- *Corresponding Author:
- Xiaorong Liu
Department of Ultrasound, Bishan Maternity & Child Hospital of Chongqing, Chongqing 402760, China
E-mail: 13648228036@163.com
| This article was originally published in a special issue, “Recent Progression in Pharmacological and Health Sciences” |
| Indian J Pharm Sci 2024:86(2) Spl Issue “237-242” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This investigation sought to examine the clinical effectiveness of utilizing both clindamycin and ultrasound in the management of chronic pelvic inflammatory disease. A retrospective review was undertaken for 102 patients diagnosed with chronic pelvic inflammatory disease who were hospitalized at our facility from August 2020 to August 2023. Clindamycin and conventional nutritional support were provided to the control group, whereas the treatment group received supplementary ultrasound therapy in addition to the control group regimen, and both groups were subjected to uninterrupted treatment for duration of 1 mo. The duration of symptom alleviation, encompassing lumbosacral pain, fatigue, and increased vaginal discharge, was documented during the treatment period. Pre- and post-treatment assessments included the measurement of inflammatory markers (interleukin-1 beta, interleukin-10, and tumor necrosis factor alpha) and immune markers (cluster of differentiation 3+, cluster of differentiation 4+ and cluster of differentiation 8+). In addition, Doppler ultrasound was employed to analyze the pelvic blood flow dynamics indicators (pulsatility index, resistance index, peak systolic velocity) of the ovarian and uterine arteries. In comparison to the control group, there was a notable enhancement in the blood flow dynamics indicators (pulsatility index, resistance index, peak systolic velocity) of the ovarian and uterine arteries in the treatment group (p<0.05). Moreover, there was no noteworthy variance in the occurrence of adverse reactions between the two groups. The use of clindamycin in conjunction with ultrasound therapy demonstrated favorable clinical effectiveness among individuals with chronic pelvic inflammatory disease by alleviating symptoms, ameliorating the inflammatory condition, adjusting immune function, and enhancing blood circulation.
Keywords
Chronic pelvic inflammatory disease, clindamycin, ultrasound therapy, clinical efficacy
Chronic Pelvic Inflammatory Disease (PID) is a prevalent gynecological condition with a high frequency of occurrence. It often stems from the inadequate treatment of acute PID. Patients with chronic PID frequently experience symptoms such as lower abdominal distension and persistent lumbosacral pain, significantly impacting their daily routines. Additionally, neglecting timely intervention over an extended period can culminate in infertility, profoundly influencing the physical and mental health of patients. Hence, proactive treatment is imperative in clinical practice[1,2].
Presently, the treatment modalities for chronic PID encompass antibiotic therapy, physiotherapy, and surgical measures[3-8]. Classified under the lincosamide class of antibiotics, clindamycin phosphate exerts its antibacterial effects by converting into clindamycin in the body. Acknowledged for its robust antibacterial efficacy and minimal undesired effects, it is acknowledged in medical practice[9,10]. Nevertheless, protracted and excessive use of antibiotics may result in bacterial resistance and secondary infections, ultimately leading to suboptimal overall effectiveness[11]. Additionally, the invasiveness and protracted recovery time constrain the utilization of surgical intervention[12].
In recent times, ultrasound therapy has been broadly adopted as a non-invasive treatment modality for addressing PID[13,14]. Demonstrating antimicrobial, sterilizing, anti-inflammatory, and analgesic properties, ultrasound aids in resolving inflammation and ameliorating pelvic circulation, thereby producing therapeutic outcomes for chronic PID[15,16]. However, stand-alone ultrasound therapy might not be the best option for some patients with chronic PID. Thus, the focus of this study is to investigate the clinical effectiveness of integrating clindamycin with ultrasound therapy for individuals dealing with chronic PID.
Materials and Methods
General information:
A retrospective review of 102 patients with chronic PID who were admitted to our hospital from August 2020 to August 2023 was conducted.
Inclusion criteria: Patients who met the diagnostic criteria and signed the informed consent form; ages between 20 y and 40 y; approved by the hospital’s relevant ethical committee.
Exclusion criteria: Patients allergic to study drugs or intolerant to ultrasound treatment; patients in pregnancy or lactation; those with other visceral diseases and abdominal hemorrhagic diseases; patients with gynecological tumors and patients with incomplete medical records. In the group of 102 cases, the mean age was recorded as 29.75±4.57 y, with a disease duration spanning from 1 y to 6 y and averaging 3.24±0.58 y. The severity of the condition was classified as 32 cases of mild, 60 cases of moderate and 10 cases of severe. Through random grouping, the patients were distributed into an observation group (50 cases) and a control group (52 cases). The absence of remarkable variations in age, disease duration, and severity between the two groups (p>0.05) underscores their comparability.
Treatment methods:
Patients in the control group were treated with clindamycin and routine nutritional support. Clindamycin phosphate capsules (produced by North China Pharmaceutical Co., Ltd., H20070164) were administered at a dosage of 150-300 mg, orally for every 6 h and patients were offered comprehensive support, including standard nursing care, a scientifically balanced diet, and measures to enhance the quality of sleep. The treatment persisted for a month, with responsive measures implemented to manage any adverse reactions during this timeframe.
Ultrasound therapy was administered to the treatment group patients, utilizing domestically produced ultrasound equipment operating at a frequency of 800 kHz and an intensity of 1.2-1.5 w/cm², in addition to the treatments provided to the control group. The continuous wave and movement technique was employed on the abdomen for 15 min per session, once a day, with treatment briefly halted during the patient’s menstrual period. This uninterrupted treatment continued for 1 mo.
Observational indicators and evaluation criteria:
Record the timeframe of symptom alleviation, spanning lumbosacral pain, fatigue, and increased vaginal discharge, throughout the treatment period for both groups.
Prior to and following the treatment, the patient’s fasting basilic vein blood to the tune of 6 ml was gathered, with 3 ml of this being subjected to centrifugation (at a radius of 9 cm, 2900 r/min for 13 min) and the resulting serum stored in a -20° refrigerator for future analysis. The enzyme-linked immunosorbent assay kits (produced by Shanghai Enzyme-linked Biotechnology Co., Ltd.) will be employed to detect Interleukin-1 Beta (IL-1β), Interleukin-10 (IL-10), and Tumor Necrosis Factor Alpha (TNF-α). Additionally, 3 ml will be tested using the ZS-AE7S flow cytometer (produced by Zhongsheng (Suzhou) Medical Technology Co., Ltd.) to measure Cluster of Differentiation (CD) 3+, CD4+, and CD8+, and calculate CD4+/CD8+.
Before and after treatment, use the DW-T3 Doppler ultrasound diagnostic instrument (produced by Dawei Medical (Jiangsu) Co., Ltd.) to investigate the pelvic blood flow dynamics in both the ovarian and uterine arteries, measuring the Pulsatility Index (PI), Resistance Index (RI), and Peak Systolic Velocity (PSV).
Document any adverse reactions that may arise during the treatment period for both group, encompassing gastrointestinal discomfort, drowsiness, nausea and vomiting, and skin allergies.
Statistical analysis:
Utilizing the Statistical Package for the Social Sciences (SPSS) 25.0 statistical software, the analysis was performed. By utilizing an independent sample t-test, a comparison between the two groups was performed using the measurement data presented as mean±standard deviation. Utilizing the Chi-square (χ²) test, the count data were analyzed. The criteria for establishing statistical significance were defined at a level of p<0.05.
Results and Discussion
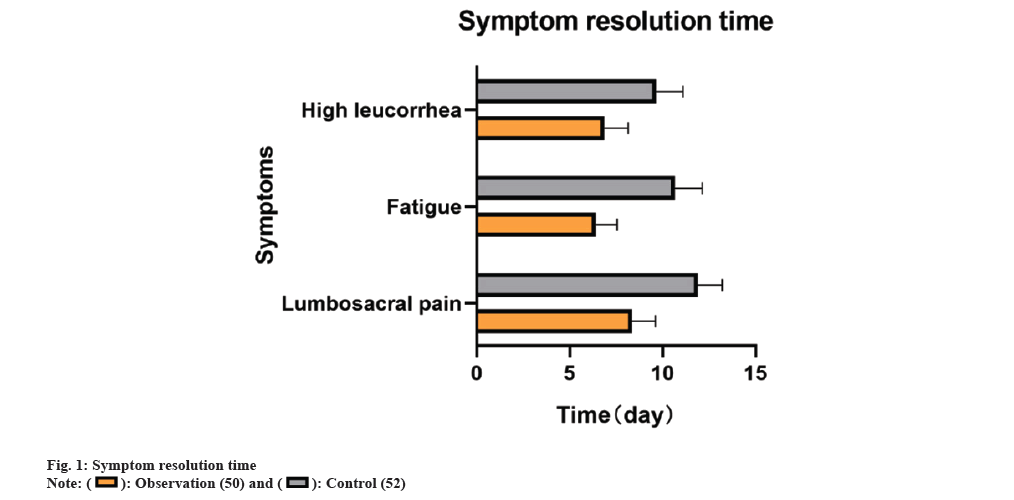
Observations revealed that the observation group experienced shorter durations of relief for lumbosacral pain, fatigue, and high leucorrhea at 8.36±1.26, 6.42±1.14, and 6.86±1.28 d, respectively, compared to the control group (11.90±1.30, 10.65±1.49, 9.65±1.44 d) (p<0.05) as shown in fig. 1.
Prior to the treatment, there was no noteworthy distinction in the levels of IL-1β, TNF-α, and IL-10 between the two groups (p>0.05). Following the treatment, IL-10 levels increased, whereas IL-1β and TNF-α levels decreased in both groups (p<0.05). Additionally, compared to the control group, the observation group exhibited higher IL-10 levels and lower IL-1β and TNF-α levels post-treatment (p<0.05) as shown in Table 1.
| Group (n) | IL-1β (pg/ml) | TNF-α (pg/ml) | IL-10 (ng/l) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Observation (50) | 18.30±2.32 | 8.42±1.39* | 8.07±1.10 | 2.44±0.45* | 18.02±3.68 | 25.58±3.91* |
| Control (52) | 17.95±2.63 | 12.29±2.31* | 8.11±0.99 | 6.85±0.84* | 18.92±3.65 | 21.24±3.53* |
| t | -0.724 | 10.203 | 0.182 | 33.992 | 1.252 | -5.877 |
| p | 0.470 | 0.000 | 0.856 | 0.000 | 0.214 | 0.000 |
Note: (*): Indicates noteworthy difference following treatment compared with prior to treatment
Table 1: Inflammatory cytokine levels.
Prior to the treatment, there were no variations detected in the levels of CD4+, CD8+, and CD4+/CD8+ between the two groups (p>0.05). Post-treatment, CD8+ levels declined, while CD4+ and CD4+/CD8+ levels increased in both groups (p<0.05). Furthermore, the observation group demonstrated decreased CD8+ levels and heightened CD4+ and CD4+/CD8+ levels compared to the control group after treatment (p<0.05) as shown in Table 2.
| Group (n) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Observation (50) | 30.95±4.65 | 44.47±4.97 | 29.02±5.21 | 24.19±4.93 | 1.18±0.28 | 1.92±0.44 |
| Control (52) | 29.32±5.30 | 41.73±5.79 | 29.58±5.33 | 26.05±4.37 | 1.02±0.25 | 1.64±0.35 |
| t | -1.645 | 6.930 | 0.538 | 2.020 | -1.453 | -3.452 |
| p | 0.103 | 0.000 | 0.592 | 0.046 | 0.149 | 0.001 |
Table 2: T Lymphocyte subsets.
Prior to the treatment, there were no variances observed in the PI, RI, and PSV of the ovarian and uterine arteries between the two groups (p>0.05). Subsequent to the treatment, there was a reduction in RI, and an increase in PI and PSV in both the ovarian and uterine arteries (p<0.05). In comparison with the control group, the research group exhibited lower RI in the ovarian and uterine arteries after treatment, along with higher PI and PSV in both arteries compared to the control group (p<0.05) as shown in Table 3.
| Group (n) | Ovarian artery | Uterine artery | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PI | RI | PSV (cm³/s) | PI | RI | PSV (cm³/s) | |||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | |
| Observation (50) | 0.50±0.11 | 0.65±0.15 | 0.69±0.14 | 0.54±0.13 | 12.93±1.37 | 14.51±1.52 | 0.56±0.14 | 0.70±0.15 | 0.72±0.17 | 0.56±0.14 | 12.69±1.42 | 17.89±1.68 |
| Control (52) | 0.51±0.12 | 0.60±0.15 | 0.69±0.14 | 0.64±0.10 | 13.03±1.33 | 17.47±1.54 | 0.57±0.11 | 0.64±0.14 | 0.74±0.19 | 0.69±0.17 | 12.48±1.53 | 15.46±1.73 |
| t | 0.479 | -1.602 | -0.053 | 4.151 | 0.377 | 9.758 | 0.332 | -2.153 | 0.568 | 4.014 | -0.724 | -7.211 |
| p | 0.633 | 0.112 | 0.958 | 0.000 | 0.707 | 0.000 | 0.740 | 0.034 | 0.571 | 0.000 | 0.471 | 0.000 |
Table 3: Pelvic Hemodynamics (x±s).
No noteworthy contrast was observed in the occurrence of adverse reactions between the two groups (p>0.05) as shown in Table 4.
| Group (n) | Gastrointestinal discomfort | Drowsiness | Nausea and vomiting | Skin sensitivity | Overall incidence |
|---|---|---|---|---|---|
| Observation (50) | 2 (4.00) | 1 (2.00) | 2 (4.00) | 2 (4.00) | 7 (14.00) |
| Control (52) | 3 (5.77) | 2 (3.85) | 3 (5.77) | 1 (1.92) | 9 (17.31) |
| χ² | 0.211 | ||||
| p | 0.646 |
Table 4: Complications n (%).
Chronic PID is an enduring inflammatory reaction of the internal reproductive organs and surrounding connective tissues, stemming from inadequate or delayed management of acute PID. It is characterized by a concealed, recurrent, and prolonged course[17]. The prevailing belief is that chronic PID is connected to bacterial invasion and weakened host resistance. However, recent clinical investigations have revealed that chronic PID is also related to the onset of sterile inflammation as a result of repeated infections, leading to the infiltration of mucosal, tissue, and organs, causing tissue edema, adhesions, and fibrosis. Aseptic inflammation is not sensitive to antibiotic drugs, and the clinical effect of antibiotic treatment alone is limited. Ultrasound’s high-frequency mechanical vibrations can generate micro-massage within the extremely small cell structures, facilitating the uptake and dispersion of inflammation within the fallopian tubes[18,19]. This study sought to evaluate and compare the clinical efficacy of utilizing the combined treatment of clindamycin and ultrasound with the single treatment of clindamycin in patients with chronic PID. Engaging in observation and analysis of the duration of symptom relief, inflammatory markers, immune indicators, and hemodynamic indicators during the treatment led to the uncovering of interesting results.
Initially, the duration of relief from lumbosacral pain, fatigue, and high leucorrhea in the observation group was notably shorter than that in the control group. This implies that the combined treatment with clindamycin and ultrasound therapy may more rapidly alleviate the symptoms of patients, consequently enhancing their quality of life.
Chronic PID encompasses fluctuations in both anti-inflammatory and pro-inflammatory cytokines, with IL-1β, TNF-α, and IL-10 playing pivotal roles in the onset and advancement of the disease. IL-1β can induce the expression and aggregation of pro-inflammatory cytokines in endothelial cells and is involved in the pathological process of chronic PID. TNF-α is pivotal in orchestrating the generation and clustering of inflammatory cells, adhesion, and inflammation. Serving as an endogenous anti-inflammatory cytokine, IL-10 has a negative feedback influence that regulates the inflammatory response[20]. After treatment, the observation group showcased higher levels of IL-10 than the control group, while displaying lower levels of IL-1β and TNF-α. This underscores the potential of combined treatment with clindamycin and ultrasound therapy to regulate the immune response, diminish the level of inflammation, and thereby improve the effectiveness of chronic PID treatment.
Lymphocytes, key immune cells, partake in various immune activities including infection, inflammation, and tumors. T lymphocytes, in particular, are engaged in numerous stages[21]. The study revealed a decrease in the level of CD8+ cells and an increase in CD4+ and CD4+/CD8+ ratios after treatment in the observation group compared to the control group. This indicates the potential regulatory effect of combined treatment with clindamycin and ultrasound therapy on the immune system, augmenting immune function and ameliorating the inflammatory state.
Hemodynamics encompasses the exploration of blood and blood vessels, and the recognition of hemodynamic irregularities can point to problems such as poor blood perfusion due to disease[22]. Previous studies have corroborated the presence of hemodynamic irregularities in patients with chronic PID. This study evidenced considerable modifications in the blood flow dynamics indicators (RI, PI, and PSV) of the ovarian and uterine arteries following treatment in the observation group. The reduction in RI and elevation in PI and PSV imply that the combined treatment with clindamycin and ultrasound therapy might enhance pelvic blood circulation, boost oxygen and nutrient supply to the tissues, and facilitate the absorption and resolution of inflammation.
In conclusion, the combined treatment with clindamycin and ultrasound therapy exhibited favorable clinical effectiveness in patients with chronic PID. Through the regulation of the immune response, reduction of inflammation levels, enhancement of immune function, and improvement of blood circulation, this combined treatment can more effectively address the symptoms of chronic PID and accelerate the absorption and recovery of inflammation. However, the limitations of this study lie in its retrospective nature and the limited number of cases. Subsequent research could expand the sample size and utilize a randomized controlled study design to further affirm the efficacy and safety of the combined treatment with clindamycin and ultrasound therapy in chronic PID.
Author’s contributions:
Xiangqin Tang and Yanli Hu have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Woodhall SC, Gorwitz RJ, Migchelsen SJ, Gottlieb SL, Horner PJ, Geisler WM, et al. Advancing the public health applications of Chlamydia trachomatis serology. Lancet Infect Dis 2018;18(12):e399-407.
[Crossref] [Google Scholar] [PubMed]
- Stevens JS, Criss AK. Pathogenesis of Neisseria gonorrhoeae in the female reproductive tract: Neutrophilic host response, sustained infection, and clinical sequelae. Curr Opin Hematol 2018;25(1):13.
[Crossref] [Google Scholar] [PubMed]
- Zhang Q, Yang G, Xiong Y, Tan J, Chen C, Gu F, et al. Impact of antibiotic cured chronic endometritis on perinatal outcomes: Re-evaluation of a cohort study with a detailed follow-up. Am J Reprod Immunol 2023;90(2):e13751.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Wu S, Wang ZK, Liu X, Liu W, Du Y, et al. Effect of different antibiotic therapies on the reproductive outcomes of fresh embryo transfer for chronic endometritis: A retrospective cohort study. Am J Reprod Immunol 2023;89(3):e13669.
[Crossref] [Google Scholar] [PubMed]
- Adamian L, Urits I, Orhurhu V, Hoyt D, Driessen R, Freeman JA, et al. A comprehensive review of the diagnosis, treatment, and management of urologic chronic pelvic pain syndrome. Curr Pain Headache Rep 2020;24:27.
[Crossref] [Google Scholar] [PubMed]
- Liang Z, Wang X, Liu YH, Zhang DM, Shi L. Analgesic effect of electroacupuncture on chronic pelvic pain in patients with sequelae of pelvic inflammatory disease. Zhongguo Zhen Jiu 2021;41(4):395-9.
[Crossref] [Google Scholar] [PubMed]
- Carlson S, Batra S, Billow M, El-Nashar SA, Chapman G. Perioperative complications of laparoscopic vs. open surgery for pelvic inflammatory disease. J Minim Invasive Gynecol 2021;28(5):1060-5.
[Crossref] [Google Scholar] [PubMed]
- Asgari Z, Moini A, Montazeri A, Tavoli Z, Hosseini L, Hosseini R, et al. Comparing the effect of adjunctive N-acetylcysteine plus low dose contraceptive with low dose contraceptive alone on recurrence of ovarian endometrioma and chronic pelvic pain after conservative laparoscopic surgery: A randomised clinical trial study. J Obstetr Gynaecol 2022;42(5):1493-7.
[Crossref] [Google Scholar] [PubMed]
- Sammour RM, Khan G, Sameer S, Khan S, Zohair T, Saraya S, et al. Development of clindamycin loaded oral microsponges (Clindasponges) for antimicrobial enhancement: In vitro characterization and simulated in vivo studies. Biol Pharm Bull 2023;46(8):1088-97.
[Crossref] [Google Scholar] [PubMed]
- Gold LS, Lain E, Del Rosso JQ, Gold M, Draelos ZD, Eichenfield LF, et al. Clindamycin phosphate 1.2 %/adapalene 0.15 %/benzoyl peroxide 3.1 % gel for moderate-to-severe acne: Efficacy and safety results from two randomized phase 3 trials. J Am Acad Dermatol 2023;89(5):927-35.
[Crossref] [Google Scholar] [PubMed]
- Bhardwaj S, Mehra P, Dhanjal DS, Sharma P, Sharma V, Singh R, et al. Antibiotics and antibiotic resistance-flipsides of the same coin. Curr Pharm Des 2022;28(28):2312-29.
[Crossref] [Google Scholar] [PubMed]
- Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol 2019;15(11):666-82.
[Crossref] [Google Scholar] [PubMed]
- Kovaleva YV. The application of low-frequency ultrasound for the comprehensive treatment and rehabilitation of the patients presenting with chronic endometritis. Vopr Kurortol Fizioter Lech Fiz Kult 2017;94(3):32-8.
[Google Scholar] [PubMed]
- Zasieda Y, Solomianyi R. Chronic prostatitis therapy combined with electromyostimulation-aspiration, transurethral electrophonophoresis of platelet-rich plasma and transrectal low-intensity pulsed ultrasound. Georgian Med News 2020;299:29-33.
[Google Scholar] [PubMed]
- Jiang X, Savchenko O, Li Y, Qi S, Yang T, Zhang W, et al. A review of low-intensity pulsed ultrasound for therapeutic applications. IEEE Tran Biomed Eng 2018;66(10):2704-18.
[Crossref] [Google Scholar] [PubMed]
- de Lucas B, Pérez LM, Bernal A, Gálvez BG. Ultrasound therapy: Experiences and perspectives for regenerative medicine. Genes 2020;11(9):1086.
[Crossref] [Google Scholar] [PubMed]
- Jennings LK, Krywko DM. Pelvic Inflammatory Disease, StatPearls, Treasure Island (FL) ineligible companies. Disclosure: Diann Krywko declares no relevant financial relationships with ineligible companies: StatPearls Publishing Copyright© 2023, StatPearls Publishing LLC 2023.
- d'Agostino MC, Craig K, Tibalt E, Respizzi S. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int J Surg 2015;24:147-53.
[Crossref] [Google Scholar] [PubMed]
- Krukowska J, Wrona J, Sienkiewicz M, Czernicki J. A comparative analysis of analgesic efficacy of ultrasound and shock wave therapy in the treatment of patients with inflammation of the attachment of the plantar fascia in the course of calcaneal spurs. Arch Orthop Trauma Surg 2016;136(9):1289-96.
[Crossref] [Google Scholar] [PubMed]
- Curry A, Williams T, Penny ML. Pelvic inflammatory disease: Diagnosis, management, and prevention. Am Family Phys 2019;100(6):357-64.
[Google Scholar] [PubMed]
- Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity 2018;48(2):202-13.
[Crossref] [Google Scholar] [PubMed]
- McLean AS. Editorial: Haemodynamic monitoring: The why, when, which and what. Curr Opin Crit Care 2019;25(3):244-5.
[Crossref] [Google Scholar] [PubMed]
 .
.



