- *Corresponding Author:
- M. Liu
Department of Gastrointestinal and Hepatobiliary Surgery, Wuhan Wuchang Hospital, Wuchang Hospital Affiliated to Wuhan University of Science and Technoloy, Wuhan, Hubei Province 430063, China
E-mail: 15827187072@163.com
| This article was originally published in a special issue, “Exploring the Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(1) Spl Issue “235-243” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To authenticate the clinical outcome of camrelizumab united with raltitrexed in the therapy of advanced gastric cancer and postoperative recurrent and metastatic gastric cancer. From January 2018 to March 2021, the clinical data of 80 sufferers with advanced gastric cancer and postoperative recurrent and metastatic gastric cancer treated in our hospital were estimated retrospectively. According to the different therapy methods, the sufferers were separated into two subgroups. 42 sufferers in the survey subgroup were treated with camrelizumab monoclonal antibody plus raltitrexed plus routine chemotherapy, and 38 sufferers in the control subgroup were treated with raltitrexed plus routine chemotherapy for 3 consecutive cycles. The short-term efficacy, serum tumor markers (carcinoembryonic antigen, carbohydrate antigen 19-9), adverse reactions and survival were compared among the two subgroups. The objective response rate and disease control rate in the survey subgroup were 54.76 % and 80.95 % respectively, which were notablely boosted than those in the control subgroup (31.58 % and 57.89 %). After therapy, the concentrations of serum carcinoembryonic antigen and carbohydrate antigen 19-9 in the survey subgroup were notablely lessened than those in the control subgroup. There were no IV grade serious adverse reactions and fewer III grade adverse reactions among the two subgroups. There was no notable divergence in the incidence of common adverse reactions such as nausea and vomiting, liver and kidney function damage, myelosuppression, skin rash, anemia and reactive cutaneous capillary endothelial proliferation among the two subgroups. The median progression-free survival in the survey subgroup and the control subgroup was 9.31 mo and 6.57 mo respectively, and the divergence was statistically notable. Camrelizumab united with raltitrexed can improve the short-term and long-term efficacy of advanced gastric cancer and postoperative recurrent and metastatic gastric cancer with high safety and no increase of drug side outcomes.
Keywords
Advanced gastric cancer, mortality, gastric cancer, camrelizumab, raltitrexed, tumor markers, chemotherapy
According to the epidemiological survey[1,2], the incidence of gastric cancer ranks 5th in the world, and the mortality rate ranks 4th in the world. Its morbidity and mortality rank 2nd in malignant tumors in China, which seriously threatens people’s lives and health. Early gastric cancer can be cured by surgery, but most of the sufferers with gastric cancer in our country have entered the middle and advanced stage at the time of diagnosis and cannot undergo radical operation[3]. The median survival time of metastatic gastric cancer is <1 y, but targeted drugs can be used to improve survival and reduce toxicity[4]. There is no definite and effective therapy for advanced gastric cancer or postoperative recurrent and metastatic gastric cancer, mainly chemotherapy[5]. Although chemotherapy drugs used in the treatment of unrespectable advanced or metastatic gastric cancer patients will lead to adverse reactions of physical function, patients with poor tolerance and poor prognosis, but chemotherapy remains a major obstacle that reduces efficacy[6,7].
Raltitrexed, a specific Thymidylate Synthase (TS) inhibitor, can inhibit Deoxyribonucleic Acid (DNA) synthesis and promote tumor cell apoptosis by inhibiting TS. It has been approved for advanced colorectal cancer[8]. Related clinical trials found that raltitrexed also has a certain therapeutic outcome on advanced gastric cancer[9], and the side outcomes can be tolerated. Advanced gastric cancer and postoperative recurrent and metastatic gastric cancer have always been the focus of clinical attention. However, treatment-related mortality is high in metastatic colorectal cancer patients taking raltitrexed, which restricts the use of raltitrexed[10]. A study found that the combination of cisplatin and raltitrexed improved the prognosis of patients with mesothelioma without affecting the quality of life[11]. In addition, both S1 and 5-Fluorouracil (5-FU) were used in combination with raltitrexed, thus improving the therapeutic effect of raltitrexed[12,13]. Therefore, it is particularly important to explore a new combination of raltitrexed therapy in the treatment of advanced gastric cancer.
In recent years, Immune Checkpoint Inhibitors (ICIs) have been favored. Camrelizumab, a Programmed Death receptor 1 (PD-1) inhibitor developed by China, was approved to market on May 29th, 2019. The main indications are recurrent or refractory Hodgkin’s lymphoma, esophageal cancer, lung cancer and liver cancer[14]. Clinical trials found that camrelizumab corroborated good anti-tumor outcome on a variety of solid malignant tumors[15]. Camrelizumab combined with trastuzumab has been reported to be beneficial and well tolerated in Human Epidermal Growth Factor Receptor 2 (HER2) positive patients with advanced gastric cancer[16]. In addition, camrelizumab combined with abraxane+carboplatin has been found to be an effective method for the treatment of advanced gastric cancer and can improve the survival rate of patients[17]. However, the effect of camrelizumab united with raltitrexed in the therapy of advanced gastric cancer and postoperative recurrent and metastatic gastric cancer is unknown. At present, camrelizumab is rarely utilized in advanced gastric cancer and postoperative recurrent and metastatic gastric cancer. Based on this, this report explored the efficacy of camrelizumab united with raltitrexed in the therapy of advanced gastric cancer and postoperative recurrent and metastatic gastric cancer, in order to provide ideas and references for clinical optimization of therapy.
Materials and Methods
General information:
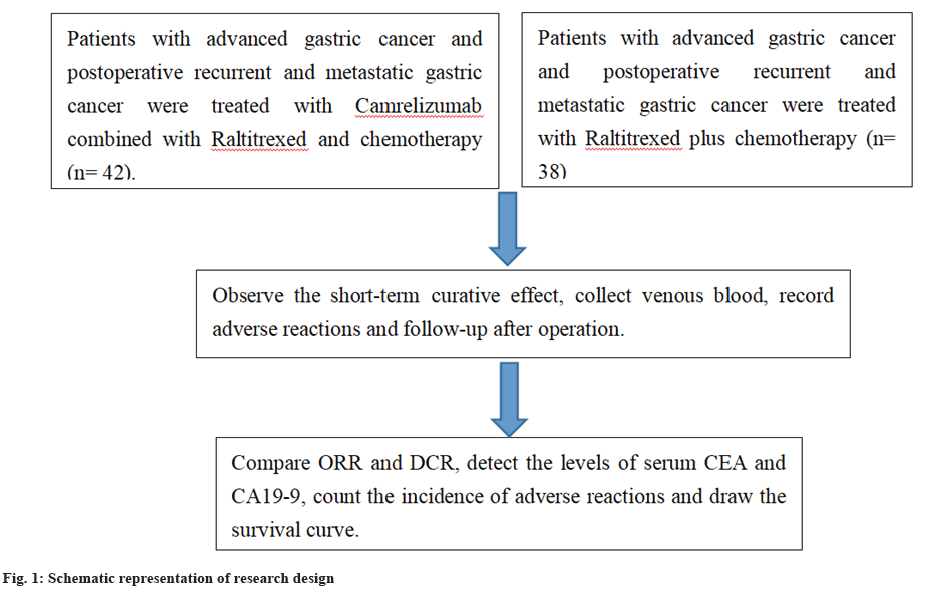
From January 2018 to March 2021, the clinical data of sufferers with advanced gastric cancer and postoperative recurrent and metastatic gastric cancer treated in our hospital were estimated retrospectively.
Inclusion criteria: 18 y-70 y old; diagnosed as advanced gastric cancer or postoperative recurrent and metastatic gastric cancer by gastroscopy or pathological examination; at least one detectable lesion with an estimated survival time of >3 mo; Karnofsky (KPS) ≥70; took camrelizumab united with raltitrexed and routine chemotherapy or raltitrexed and routine chemotherapy and the clinical data were complete[18].
Exclusion criteria: Sufferers merged with other malignant tumors; sufferers with abnormal function of heart, liver and kidney; sufferers who were treated with other regimens midway and pregnant or lactating female sufferers. Finally, 80 sufferers were included and divided into two subgroups according to different therapy schemes. 42 sufferers in the survey subgroup were treated with camrelizumab monoclonal antibody plus raltitrexed plus routine chemotherapy, and 38 sufferers in the control subgroup were treated with raltitrexed plus routine chemotherapy for 3 consecutive cycles. In the survey subgroup, there were 25 males and 17 females, 20 instances of advanced gastric cancer, 22 instances of recurrent and metastatic gastric cancer after operation, the age was 35 y to 68 y old, mean (54.81±12.34) y, and the pathological type was adenocarcinoma (n=37) and other 5 instances. In the control subgroup, there were 22 males and 16 females, 17 sufferers with advanced gastric cancer, 21 sufferers with recurrent and metastatic gastric cancer after operation, the age was 38 y-67 y old, mean (53.52±13.80) y, and the pathological type was adenocarcinoma (32 instances) and other 6 instances. There was no notable divergence in the general data among the two subgroups (p>0.05)[19].
Therapy methods:
The control subgroup was treated with raltitrexed plus routine chemotherapy (platinum+fluorouracil). The dose of raltitrexed was 3 mg/m², which was infused intravenously on the day of chemotherapy. In the survey subgroup, on the basis of the control subgroup, united with camrelizumab monoclonal antibody, the dose was 200 mg, intravenous drip on the day of chemotherapy. Both subgroups took 3 w courses for 3 consecutive courses of therapy.
During the therapy, symptomatic therapies were given, such as anti-allergy, anti-vomiting, protection of gastric mucosa, liver protection and maintenance of water and electrolyte balance.
After each course of therapy, blood routine examination and Computed Tomography (CT) examination were performed to record the occurrence of adverse reactions. After the therapy, the sufferers were followed up by telephone once a month until March 2022.
Observation indicators:
Short-term efficacy: Referred to the evaluation criteria of Response Evaluation Criteria in Solid Tumors (RECIST)[20] to evaluate the short-term efficacy of the two subgroups, Complete Remission (CR) means tumor lesions disappeared and no new lesions were found; Partial Remission (PR) means the maximum diameter of tumor was reduced by >30 %; Disease Stabilization (SD) means the maximum diameter of the tumor shrank by <30 % or increased by <20 % and Disease Progression (PD) means the maximum diameter of the tumor increased by >20 % or new lesions were found.
Objective Response Rate (ORR)=(CR+PR) number of instances/total number of instances×100 %
Disease Control Rate (DCR)=(CR+PR+SD)/total number of instances×100 %
Concentration of serum tumor markers: Fasting venous blood 5 ml was collected before therapy and within 1 w after therapy, the supernatant was collected after centrifugation. The expression of Carcinoembryonic Antigen (CEA) and Carbohydrate Antigen 19-9 (CA19-9) in serum was detected by Chemiluminescence immunoassay.
Adverse reactions: The intensity of adverse reactions (I-IV) of the two subgroups was evaluated according to the classification and evaluation standard of common toxic and side outcomes of anticancer drugs (National Cancer Institute Common Toxicity Criteria (NCICTC) 3.0) formulated by World Health Organization (WHO)[21]. The common adverse reactions included nausea and vomiting, liver and kidney function damage, bone marrow suppression, rash, anemia, Reactive Cutaneous Capillary Endothelial Proliferation (RCCEP), etc.
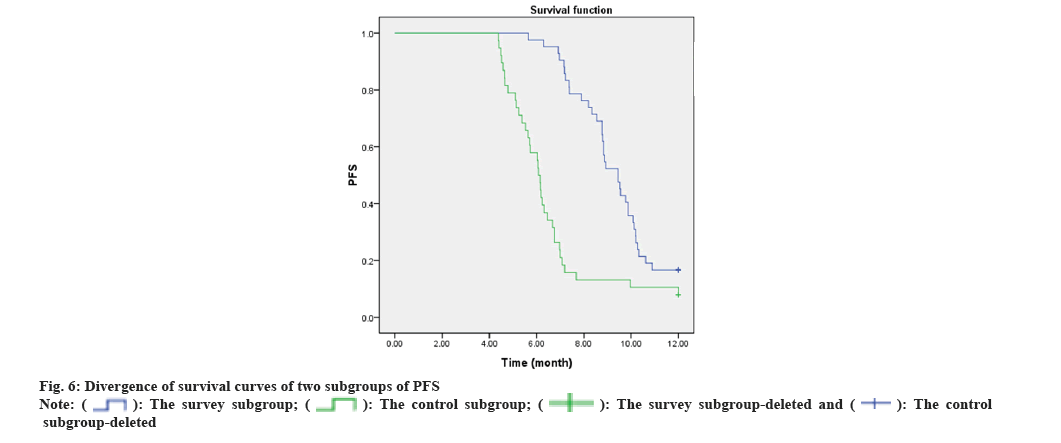
Long term efficacy: Estimated the follow-up data of the two subgroups within 1 y after the end of therapy, counted the survival of the sufferers, and estimated the median Progression Free Survival (PFS) (the time from the start of therapy to the time when the illness progresses or dies) by drawing the survival curve.
Data statistics:
Statistical Package for the Social Sciences (SPSS) 22.0 software was utilized to analyze the data. The measurement data conformed to the normal distribution and had uniform variance, which was expressed by (x̄±s) and tested by independent sample t-value; the counting data were expressed by n (%) and tested by Chi-square (χ²)/Fisher exact probability method; Kaplan Meier method was utilized to plot the survival curve, and log-rank test was performed. With p<0.05 as the statistical significance was shown in fig. 1.
Results and Discussion
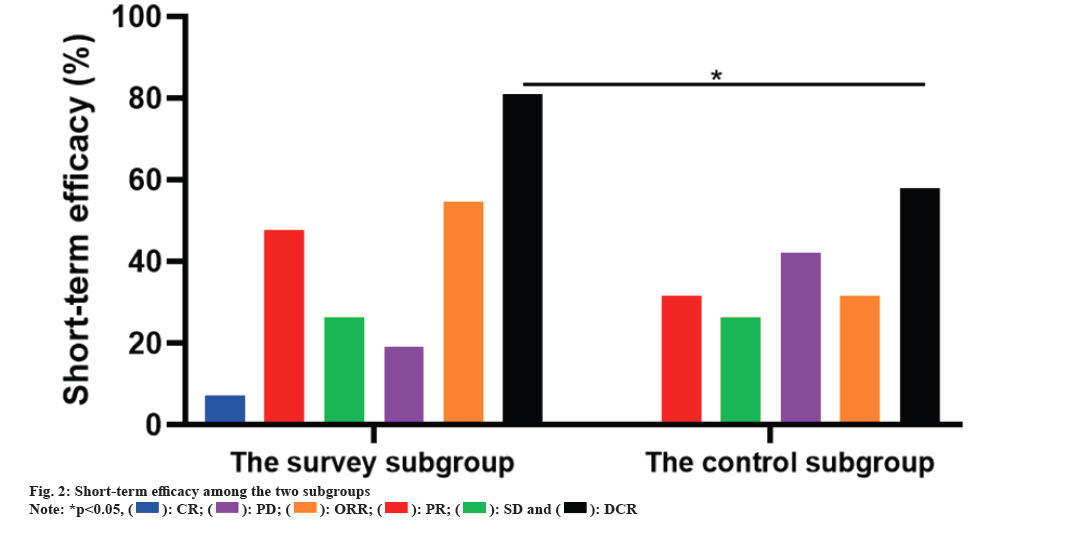
The ORR and DCR of the survey subgroup were 54.76 % and 80.95 % respectively, which were notably boosted than those of the control subgroup (31.58 % and 57.89 %, p<0.05). As corroborated in Table 1 and fig. 2.
| Group | n | CR | PR | SD | PD | ORR | DCR |
|---|---|---|---|---|---|---|---|
| Survey | 42 | 3 (7.14) | 20 (47.62) | 11 (26.19) | 8 (19.05) | 23 (54.76) | 34 (80.95) |
| Control | 38 | 0 (0.00) | 12 (31.58) | 10 (26.32) | 16 (42.11) | 12 (31.58) | 22 (57.89) |
| χ² | 4.357 | 5.051 | |||||
| p | 0.037 | 0.025 |
Table 1: Divergence of short-term efficacy among the two subgroups n (%).
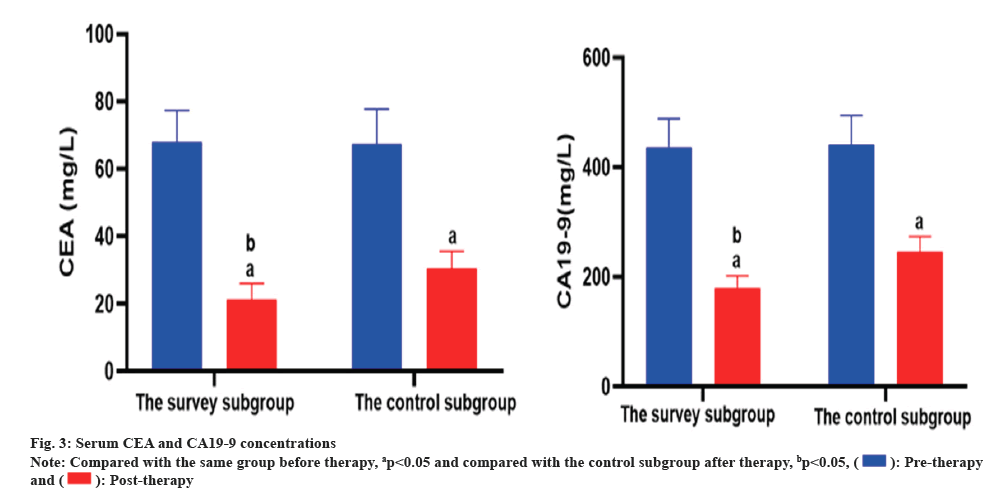
Before therapy, there was no notable divergence in the concentrations of serum CEA and CA19-9 among the two subgroups (p>0.05), but after therapy, the concentrations of serum CEA and CA19-9 in the two subgroups were notably lessened than those before therapy (p<0.05), and the concentrations in the survey subgroup were notably lessened than those in the control subgroup (p<0.05). As corroborated in Table 2 and fig. 3.
| Group | n | CEA | CA19-9 | ||
|---|---|---|---|---|---|
| Pre-therapy | Post-therapy | Pre-therapy | Post-therapy | ||
| Survey | 42 | 67.85±9.54 | 21.27±4.81a | 436.50±52.13 | 180.34±22.01a |
| Control | 38 | 67.46±10.33 | 30.38±5.25a | 440.62±54.00 | 245.62±28.05a |
| t | 0.176 | 8.1 | 0.347 | 11.636 | |
| p | 0.861 | <0.001 | 0.73 | <0.001 | |
Note: ap<0.05, contrasted to the same subgroup before therapy
Table 2: Divergence of serum CEA and CA19-9 concentrations among the two subgroups (x̄±s, mg/l).
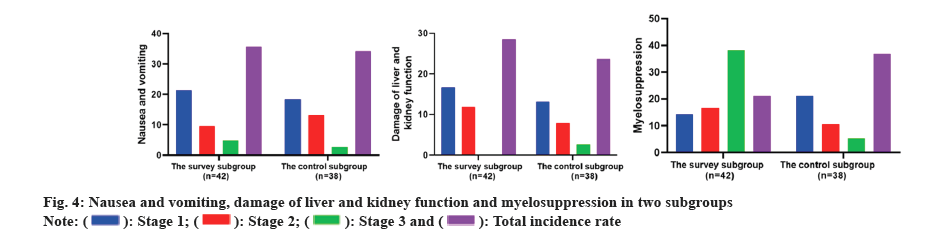
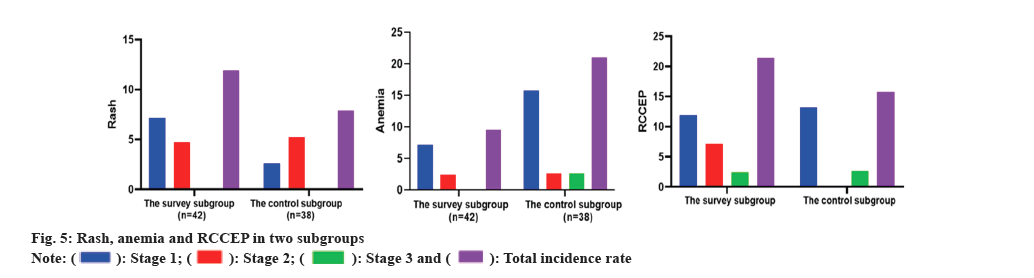
There was no serious adverse reaction of grade IV among the two subgroups, and there was no notable divergence in the incidence of nausea and vomiting, liver and kidney function damage, myelosuppression, rash, anemia and RCCEP among the two subgroups. As corroborated in Table 3 and, fig. 4 and fig. 5.
| Adverse reaction | Survey subgroup (n=42) | Control subgroup (n=38) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | Total incidence rate | Stage I | Stage II | Stage III | Total incidence rate | χ² | p | |
| Nausea and vomiting | 9 (21.43) | 4 (9.52) | 2 (4.76) | 15 (35.71) | 7 (18.46) | 5 (13.16) | 1 (2.63) | 13 (34.21) | 0.02 | 0.888 |
| Damage of liver and kidney function | 7 (16.67) | 5 (11.90) | 0 (0.00) | 12 (28.57) | 5 (13.16) | 3 (7.89) | 1 (2.63) | 9 (23.68) | 0.246 | 0.62 |
| Myelosuppression | 6 (14.29) | 7 (16.67) | 3 (7.14) | 16 (38.10) | 8 (21.05) | 4 (10.53) | 2 (5.26) | 14 (36.84) | 0.013 | 0.908 |
| Rash | 3 (7.14) | 2 (4.76) | 0 (0.00) | 5 (11.90) | 1 (2.63) | 2 (5.26) | 0 (0.00) | 3 (7.89) | - | 0.715 |
| Anemia | 3 (7.14) | 1 (2.38) | 0 (0.00) | 4 (9.52) | 6 (15.79) | 1 (2.63) | 1 (2.63) | 8 (21.05) | 2.08 | 0.419 |
| RCCEP | 5 (11.90) | 3 (7.14) | 1 (2.38) | 9 (21.43) | 5 (13.16) | 0 (0.00) | 1 (2.63) | 6 (15.79) | 0.416 | 0.519 |
Note: (-): Fisher exact probability method
Table 3: Divergence of adverse reactions among the two subgroups n (%).
The median PFS of the survey subgroup was 9.31 mo (95 % CI 8.797~9.827), and that of the control subgroup was 6.57 mo (95 % CI 5.873~7.264). The median PFS of the survey subgroup was notably longer than that of the control subgroup, and the divergence was statistically notable (χ²=20631, p<0.001). As corroborated in fig. 6.
With the development of medical science and technology, and the change of diet life structure, malignant tumor has become a common clinical illness, and tens of millions of people are plagued by malignant tumor every year. According to the report[22], the incidence of gastric cancer in China is the highest in the world, and the 5 y survival rate is only 27.4 %. Although the mortality rate has declined in recent years, it is becoming younger, and the situation is not optimistic. The reason why gastric cancer has become a difficult problem in clinical diagnosis and therapy is that some sufferers are already in the late stage, and they are easy to relapse and metastasis after operation, so they cannot be cured by operation. At the same time, the outcome of systemic chemotherapy is limited. At present, there is no standardized therapy plan in the world. The Chinese society of Clinical Oncology (CSCO) guidelines for the diagnosis and therapy of primary gastric cancer proposed that the first-line therapy scheme should be determined according to the expression of HER2 in sufferers with advanced gastric cancer[23]. Trastuzumab+cisplatin+fluorouracil should be utilized for HER2 positive sufferers, and cisplatin+fluorouracil should be utilized for HER2 negative sufferers; paclitaxel+ramucirumab is recommended as the second-line therapy scheme; the third line of therapy is apatinib and pembrolizumab. In conclusion, the clinical therapy of advanced gastric cancer and postoperative recurrent and metastatic gastric cancer still needs to be breakthrough.
On the basis of conventional chemotherapy regimen, this report compared the efficacy of camrelizumab combined with raltitrexed and raltitrexed alone in the therapy of advanced gastric cancer and postoperative recurrent and metastatic gastric cancer, the results corroborated that the ORR and DCR of the survey subgroup were 54.76 % and 80.95 %, which were boosted than those of the control subgroup (31.58 % and 57.89 %), indicating that camrelizumab monoclonal antibody united with raltitrexed could improve the short-term outcome of advanced gastric cancer. Wang et al.[24] also found that after the observation group was treated with irinotecan on the basis of chemotherapy in the control group, the short-term effect of the observation group was better than that of the control group. Systemic chemotherapy is the main therapy for advanced gastric cancer, but conventional chemotherapy program with platinum+fluorouracil is not effective and the curative outcome is limited[25]. With the emergence of new anti-tumor drugs, chemotherapy combined regimens have improved and advanced the therapy of advanced gastric cancer. As an anti-tumor drug officially approved to be marketed in China in 2010, raltitrexed was initially utilized to treat sufferers with advanced colorectal cancer who were intolerant to 5-FU/calcium folinate, and then it was also utilized in esophageal cancer, advanced gastric cancer, malignant pleural mesothelioma and other solid tumors. It has been proven that it can improve the short-term efficacy of sufferers[26]. After entering the cells, raltitrexed is metabolized into polyglutamic acid under the action of folylpolyglutamate synthase, which inhibits DNA synthesis of tumor cells by inhibiting thymus synthetize, thus promoting cell death and playing an anti-tumor role. At the same time, the drug does not need activation, has a long half-life and is well tolerated[27]. A report has corroborated that contrasted to 5-FU+oxaliplatin, raltitrexed+oxaliplatin has more advantages in the therapy of advanced gastric cancer[28], with higher ORR (47.6 % vs. 31.8 %), longer PFS (7.8 mo vs. 6.6 mo) and tolerable side outcomes. Camrelizumab is a PD-1 inhibitor independently developed in China. It can target and bind PD-1 molecules, block the binding of PD-1 with its ligands PD-L1 and PD-L2, thereby relieving the immunosuppression mediated by this signaling pathway, activating T lymphocytes, and continuously exerting anti-tumor outcomes[29]. The anti-tumor mechanism of camrelizumab is different from that of raltitrexed. The combination of camrelizumab and raltitrexed has synergistic outcome, stronger anti-tumor outcome and better curative outcome. Recent related report also pointed out that PD-1 inhibitor united with apatinib and chemotherapy had better efficacy in the therapy of HER2 negative advanced gastric cancer, and the DCR increased from 44.4 % to 75.7 %[30]. In this report, the serum concentrations of CEA and CA19-9 in the survey subgroup were notably lessened than those in the control subgroup after therapy, which also shows that the combination of camrelizumab and raltitrexed can improve the short-term efficacy of advanced gastric cancer, and postoperative recurrent and metastatic gastric cancer.
Adverse reactions are the main factors affecting the anti-tumor outcome of drugs, a report on the safety of clinical application of camrelizumab pointed out that the main adverse reactions of camrelizumab were immune related adverse events[31], which could involve the skin, lung, liver and endocrine system, most of which were mild to moderate, and few were severe adverse reactions. The results of this report corroborated that there were no grade IV adverse reactions in the two subgroups, and there was no statistical divergence in the incidence of nausea and vomiting, liver and kidney function damage, myelosuppression, rash, anemia, RCCEP and other common adverse reactions, indicating that camrelizumab is safe and effective in the therapy of advanced gastric cancer, and postoperative recurrent and metastatic gastric cancer, and the sufferers have good tolerance. Some studies believe that camrelizumab will increase the risk of grade III or higher REECP[32-34], which may be caused by the imbalance among angiogenesis enhancers and inhibitors, resulting in capillary endothelial proliferation, but it is notably improved after symptomatic therapy, which also shows that camrelizumab is safe and feasible for the therapy of advanced gastric cancer. This report also estimated that the median PFS of the survey subgroup was longer than that of the control subgroup (9.31 mo vs. 6.57 mo), indicating that camrelizumab united with raltitrexed can benefit the survival of sufferers with advanced gastric cancer and postoperative recurrent and metastatic gastric cancer. The recent ATTRACTION-3 clinical trial corroborated that the PD-1 inhibitor nivolumab united with chemotherapy can prolong the PFS of sufferers with HER2 negative advanced gastric cancer (10.45 mo vs. 8.34 mo), which is similar to the results of this report[35].
To conclude, camrelizumab united with raltitrexed can control the illness progression of sufferers with advanced gastric cancer and postoperative recurrent and metastatic gastric cancer, prolong PFS, and tolerate toxic and side outcomes. At the same time, there are some limitations in this report, such as the small number of included instances and the short follow-up time. The results need to be further verified by large sample size and multi center studies.
Author contributions:
Chao Liu was major contributors in writing the manuscript. Tianping Chen and Mingkui Liu collected the patient data, did literature searches, and revised the manuscript. All authors read and approved the final manuscript. And Chao Liu and Tianping Chen have contributed equally to this work and share first authorship.
Conflict of interests:
The authors declared no conflict of interests.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66(2):115-32.
- Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34(13):1448-54.
- Patel TH, Cecchini M. Targeted therapies in advanced gastric cancer. Curr Treat Options Oncol 2020;21:1-4.
- Liu X, Guo W, Zhang W, Yin J, Zhang J, Zhu X, et al. A multi-center phase II study and biomarker analysis of combined cetuximab and modified FOLFIRI as second-line treatment in patients with metastatic gastric cancer. BMC Cancer 2017;17(1):188.
- Tabernero J, Ohtsu A, Muro K, van Cutsem E, Oh SC, Bodoky G, et al. Exposure-response analyses of ramucirumab from two randomized, phase III trials of second-line treatment for advanced gastric or gastroesophageal junction cancer. Mol Cancer Ther 2017;16(10):2215-22.
- Wang J, Zhang G, Dai C, Gao X, Wu J, Shen L, et al. Cryptotanshinone potentiates the antitumor effects of doxorubicin on gastric cancer cells via inhibition of STAT3 activity. J Int Med Res 2017;45(1):220-30.
- Avallone A, Gennaro ED, Silvestro L, Iaffaioli VR, Budillon A. Targeting thymidylate synthase in colorectal cancer: Critical re-evaluation and emerging therapeutic role of raltitrexed. Expert Opin Drug Saf 2014;13(1):113-29.
- Yue S, Zhang D, Zhou L. Clinical efficacy of raltitrexed combined with docetaxel for advanced gastric cacinoma. Cancer Res Prev Treat 2014;41(2):160-2.
- Wilson KS, Malfair Taylor SC. Raltitrexed: Optimism and reality. Expert Opin Drug Metab Toxicol 2009;5(11):1447-54.
- Surmont VF, van Meerbeeck JP. Raltitrexed in mesothelioma. Expert Rev Anticancer Ther 2011;11(10):1481-90.
- Chen Y, Wu J, Cheng K, Li ZP, Luo DY, Qiu M, et al. S-1 plus raltitrexed for refractory metastatic colorectal cancer: A phase II trial. Oncologist 2019;24(5):591-65.
- Schwartz GK, Harstrick A, Baron MG. Raltitrexed (Tomudex™) in combination with 5-fluorouracil for the treatment of patients with advanced colorectal cancer: Preliminary results from phase I clinical trials. Eur J Cancer 1999;35(1):9-13.
- Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther 2015;9:6075-81.
- Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): An open-label, single-arm, phase 2 trial. Lancet Oncol 2020;21(8):1057-65.
- Xu M, Meng X, Lu Y, Wang F. Efficacy and safety of camrelizumab in combination with trastuzumab and chemotherapy as the first-line treatment for patients with HER2-positive advanced gastric cancer. J Gastrointest Oncol 2022;13(2):548-58.
- Ma J, Zhang W, Du J, Li J, Lin G, Tian Y. Efficacy and safety of camrelizumab combined with abraxane+lobaplatin regimen for advanced gastric cancer. Am J Transl Res 2023;15(2):1485-93.
- Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Effect of laparoscopic vs. open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: The CLASS-01 randomized clinical trial. JAMA 2019;321(20):1983-92.
- Luo R, Liu D, Ye S, Tang H, Zhu W, He P, et al. Short-and long-term outcomes of totally robotic vs. robotic-assisted radical distal gastrectomy for advanced gastric cancer: A mono-institution retrospective study. World J Surg Oncol 2019;17(1):188.
- Eisenhauer E, Therasse P, Bogaerts J, Schwartz L, Sargent D, Ford R, et al. 32 INVITED New response evaluation criteria in solid tumors: Revised RECIST guideline version 1.1. Eur J Cancer 2008;12(6):13.
- Program C. Common terminology criteria for adverse events v3.0 (CTCAE). Int J Clin Oncol 2004.
- Qiu MZ, Wang ZX, Zhou YX, Yang DJ, Wang FH, Xu RH. Proposal for a new TNM stage based on the 7th and 8th American Joint Committee on Cancer pTNM staging classification for gastric cancer. J Cancer 2018;9(19):3570.
- Xu RH, Zhou ZW, Shen L. Original Chinese Society of Clinical Oncology (CSCO) guidelines for the diagnosis and treatment of primary gastric cancer (2017.V1). Beijing: People's Health Publishing House Society; 2017.p. 42-8.
- Wang JP, Du JL, Li YY. Short-term efficacy and influencing factors of conventional chemotherapy combined with irinotecan in patients with advanced gastric cancer. World J Gastrointest Oncol 2023;15(1):143.
- Solomon BL, Garrido-Laguna I. Upper gastrointestinal malignancies in 2017: Current perspectives and future approaches. Future Oncol 2018;14(10):947-62.
- Wu T, Chen D, Zhi J. Clinical use of raltitrexed. Chin J Mod Appl Pharm 2014;31(5):643-6.
- Scott LJ. Apatinib: A review in advanced gastric cancer and other advanced cancers. Drugs 2018;78(7):747-58.
- Qiu X, Li J, Zhou H, Zhang M, Jiang C, Shen Z, et al. Concurrent chemoradiotherapy with raltitrexed and nedaplatin regimen for esophageal squamous cell carcinoma. Medicine 2020;99(4):e18732.
- He Y, Peng YZ, Ji ZN. Clinical study of oxaliplatin plus raltitrexed vs. oxaliplatin plus fluorouracil as first-line treatment for advanced gastric cancer. Chin J Clin Pharmacol Ther 2015;20(2):194-8.
- Tang L, Wu Q. Clinical outcomes and safety analysis for PD-1 inhibitor plus apatinib and chemotherapy in HER2 negative advanced gastric cancer. Chin J Cancer Prev Treat 2022,29(9):674-86.
- Huang C, Qi LM, Li DH. A analysis of safety of 73 users of carrelizumab. Chin J Pharmacovigil 2022;19(3):292-301.
- Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim IK, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 2017;544(7649):250-4.
- Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Wyser Rmili C, Kiialainen A, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med 2017;9(385): 9670.
- Chen X, Ma L, Wang X, Mo H, Wu D, Lan B, et al. Reactive capillary hemangiomas: A novel dermatologic toxicity following anti-PD-1 treatment with SHR-1210. Cancer Biol Med 2019;16(1):173-81.
- Boku NM, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, et al. LBA7_PR Nivolumab plus chemotherapy vs. chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538-37) study. Ann Oncol 2020;31:S1192.
 .
.

 .
.
 .
.
 .
.
 subgroup-deleted.
subgroup-deleted.



