- *Corresponding Author:
- Aimei He
Department of Internal Medicine, Physical Examination Center, Lishui Maternity and Child Health Care Hospital, Lishui, Zhejiang 323000, China
E-mail: 13587181110@163.com
| This article was originally published in a special issue, “Exploring the Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(1) Spl Issue “72-78” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the efficacy of labetalol combined with magnesium sulfate in the treatment of hypertension during pregnancy and analyze the effects of such treatment on serum pregnancy-associated plasma protein A and placental growth factor levels. 90 patients with pregnancy hypertension who were treated in our hospital from January 2020 to June 2020 were selected as research objects. Patients are randomly divided into the research group (n=45, received magnesium sulfate combined with labetalol) and the control group (n=45, simply received magnesium sulfate treatment). Then we compared the differences in blood pressure, serum pregnancyassociated plasma protein A and placental growth factor levels, and adverse reactions in the treatment of two groups. There was no statistically significant difference between the two groups of patients (p>0.05). The total incidence of adverse reactions in the study group was 6.67 % (3/45), which was significantly lower than the 24.44 % (11/45) of the patients in the control group. The incidence of neonatal suffocation, fetal distress, postpartum infection and postpartum bleeding in the research group are significantly lower than those in the control group, and the differences between the groups are statistically significant (p<0.05). The serum levels of patients in the research group with pregnancy-associated plasma protein A and placental growth factor levels were significantly higher than the control group (p<0.05). The treatment effect of hypertension during pregnancy is affirmed by the labetalol and magnesium sulfate. After treatment, patients have normal blood pressure, serum pregnancy-associated plasma protein A and placental growth factor levels have improved significantly. Furthermore, the combined treatment can effectively reduce the incidence of adverse reactions.
Keywords
Labetalol, magnesium sulfate, pregnancy hypertension, pregnancy-associated plasma protein A, placental growth factor
Gestational hypertension during pregnancy is one of the endemic diseases[1], the disease usually occurs within 2 w after pregnancy, the number of illness that accounts for about 5 %, common clinical symptoms include headache, dizziness, nausea, leg edema, etc[2]. At first, the symptoms of the disease show moderately elevated blood pressure. Then, it will gradually develop to proteinuria and hypertension. Severe cases may even develop eclampsia[3,4]. According to related statistics, the incidence of gestational hypertension is about 5 %-10 % in foreign countries and 9.4 %-10.4 % in China[5]. With the changes in lifestyle and diet structure, the incidence has an increasing trend[6]. Pregnancy hypertension not only causes clinical features such as proteinuria, hypertension, etc. but also totally increase the incidence of newborn dangerous events such as suffocation, premature fetal membrane and limited growth. So, early intervention has great meaning for improving both maternal and neonatal conditions[7,8].
Magnesium sulfate is a commonly used antihypertensive drug in clinical practice[9]. It is affirmed in hypertension during pregnancy, especially medium to severe pregnancy and hypertension, but some scholars have found that the magnesium sulfate concentration is too high to cause gastrointestinal tract reactions and nervous system response to the gastrointestinal tract reactions, which may endanger the safety of the fetus[10], so some scholars have been committed to seek a new type of antihypertensive drug in recent years. Labetalol is an antihypertensive drug which acts as both alpha (α) and beta (β) adrenergic receptor blocker. Its mechanism is to block adrenaline receptors, slow down sinus arrhythmia and resist it by reducing peripheral vascular resistance. At present, there are still few researches on the combination of labetalol and magnesium sulfate in the treatment of pregnancy hypertension[11]. This is because other researches always focus on one aspect, such as only labetalol or magnesium sulfate. This article intends to use the control group to explore the feasibility of combining the above two drugs during pregnancy. It provides clinical reference for improving the prognosis of hypertension patients with safety.
Materials and Methods
General information:
90 patients with pregnancy hypertension who were treated in our hospital from January 2020 to June 2020 were selected as research objects. According to the random digital table, it was distinguished into the research/Study Group (SG, n=45 and administered with magnesium sulfate combined with labetalol treatment) and Control Group (CG, n=45, simply received magnesium sulfate treatment). This study has been approved by the Hospital Ethics Committee of our hospital.
Inclusion criteria: Age 35-40 y old; clear consciousness; can cooperate with investigations; provide complete medical records and no use of other medications.
Exclusion criteria: Patients with mental illness; those who have consciousness disorder; patients with diseases such as coronary heart disease and kidney failure; poor compliance; alcohol or drug dependence and participating in other unreasonable clinical investigations.
Interventional methods:
Both patients were admitted to the hospital for basic intervention, such as restrictions on activities and liquid intake, and guided patients to eat more vitamin-rich foods.
Patients in the control group were administered with magnesium sulfate on the basis of the above intervention method (manufacturer: Tianjin Jinyao Pharmaceutical Co., Ltd., approval number: National medicine quasi-H12020994, specification 10 ml: 2.5 g). For intravenous drip, use 10 ml of 25 % magnesium sulfate+100 ml of 5 % glucose injection. The injection was given within 30 min and then 60-80 ml of 25 % magnesium sulfate+1000 ml of 5 % glucose injection were given for maintenance, 1 time/d, and continuous treatment was given for 30 d.
Based on the control group, the research group use both magnesium sulfate along with 100 mg labetalol (manufacturer: Jiangsu Dino Pharmaceutical Co., Ltd., approval number: National medicine quasi-word H32026119, specifications 100 mg/ tablet) for treatment, 1 time/d. Both groups were continuously treated for 30 d.
Observation indicators and evaluation standards:
Main observation indicators: The changes in the blood pressure of the patients before and after treatment between the two groups of patients were recorded, and 30 d of compression and Diastolic Blood Pressure (DBP) between the two groups were measured. The changes in the serum Pregnancy-Associated Plasma Protein A (PAPPA) and Placental Growth Factor (PLGF) levels in the two groups of patients before and after treatment were recorded.
Secondary observation indicators: The incidence of adverse reactions in patients between the two groups after treatment was recorded. The statistics from the incidence of electrolyte disorders, fatigue, headache, dizziness, hypoproteinemia, etc. after treatment was also recorded. Compared with the two groups of patients and infants, the patients with neonatal suffocation, intrauterine distress, postpartum infection and postpartum bleeding are compared with the incidence of postpartum delivery.
Statistical methods:
Statistical Package for the Social Sciences (SPSS) 20.0 software was used to implement statistical analysis for the collected data. The experimental data are represented as mean±Standard Deviation (SD, X̄±S) and the differences between the two groups are compared using Chi square-test. The statistical analysis of continuous variables at different time points adopts student’s t-test and it is statistically significant to take p<0.05 as differences[12].
Results and Discussion
The comparison of the general clinical data of two groups of patients was shown in Table 1. Integrated with two groups of patient’s, the age, pregnancy, production, gestational week, weight and other clinical data were compared between the two groups. The results show that the comparison of the above mentioned data between the two groups of patients is not statistically significant (p>0.05).
| General clinical data | Research group (n=45) | Control group (n=45) | t/χ2 | p |
|---|---|---|---|---|
| Average age (age) | 27.82±3.60 | 27.25±4.10 | 0.701 | 0.485 |
| Average weight (kg) | 64.79±7.34 | 63.89±7.90 | 0.560 | 0.577 |
| Average pregnancy (times) | 1.08±0.54 | 1.17±0.62 | 0.734 | 0.465 |
| Average production (times) | 1.07±0.66 | 1.11±0.70 | 0.279 | 0.781 |
| Average gestational week (w) | 29.26±2.94 | 29.24±3.96 | 0.027 | 0.979 |
Table 1: The Comparison of the General Clinical Data of Two Groups of Patients X̄±S, %]
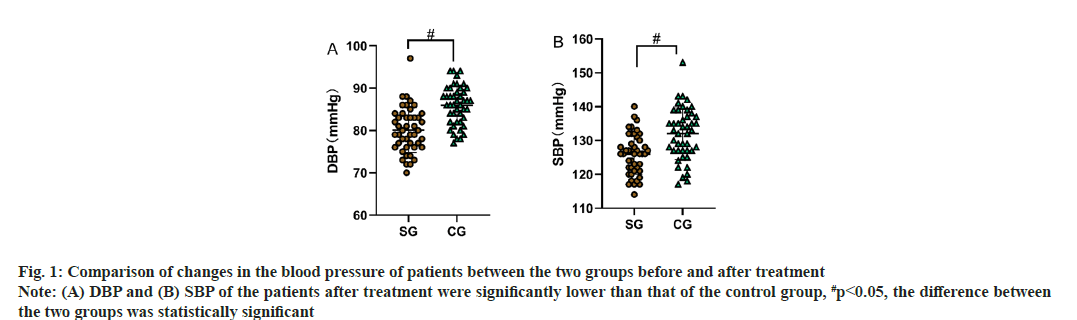
Comparison of changes in the blood pressure of patients between the two groups before and after treatment was shown in Table 2. There was no statistically significant difference between the DBP and contraction pressure in the two groups of patients (p>0.05), at the same time, the DBP and contraction voltage of the two groups were significantly reduced (p<0.05) as shown in Table 2, fig. 1A and fig. 1B.
| Group | N | DBP | SBP | ||
|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | ||
| Research group | 45 | 96.65±9.51 | 80.15±5.30a | 154.88±10.81 | 125.98±5.99a |
| Control group | 45 | 95.48±7.95 | 85.88±4.54a | 154.01±10.53 | 131.93±7.71a |
| t | - | 0.633 | 5.508 | 0.387 | 4.088 |
| p | - | 0.528 | <0.001 | 0.700 | <0.001 |
Note: Compared with before treatment, ap<0.05
Table 2: Comparison of Changes in the Blood Pressure of Patients Between the Two Groups before and after Treatment (X̄±S, Mmhg)
Fig. 1: Comparison of changes in the blood pressure of patients between the two groups before and after treatment Note: (A) DBP and (B) SBP of the patients after treatment were significantly lower than that of the control group, #p<0.05, the difference between the two groups was statistically significant
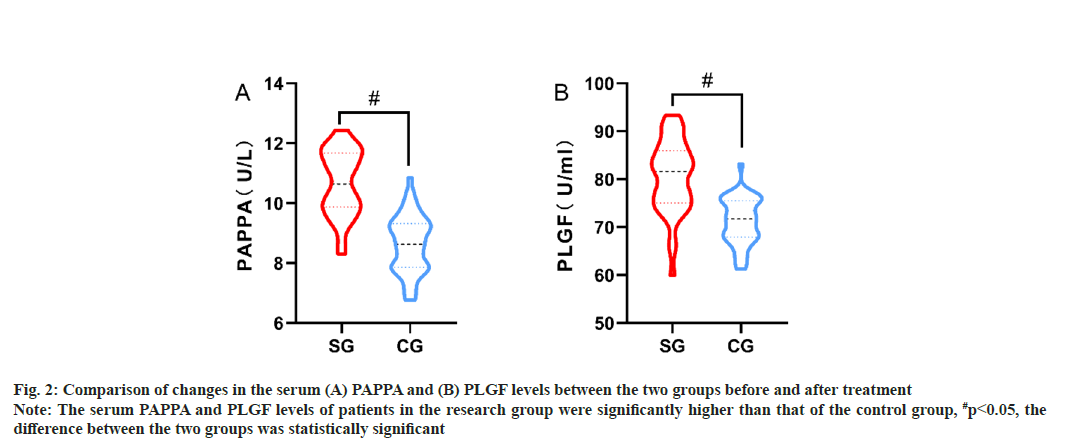
Comparison of changes in the serum PAPPA and PLGF levels between the two groups before and after treatment were shown in Table 3, fig. 2A and fig. 2B. There was no statistically significant difference between serum PAPPA and PLGF levels in the two groups of patients before treatment (p>0.05). After the treatment, the level of patients with serum PAPPA and PLGF was significantly higher than the control group (p<0.05).
| Group | N | PAPPA (U/l) | PLGF (U/l) | ||
|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | ||
| Research group | 45 | 7.42±1.10 | 10.68±1.09a | 66.26±3.45 | 80.54±7.47a |
| Control group | 45 | 7.51±1.04 | 8.65±0.96a | 66.82±4.07 | 71.23±4.85a |
| t | - | 0.399 | 9.375 | 0.704 | 7.012 |
| p | - | 0.691 | <0.001 | 0.483 | <0.001 |
Note: Compared with before treatment, ap<0.05
Table 3: Comparison of Changes in the Serum Pappa and Plgf Levels Between the Two Groups before and after Treatment (X̄±S)
Fig. 2: Comparison of changes in the serum (A) PAPPA and (B) PLGF levels between the two groups before and after treatment Note: The serum PAPPA and PLGF levels of patients in the research group were significantly higher than that of the control group, #p<0.05, the difference between the two groups was statistically significant
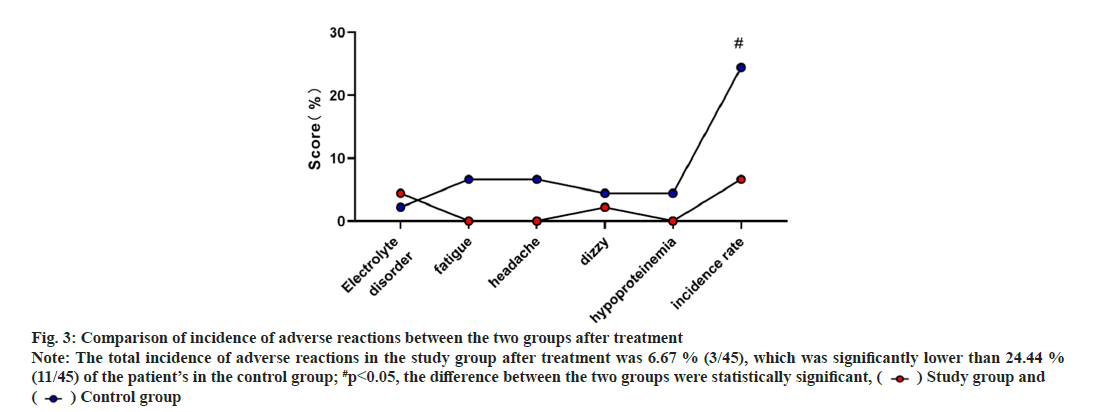
The incidence of adverse reactions between the two groups after treatment was shown in Table 4 and fig. 3. The incidence of electrolyte disorders, fatigue, headache, dizziness, hypoproteinemia, etc. after treatment was compared between the two groups. It is obviously lower than the 24.44 % (11/45) of the patient’s in the control group and the difference between the groups are statistically significant (p<0.05).
| Group | N | Electrolytic disorders | Weakness | Headache | Dizziness | Hypoproteinemia | Total incidence rate |
|---|---|---|---|---|---|---|---|
| Research group | 45 | 2 (4.44) | 0 (0.00) | 0 (0.00) | 1 (2.22) | 0 (0.00) | 3 (6.67) |
| Control group | 45 | 1 (2.22) | 3 (6.67) | 3 (6.67) | 2 (4.44) | 2 (4.44) | 11 (24.44) |
| χ2 | 5.414 | ||||||
| p | 0.020 |
Table 4: Comparison of the Incidence of Adverse Reactions Between the Two Groups after Treatment [N (%)]
Fig. 3: Comparison of incidence of adverse reactions between the two groups after treatment Note: The total incidence of adverse reactions in the study group after treatment was 6.67 % (3/45), which was significantly lower than 24.44 % (11/45) of the patient’s in the control group; #p<0.05, the difference between the two groups were statistically significant, ( ) Study group and (
) Study group and ( ) Control group
) Control group
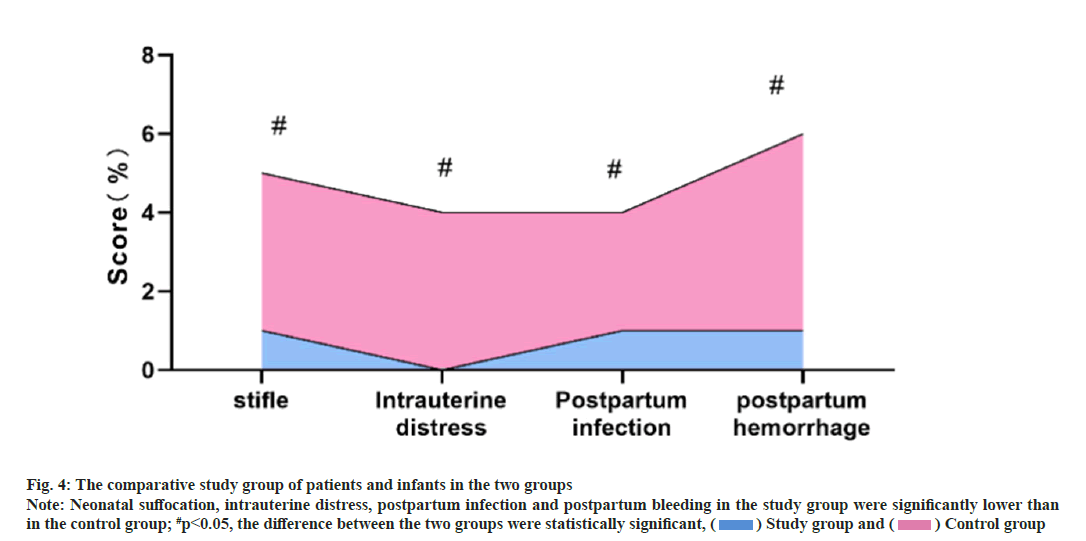
Comparison of parameters in both maternal and infant patients between the two groups was shown in fig. 4. Statistical analysis shows that the patients with neonatal suffocation, intrauterine distress, postpartum infection and postpartum bleeding were significantly lower than those in the control group, and the differences between the group were statistically significant (p<0.05).
Fig. 4: The comparative study group of patients and infants in the two groups Note: Neonatal suffocation, intrauterine distress, postpartum infection and postpartum bleeding in the study group were significantly lower than in the control group; #p<0.05, the difference between the two groups were statistically significant, ( ) Study group and (
) Study group and ( ) Control group
) Control group
With the development of society, especially lifestyle and the adjustment of diet in daily life, the patients are increasing annually[13]. This disease can be caused by uterine ischemia, proteinuria, immune and genetic parameters[14]. The emergence of pregnancy hypertension will bring about serious health threats to mothers and newborns, such as maternal vomiting, proteinuria and edema. Meanwhile, it can also increase newborn suffocation, premature birth, dead tires, premature fetal membrane breakthroughs and other dangerous events.
Drug treatment is still the main method of intervention in hypertension during pregnancy. Magnesium sulfate is used as antihypertensive drug. It is commonly used in clinical treatment of constipation, obstructive jaundice, convulsions, uremia, etc.[15]. The effect of improving hypertension during pregnancy can significantly reduce the blood pressure level of patients and reduce the incidence of various types of complications[16]. However, with the clinical promotion and application of the drugs, some studies have found that although the effect of simultaneous application of magnesium sulfate is obvious, it may induce dehydration when large doses of the drug were administered and some patients with gastric ulcers may still have severe effects[17]. Therefore, combined treatment has become a trend of hypertension during pregnancy. By setting up a control group, this study conducted an evaluation of the intervention effects of patients. The results show that compared with the patients who simply apply magnesium sulfate, the patients in the research group are obviously dominant in terms of blood pressure and serum factors after intervention. A comparative study carried out for 90 patients with hypertension during pregnancy found that labetalol combined with magnesium sulfate can significantly reduce patients with serum inflammatory factors after treatment such as high migration protein and Homocysteine (HCY). The cesarean section rate of patients with research group was significantly lower than that of the control group of simultaneous application of magnesium sulfate[18]. It is also found that labetalol combined with magnesium sulfate can reduce the average DBP of patients with hypertension during pregnancy from 111.64±8.64 mmHg to 81.30±10.04 mmHg and the average Systolic Blood Pressure (SBP) was reduced from 170.43±19.51 mmHg to 132.41±18.72 mmHg. The levels of C-Reactive Protein (CRP) and HCY were also reduced after treatment with labetalol and magnesium sulfate[19].
The author of this article analyzes that magnesium sulfate is mainly released by inhibiting central nervous activity and neurotransmitteracetylcholine release, preventing nerve conduction to inhibit the smoothing muscle contraction of the uterus, and can also relax the smooth muscle of the blood vessels and it is easy to rebound in stopping the drug[20]. Labetalol belongs to α and β-blockers. It can increase blood flow by blocking adrenaline receptors and control blood pressure. Compared to single application of magnesium sulfate, combined treatment show more effect and mild, therefore, there are significant differences in the antihypertensive effect[21]. PAPPA is a cytokine secreted by the placenta nourishing cells and its level will increase with the increase of the gestational week. This factor mainly reflects the invasion and activity of nourishing cells, which is an important serum factor in the early stage of eclampsia[22]. PLGF is a type of vascular endothelial growth factors. It is also secreted by nourishing cells by placenta, which can play as a sensitive indicator in the function of promoting cell proliferation and activation which is a placental function[23]. As mentioned earlier, hypertension during pregnancy will affect the placental functions of pregnant women and even induce fetal suffocation. Therefore, the levels of PAPPA and PLGF can intuitively reflect the premium maternal placenta state during pregnancy. The blood pressure and placenta function are better in the research group compared to the control group where PAPPA and PLGF levels are lower in the control group.
Finally, experimental results during the treatment of two groups showed that the incidence of adverse reactions during pregnancy in the research group were much lower than that in control group. In Canada, they can increase the delivery rate of women during pregnancy from 58.33 % to 75.00 %. Meanwhile, the postpartum bleeding rate can be reduced from 21.67 % to 6.67 %[24]. During that work, they have analyzed the magnesium sulfate has a good antihypertensive effect. Although magnesium sulfate can quickly reduce the pressure in a short period of time, for patients with magnesium sulfate tolerance, dose need to be increased. Moreover, a large number of magnesium sulfate will significantly increase the incidence of adverse reactions.
In conclusion, based on study above mentioned, the effect of treatment of the hypertension during pregnancy is affirmed by the treatment with labetalol and magnesium sulfate. In this study, the patient’s blood pressure can be controlled well and simultaneously serum PAPPA and PLGF levels are also improved.
Author’s contributions:
Jixian Wu made contributions to the study design, data acquisition, data analysis and initial drafting. Aimei He contributed to the study design, revisions of the initial draft and experimental supervision.
Conflict of interests:
The authors declared that they have no conflicts of interest regarding this work.
References
- Fahmy WM, Crispim CA, Cliffe S. Association between maternal death and cesarean section in Latin America: A systematic literature review. Midwifery 2018;59:88-93.
[Crossref] [Google scholar] [PubMed]
- Lu Y, Chen R, Cai J, Huang Z, Yuan H. The management of hypertension in women planning for pregnancy. Br Med Bull 2018;128(1):75-84.
[Crossref] [Google scholar] [PubMed]
- Xie X, Pan X, Zhang W, An J. A context hierarchical integrated network for medical image segmentation. Comput Electr Eng 2022;101:108029.
- Sandall J, Tribe RM, Avery L, Mola G, Visser GH, Homer CS, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet 2018;392(10155):1349-57.
[Crossref] [Google scholar] [PubMed]
- Song H, Hu K, Du X, Zhang J, Zhao S. Risk factors, changes in serum inflammatory factors and clinical prevention and control measures for puerperal infection. J Clin Lab Anal 2020;34(3):e23047.
[Crossref] [Google scholar] [PubMed]
- Woodd SL, Montoya A, Barreix M, Pi L, Calvert C, Rehman AM, Chou D, Campbell OM. Incidence of maternal peripartum infection: A systematic review and meta-analysis. PLoS Med 2019;16(12):e1002984.
[Crossref] [Google scholar] [PubMed]
- Zimring JC, Hudson KE. Cellular immune responses in red blood cell alloimmunization. Hematology Am Soc Hematol Educ Program 2016;2016(1):452-6.
[Crossref] [Google scholar] [PubMed]
- Nombela I, Ortega-Villaizan MD. Nucleated red blood cells: Immune cell mediators of the antiviral response. PLoS Pathog 2018;14(4):e1006910.
[Crossref] [Google scholar] [PubMed]
- Pessoa TD, Clemente Junior WS, Costa TX, Bezerra PK, Martins RR. Drug interactions in maternal intensive care: Prevalence, risk factors and potential risk medications. Einstein 2019;17(3):eAO4521.
[Crossref] [Google scholar] [PubMed]
- Belizaire R, Mack J, Kadauke S, Kim Y, Saidman S, Makar RS. Red blood cell alloantibodies are associated with increased alloimmunization against human leukocyte antigens. Transfusion 2019;59(7):2256-63.
[Crossref] [Google scholar] [PubMed]
- Hendrickson JE, Delaney M. Hemolytic disease of the fetus and newborn: Modern practice and future investigations. Transfus Med Rev 2016;30(4):159-64.
[Crossref] [Google scholar] [PubMed]
- Sutton AL, Harper LM, Tita AT. Hypertensive disorders in pregnancy. Obstet Gynecol Clin North Am 2018;45(2):333-47.
[Crossref] [Google scholar] [PubMed]
- Guedes-Martins L. Chronic hypertension and pregnancy. Adv Exp Med Biol 2017;956:395-407.
[Crossref] [Google scholar] [PubMed]
- Liu FM, Zhao M, Wang M, Yang HL, Li L. Effect of regular oral intake of aspirin during pregnancy on pregnancy outcome of high-risk pregnancy-induced hypertension syndrome patients. Eur Rev Med Pharmacol Sci 2016;20(23):5013-6.
[Google scholar] [PubMed]
- Xie X, Pan X, Shao F, Zhang W, An J. MCI-Net: Multi-scale context integrated network for liver CT image segmentation. Comput Electr Eng 2022;101:108085.
- Jafarzadeh A, Hadavi M, Hasanshahi G, Rezaeian M, Vazirinejad R, Aminzadeh F, et al. Cesarean or cesarean epidemic? Arch Iran Med 2019;22(11):663-70.
[Google scholar] [PubMed]
- Majangara R, Gidiri MF, Chirenje ZM. Microbiology and clinical outcomes of puerperal sepsis: A prospective cohort study. J Obstet Gynaecol 2018;38(5):635-41.
[Crossref] [Google scholar] [PubMed]
- Mohamed-Ahmed O, Hinshaw K, Knight M. Operative vaginal delivery and post-partum infection. Best Pract Res Clin Obstet Gynaecol 2019;56:93-106.
[Crossref] [Google scholar] [PubMed]
- Ngonzi J, Bebell LM, Fajardo Y, Boatin AA, Siedner MJ, Bassett IV, et al. Incidence of postpartum infection, outcomes and associated risk factors at Mbarara regional referral hospital in Uganda. BMC Pregnancy Childbirth 2018;18(1):1-11.
[Crossref] [Google scholar] [PubMed]
- Kaiser JE, Bakian AV, Silver RM, Clark EA. Clinical variables associated with adverse maternal outcomes in puerperal group a Streptococci infection. Obstet Gynaecol 2018;132(1):179-84.
- Axelsson D, Brynhildsen J, Blomberg M. Postpartum infection in relation to maternal characteristics, obstetric interventions and complications. J Perinat Med 2018;46(3):271-8.
[Crossref] [Google scholar] [PubMed]
- Mascarello KC, Horta BL, Silveira MF. Maternal complications and cesarean section without indication: Systematic review and meta-analysis. Rev Saude Publica 2017;51:105.
[Crossref] [Google scholar] [PubMed]
- Subramaniam A, Ptacek T, Lobashevsky E, Cliver S, Lefkowitz EJ, Morrow CD, et al. Midtrimester cervicovaginal microbiota: Identification of microbial variations associated with puerperal infection at term. Am J Perinatol 2016:1165-75.
[Crossref] [Google scholar] [PubMed]
- Bonet M, Ota E, Chibueze CE, Oladapo OT. Routine antibiotic prophylaxis after normal vaginal birth for reducing maternal infectious morbidity. Cochrane Database Syst Rev 2017;11(11):CD012137.
[Crossref] [Google scholar] [PubMed]