines, so as to provide reliable reference for the subsequent treatment of HZ.
Materials and Methods
Sample information:
The clinical data of 120 patients with HZ treated in our hospital between October 2020 and June 2023 were analyzed retrospectively.
Inclusion criteria:
Patients meeting the diagnostic criteria of HZ in Clinical Dermatology of China[9]; patients between 18 y and 65 y old; patients who had not received antiviral drugs during the last week before admission; patients without complicated infection; patients with detailed clinical data.
Exclusion criteria:
Patients with autoimmune or hematological diseases; patients who had received immunoglobulin, glucocorticoids, antiviral drugs or other treatments recently; patients with liver or renal insufficiency; patients with malignant tumor and patients who were allergic to the drugs used in this study.
Sample screening:
According to the above criteria, the 120 patients were screened, and 89 patients who met the requirements of this study were screened out. Among them, 42 patients treated with famciclovir were included in the control group, and the other 47 patients treated with both famciclovir and betamethasone injection were included in the study group.
Therapeutic regimen:
The control group was treated with famciclovir capsule (Sichuan MED-SHINE Pharmaceutical Co., Ltd., State Food and Drug Administration (SFDA) approval number: H19991045; specification: 0.125 g). Each patient took 0.25 g famciclovir capsule 3 times a day with warm water, for 10 d consecutively.
The study group was treated with compound betamethasone injection (Schering plough Pharmaceutical Factory, Belgium; SFDA approval number: J20080062) and famciclovir capsule. Famciclovir capsule was administrated first with the same dosage, usage and course of treatment the same as those of the control group. In addition, the patient was intramuscularly injected with 1 ml compound betamethasone injection, once a day, for 10 d consecutively. Acyclovir cream (Fujian Yongan Pharmaceutical Co., Ltd., SFDA approval number: H35021081) was used externally in both groups, and no other drugs were used during the treatment.
Outcome measures:
Primary outcome measures: Efficacy and serum inflammatory factors collectively are considered as primary outcome measures. The efficacy of the two groups was compared based on the following criteria: Cured: All blisters scabbed and the patient had no pain and basically no rash; markedly effective: Most blisters scabbed, and the patient had a slight pain and obviously relieved rash; effective: The blisters scabbed partially and the patient had obvious but tolerable pain and relieved rash; Ineffective: The blisters did not scab, and the patient had obvious and unbearable pain, and not relieved rash. Overall response rate=(number of cured cases+the number of patients with markedly effective treatment+that of patients with effective treatment)/the total number of patients×100 %. The serum inflammatory factors of the two groups were compared before and after treatment, including Interleukin (IL)-6 and IL-10. Peripheral venous blood (3 ml) was acquired from each patient before and after treatment, followed by centrifugation (3000 r/min) to separate the serum. The serum was stored at -20° for testing, and the contents of IL-6 and IL-10 were determined by enzyme-linked immunosorbent assay. The efficacy of the patient was evaluated and logistic regression analysis was conducted for analysis of risk factors impacting the patients’ prognosis.
Secondary outcome measures: Pain and adverse reactions are considered as secondary outcome measures. The Visual Analogue Scale (VAS) was adopted for evaluating the pain of the patients[10]. VAS is a scale with a 1 cm-10 cm line and a movable cursor. The left end means painless, with a score of 0 points, and the right end means severe pain, with a score of 10 points. The patient was instructed to move the cursor according to his/her own situation. A higher VAS score indicates more severe pain.
Adverse reactions: The occurrence of adverse reactions, including nausea and vomiting, headache and abdominal discomfort, in the two groups were statistically compared.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 20.0 was used for statistical analysis of the collected data and GraphPad 8 software package was used for visualization of data into required pictures. The measurement data were described by mean±standard deviation and their inter group and intro-group comparison were conducted using the independent-samples t-test and paired t-test, respectively. The counting data were described by percentage (%), analyzed using the Chi-Square (χ2) test. The risk factors affecting the patient’s prognosis were analyzed through logistics regression. p<0.05 indicates a notable difference.
Results and Discussion
According to comparison of baseline data between the two groups, the control group and study group were not greatly different in terms of age, gender, Body Mass Index (BMI), course of disease, location of skin lesions, underlying diseases, history of alcoholism and place of residence (p>0.05) as shown in Table 1.
| Factors | Study group (n=47) | Control group (n=42) | χ2 | p value | |
|---|---|---|---|---|---|
| Age | ≥55 y old | 18 | 10 | 2.159 | 0.142 |
| <55 y old | 29 | 32 | |||
| Gender | Male | 28 | 28 | 0.478 | 0.489 |
| Female | 19 | 14 | |||
| BMI | ≥23 kg/m2 | 17 | 17 | 0.174 | 0.676 |
| < 23 kg/m2 | 30 | 25 | |||
| Course of disease | ≥5 d | 16 | 11 | 0.647 | 0.421 |
| <5 d | 31 | 31 | |||
| Location of skin lesions | Waist and abdomen | 10 | 6 | 2.402 | 0.301 |
| Chest and back | 21 | 15 | |||
| Head and neck | 16 | 21 | |||
| Underlying diseases | Yes | 12 | 6 | 1.739 | 0.187 |
| No | 35 | 36 | |||
| History of alcoholism | Yes | 15 | 8 | 1.916 | 0.166 |
| No | 32 | 34 | |||
| Place of residence | Rural areas | 34 | 28 | 0.338 | 0.561 |
| Urban areas | 13 | 14 |
Table 1: Baseline Data
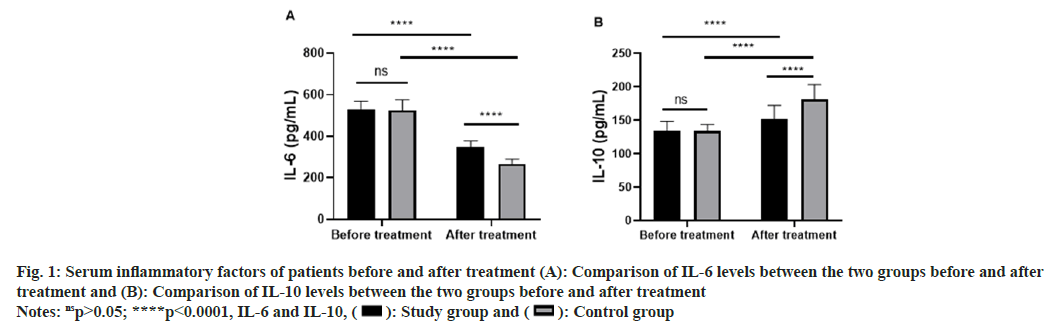
The levels of IL-6 and IL-10 in the two groups were analyzed and compared before and after treatment. According to the results, before treatment, the two groups were similar in the levels of IL-6 and IL-10 (p>0.05), while after treatment, the IL-6 level in both groups decreased notably (p<0.0001) and the IL-10 level in both groups increased notably (p<0.0001), with a more notable decrease and a more notable increase in the study groups than those in the control group (p<0.0001, fig. 1).
Fig. 1: Serum inflammatory factors of patients before and after treatment (A): Comparison of IL-6 levels between the two groups before and after treatment and (B): Comparison of IL-10 levels between the two groups before and after treatment
Notes: nsp>0.05; ****p<0.0001, IL-6 and IL-10,  Control group
Control group
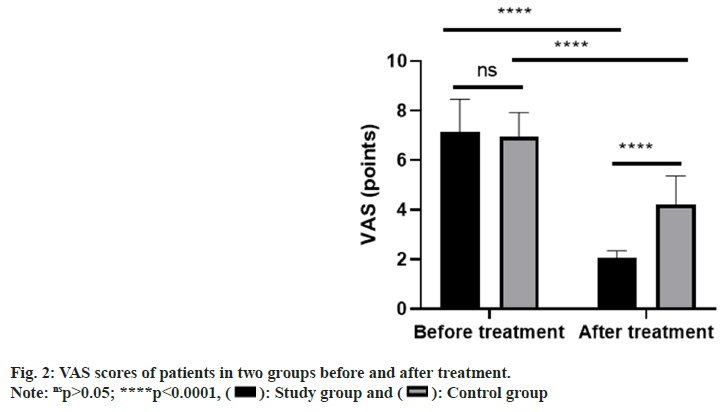
According to analysis of VAS scores of the two groups before and after treatment, before treatment, the two groups were not greatly different in VAS score (p>0.05), while after treatment, VAS scores of both groups decreased notably (p<0.0001), with a notable decrease in the study group than that in the control group (p<0.0001, fig. 2). Comparison of clinical efficacy on the two groups revealed a notably lower overall response rate in the control group than that in the study group (p=0.020, Table 2). Statistical analysis of adverse reactions in the two groups revealed no notable difference between the control group and study group in the total incidence of adverse reactions (p=0.834, Table 3).
| Group | Cured | Markedly effective | Effective | Ineffective | Overall response |
|---|---|---|---|---|---|
| Study (n=47) | 27 (57.45) | 13 (27.66) | 4 (8.51) | 3 (6.38) | 44 (93.62) |
| Control (n=42) | 13 (30.95) | 11 (26.19) | 8 (19.05) | 10 (23.81) | 32 (76.19) |
| χ2 | 6.292 | 0.0243 | 2.111 | 5.400 | 5.400 |
| p value | 0.012 | 0.876 | 0.146 | 0.020 | 0.020 |
Table 2: Comparison of Efficacy between Two Groups, n (%)
| Group | Nausea and vomiting | Headache | Abdominal discomfort | Total adverse reaction |
|---|---|---|---|---|
| Study (n=47) | 3 (6.38) | 2 (4.26) | 1 (2.13) | 6 (12.77) |
| Control (n=42) | 3 (7.14) | 1 (2.38) | 2(4.76) | 6 (14.28) |
| χ2 | 0.021 | 0.239 | 0.473 | 0.044 |
| p value | 0.887 | 0.625 | 0.492 | 0.834 |
Table 3: Incidence of Adverse Reactions, n (%)
After treatment, the treatment outcome that was cured, markedly effective or effective was judged as good prognosis, and patients with such a treatment outcome were included into a good-prognosis group (n=76). The treatment outcome that was ineffective was judged as poor prognosis, and patients with such a treatment outcome were included into a poor-prognosis group (n=13). The clinical data of the two groups were compared and subjected to univariate analysis. As a result, age, course of disease, underlying diseases, history of alcoholism and medication regimen were risk factors impacting the prognosis of patients (Table 4). The indicators with notable differences in the above were assigned (Table 5) and subjected to multivariate analysis. According to logistics regression analysis, underlying diseases and medication regimen were independent risk factors affecting the prognosis of patients as shown in Table 6.
| Factors | Good-prognosis group (n=76) | Poor-prognosis group (n=13) | χ2 | p value | |
|---|---|---|---|---|---|
| Age | ≥55 y old | 19 | 9 | 10.071 | 0.002 |
| <55 y old | 57 | 4 | |||
| Gender | Male | 50 | 6 | 1.835 | 0.176 |
| Female | 26 | 7 | |||
| BMI | ≥23 kg/m2 | 28 | 6 | 0.408 | 0.523 |
| <23 kg/m2 | 48 | 7 | |||
| Course of disease | ≥5 d | 17 | 10 | 15.631 | <0.0001 |
| <5 d | 59 | 3 | |||
| Location of skin lesions | Waist and abdomen | 12 | 4 | 2.761 | 0.252 |
| Chest and back | 30 | 6 | |||
| Head and neck | 34 | 3 | |||
| Underlying diseases | Yes | 18 | 10 | 14.591 | 0.0001 |
| No | 58 | 3 | |||
| History of alcoholism | Yes | 14 | 9 | 14.951 | 0.0001 |
| No | 62 | 4 | |||
| Place of residence | Rural areas | 54 | 8 | 0.476 | 0.491 |
| Urban areas | 22 | 5 | |||
| Medication regimen | Famciclovir | 32 | 10 | 5.400 | 0.020 |
| Famciclovir+betamethasone injection | 44 | 3 |
Table 4: Univariate Analysis
| Factors | Assessment |
|---|---|
| Age | <55 y old=0, ≥55 y old=1 |
| Course of disease | <5 d=0, ≥5 d=1 |
| Underlying diseases | None=0, Yes=1 |
| History of alcoholism | None=0, Yes=1 |
| Medication regimen | Famciclovir+betamethasone injection=0, famciclovir=1 |
| Prognosis | Good prognosis=0, poor prognosis=1 |
Table 5: Multivariate Analysis
| Factors | B | Standard error | Wald test | Difference | Significance | Odds ratio | 95 % Confidence Interval (CI) | |
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||
| Age | 0.417 | 0.674 | 0.382 | 1 | 0.536 | 1.517 | 0.405 | 5.682 |
| Course of disease | -0.277 | 0.735 | 0.142 | 1 | 0.706 | 0.758 | 0.179 | 3.2 |
| Underlying diseases | 1.298 | 0.651 | 3.974 | 1 | 0.046 | 3.661 | 1.022 | 13.112 |
| History of alcoholism | -0.445 | 0.86 | 0.267 | 1 | 0.605 | 0.641 | 0.119 | 3.46 |
| Medication regimen | -1.947 | 0.823 | 5.595 | 1 | 0.018 | 0.143 | 0.028 | 0.716 |
Table 6: Multivariate Logistic Regression Analysis
HZ is an infectious skin disease caused by varicella-zoster virus when human cellular immunity is low[11]. The virus can lurk in the dorsal root neurons of the spinal nerve for a long time and is neutrophilic. It grows again when the patient has a cold, fever, infection or low resistance, and transfers to the skin along the nerve fiber, causing a strong inflammatory reaction between the involved nerve and the skin[12,13]. The skin rash of this disease is mostly unilateral and has features including nerve segmental distribution, and its incidence increases with age[5]. Famciclovir and betamethasone injection are frequently adopted in the treatment of HZ, both of which have demonstrated good efficacy[14,15]. This study explored the application effect of the combination of the two drugs on HZ and its influence on cytokines.
The development of HZ is strongly bound up with the level of inflammatory factors[16]. In the development of the disease, IL-6 can stimulate neuronal excitement and cause pain[17-19], while IL-10 is an inhibitory regulator that can block the release of proinflammatory factors and alleviate nerve pain symptoms[20]. In this study, after treatment, IL-6 in the two groups decreased significantly, while the IL- 10 in them increased significantly, with a notably higher IL-6 level and a notably lower IL-10 level in the control group than those in the study group. The results indicate that betamethasone injection combined with famciclovir can more strongly improve the level of inflammatory factors and enhance the immunity of patients with HZ. This study analyzed the VAS score of the two groups before and after treatment, and found a notable decrease in VAS score in both groups and a more notable decrease in the study group than that in the control group, suggesting that betamethasone injection combined with famciclovir can alleviate the pain degree of HZ patients. The reason for the above results may be as follows: Compound betamethasone injection is composed of soluble betamethasone ester and slightly soluble betamethasone ester. After intramuscular injection, soluble betamethasone ester is absorbed rapidly, which continuously produces effects, so that the symptoms can be effectively controlled. And the operation is very simple, with requirement of only local injection, and the analgesic effect can be enhanced at the same time as anti-inflammation[21]. This study also compared the overall response rate and the total incidence of adverse reactions between the two groups, and found a notably lower overall response rate in the control group than that in the study group, but found no notable difference between the two groups in the total incidence of adverse reactions. It shows that betamethasone injection combined with famciclovir can effectively treat HZ without increasing adverse reactions. The possible reasons are as follows: Compound betamethasone injection is betamethasone dipropionate and sodium betamethasone phosphate. The application of compound betamethasone injection on the basis of antiviral drugs in the acute attack of HZ has the advantages of reducing the production of antibodies, inhibiting inflammatory cell chemotaxis, relieving the inflammatory reaction of involved nerve fibers, relieving pain quickly and reducing the occurrence of residual neuralgia[22]. Arenas-Archila et al.[15] have found that pre-auricular injection of betamethasone combined with adoption of acyclovir can effectively treat acute ocular HZ, which is consistent with the conclusion of this study. Finally, this study analyzed the factors affecting the prognosis of patients, and found age, course of disease, underlying diseases, history of alcoholism and medication regimen were risk factors affecting the prognosis of patients. According to logistics regression analysis, underlying diseases and medication regimen were independent risk factors affecting the prognosis of patients.
This study has confirmed the effect of betamethasone injection combined with famciclovir in the treatment of HZ and its influences on cytokines through retrospective analysis, but it still has some limitations. First of all, the limited sample size of this study may result in some deviation in the conclusion of the study. In addition, the dosage of the two drugs has not been studied in this study, so the optimal dosage of the two drugs needs further study. In addition, the long-term prognosis of the two groups of patients has not been studied, so the influence of the combination of the two on the long-term prognosis of HZ needs further exploration. Therefore, we hope to conduct a more comprehensive analysis on the application of betamethasone injection combined with famciclovir in HZ in the future to obtain more effective experimental results.
To sum up, compared with famciclovir alone, betamethasone injection combined with famciclovir is more effective in treating patients with HZ, which can effectively improve the inflammatory factors and can relieve the pain of patients, without increasing adverse reactions. In addition, underlying diseases and medication regimens are independent risk factors for the prognosis of patients with HZ.
Ethical statement:
This study was performed with permission from the Medical Ethics Committee of our hospital.
Conflict of interests:
The authors declared no conflict of interests.
References
- Schmader K. Herpes Zoster (Japanese Version). Ann Intern Med 2018;169(3):ITC17-32.
[Crossref] [Google Scholar] [PubMed]
- Rosamilia LL. Herpes zoster presentation, management, and prevention: A modern case-based review. Am J Clin Dermatol 2020;21(1):97-107.
[Crossref] [Google Scholar] [PubMed]
- Evans DD, Kaikai SM. Presentation, management, and prevention of herpes zoster. Adv Emerg Nurs J 2022;44(1):3-10.
[Crossref] [Google Scholar] [PubMed]
- Czech T, Nishimura Y. Characteristics of herpes zoster infection in patients with COVID‐19: A systematic scoping review. Int J Dermatol 2022;61(9):1087-92.
[Crossref] [Google Scholar] [PubMed]
- Asada H. Recent topics in the management of herpes zoster. J Dermatol 2023;50(3):305-10.
[Crossref] [Google Scholar] [PubMed]
- Lehrer S, Rheinstein PH. Herpes zoster vaccination reduces risk of dementia. In Vivo 2021;35(6):3271-5.
[Crossref] [Google Scholar] [PubMed]
- Junior HP, de Oliveira MF, Gambero S, Amazonas RB. Randomized clinical trial of famciclovir or acyclovir for the treatment of herpes zoster in adults. Int J Infect Dis 2018;72:11-5.
[Crossref] [Google Scholar] [PubMed]
- Gold LS, Bagel J, Allenby K, Sidgiddi S. Betamethasone dipropionate spray 0.05 % alleviates troublesome symptoms of plaque psoriasis. Cutis 2020;105(2):97-E1.
[Google Scholar] [PubMed]
- Sun X, Wei Z, Lin H, Jit M, Li Z, Fu C. Incidence and disease burden of herpes zoster in the population aged ≥50 years in China: Data from an integrated health care network. J Infect 2021;82(2):253-60.
[Crossref] [Google Scholar] [PubMed]
- Chiarotto A, Maxwell LJ, Ostelo RW, Boers M, Tugwell P, Terwee CB. Measurement properties of visual analogue scale, numeric rating scale, and pain severity subscale of the brief pain inventory in patients with low back pain: A systematic review. J Pain 2019;20(3):245-63.
[Crossref] [Google Scholar] [PubMed]
- Kelley A. Herpes zoster: A primary care approach to diagnosis and treatment. JAAPA 2022;35(12):13-8.
[Crossref] [Google Scholar] [PubMed]
- Joo T, Lee YC, Kim TG. Herpes zoster involving the abducens and vagus nerves without typical skin rash: A case report and literature review. Medicine 2019;98(19):e15619.
[Crossref] [Google Scholar] [PubMed]
- Park JM, Kim SE, Yang HC. Clinical characteristics of herpes zoster laryngitis. Eur Arch Laryngol 2020;277:2907-12.
[Crossref] [Google Scholar] [PubMed]
- Bist A, Savitha A, Gumma KM. Efficacy of valacyclovir and famciclovir in herpes zoster: A comparative study. Indian J Pharmacol 2020;52(6):472.
[Crossref] [Google Scholar] [PubMed]
- Arenas-Archila E, Alvizu F, Muñoz-Sarmiento D. Preauricular injection of betamethasone depot and acyclovir for the treatment of acute herpes zoster ophthalmicus. Arch Soc Esp Oftalmol 2015;90(4):195-7.
[Crossref] [Google Scholar] [PubMed]
- Crouch AE, Hohman MH, Moody MP, Andaloro C. Ramsay Hunt Syndrome. In: StatPearls 2023.
- Peng L, Du B, Sun L, Zhao Y, Zhang X. Short‑term efficacy and safety of prednisone in herpes zoster and the effects on IL‑6 and IL‑10. Exp Ther Med 2019;18(4):2893-900.
[Crossref] [Google Scholar] [PubMed]
- Ricke DO. Pain adverse events, Bell’s palsy, and Guillain-Barré syndrome following vaccination. J Mod Biol Drug Discov 2022;1:3.
- Liang J, Tian XF, Yang W. Psychological care can reduce pain intensity, relieve negative emotions, and improve the quality of life of patients with advanced gastrointestinal cancer. J Mod Nurs Pract Res 2021;1(1): 6.
- Liu K, Yin Y, Zhou X, Zhu K, Luo Z. Expression and correlation of IL-2, IL-10 and TNF-α in patients with multiple myeloma-infected herpes zoster treated by bortezomib-containing regimen. Am J Transl Res 2021;13(12):13732.
[Google Scholar] [PubMed]
- Maeda T, Yoshizawa S, Hirayama T, Saga T, Tateda K, Urita Y. Neurosyphilis mimicking Ramsay Hunt syndrome. J Nippon Med Sch 2015;82(5):254-6.
[Crossref] [Google Scholar] [PubMed]
- Xiao L, Zou J, Fang F. Study of the therapeutic effects of betamethasone injection combined with musculoskeletal ultrasonography compared with radial shock wave therapy in the treatment of tenosynovitis of the long head of the biceps brachii. Am J Transl Res 2021;13(3):1734-41.
[Google Scholar] [PubMed]
 Control group
Control group



