- *Corresponding Author:
- Xia Peng
Department of Stomatology, Chongqing Yubei District Second People's Hospital, Chongqing 401120, China
E-mail: 19923080086@163.com
| This article was originally published in a special issue, “Recent Progression in Pharmacological and Health Sciences” |
| Indian J Pharm Sci 2024:86(2) Spl Issue “299-304” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Examining the efficacy of the combined application of minocycline hydrochloride ointment and metronidazole for treating chronic periodontitis and studying its impact on periodontal microecology. The research involved dividing 235 chronic periodontitis patients into an observation group and a control group for further analysis. Both sets of participants underwent fundamental periodontal therapy, with the control cohort undergoing metronidazole treatment, and the observation cohort undergoing combined minocycline hydrochloride ointment and metronidazole treatment. A month into the treatment, the treatment effectiveness was appraised, and an evaluation of the periodontal indices, such as the gingival index, plaque index and probing depth, was carried out. Collection of gingival crevicular fluid samples for assessment of inflammatory factors and immune cell levels was conducted, alongside the documentation of bacterial colony counts in the periodontal microecology. The overall effectiveness rate in the observation group stood at 96.00 %, a significant increase compared to the 84.00 % noted in the control group. Decreases in the gingival index, plaque index and probing depth indices were observed in the observation group, and these reductions were greater than those in the control group. Furthermore, the reduction in inflammatory factor levels in the gingival crevicular fluid was more notable in the observation group than in the control group. Greater declines in the levels of Th17 and Treg cells were observed in the observation group relative to the control group. Additionally, the observation group saw a reduction in the bacterial colony count of harmful bacteria in periodontal microecology, paired with an increase in beneficial bacteria. The use of minocycline hydrochloride ointment in conjunction with metronidazole in chronic periodontitis patients leads to a notable improvement in periodontal indices, a reduction in inflammatory responses, the modulation of Th17 and Treg cell levels, and the preservation of periodontal microecology.
Keywords
Chronic periodontitis, minocycline hydrochloride, metronidazole, efficacy, microecology
Originating from oral microbial infection, chronic periodontitis is typified by persistent inflammation of the periodontal tissues. Although it can emerge at any age, its prevalence is more prominent in adults, with a marked spike in incidence post the age of 35 y[1,2]. Inadequate and untimely treatment may lead to the degradation of the periodontal tissues, impaired tooth stability, and the potential for tooth loss[3]. Managing chronic periodontitis effectively involves mitigating inflammation and preventing infections within the periodontal tissues[4].
Mechanical removal of dental plaque remains the primary approach in addressing chronic periodontitis[5]. However, dependence solely on mechanical cleaning methods can present challenges in fully eradicating periodontal pathogens, and patients may encounter difficulties in performing these procedures. Hence, the search for locally administered treatment options for chronic periodontitis is of great importance.
Minocycline hydrochloride ointment is extensively utilized in oral disease treatment regimens[6,7].
Scientific investigations have demonstrated minocycline hydrochloride’s antibacterial, antiinflammatory, and anti-fibrinolytic qualities, providing noteworthy efficacy in periodontitis management[8,9]. In contrast, metronidazole serves as an antimicrobial therapy, effectively inhibiting periodontal pathogens and alleviating the symptoms of chronic periodontitis[10]. The combined administration of these two treatments may generate a synergistic influence, enhancing treatment efficacy.
Conversely, periodontal microecology stands as a critical factor in upholding periodontal wellbeing[11]. Establishing and maintaining a balanced and healthy periodontal microecology is imperative in forestalling the emergence and advancement of periodontitis[12]. Nevertheless, at present, there is a dearth of extensive and methodical studies regarding the influence of concurrently using minocycline hydrochloride ointment and metronidazole on periodontal microecology in addressing chronic periodontitis.
As a result, this study sought to assess the effectiveness of combining minocycline hydrochloride ointment with metronidazole in managing chronic periodontitis, and explore its influence on periodontal microecology. Our objective is to contribute to the enhancement of individualized and effective treatment alternatives for chronic periodontitis and to make a valuable contribution to sustaining oral health by obtaining a more thorough grasp of the treatment effectiveness and its impact on periodontal microecology through this combined therapy.
Materials and Methods
General information:
235 patients diagnosed with chronic periodontitis, who were admitted to our hospital from June 2019 to October 2023, were chosen for the study under the endorsement of the Medical Ethics Committee.
Inclusion criteria: Meeting the diagnostic criteria for chronic periodontitis; having >20 remaining teeth, and periodontal pocket depth ≥4 mm; no history of periodontal treatment or antibacterial drug intake in the 3 mo prior to the start of the study; absence of occlusal trauma and poor restorations, and patients provided informed consent.
Exclusion criteria: Severe cardiac, hepatic, or renal dysfunction; periodontal tissue trauma; concomitant respiratory or digestive system infections and cognitive impairment were excluded from this study. The patients were divided into an observation group (n=120) and a control group (n=115) using a random number table.
Methods:
Both patient groups underwent fundamental periodontal treatment, which encompassed supragingival scaling, subgingival curettage, and plaque control. Notably, the control group was administered metronidazole, where metronidazole tablets (from Wudang Hubei Kuihua Pharmaceutical Group Co., Ltd., National Drug Approval Number H20045869, specification of 0.2 g) were orally administered at a dose of 0.4 g, three times a day. Minocycline hydrochloride ointment combined with metronidazole treatment was administered to the observation group, following the same protocol for metronidazole as the control group. Subsequent to routine essential care, periodontal pockets were weekly filled with minocycline hydrochloride ointment (from Japan Shin-Etsu Chemical Co., Ltd., approval number: H20100244, specification: 0.5 g) until an overflow occurred. The treatment was continuously upheld for both groups over a period of 1 mo.
Observation indices:
Clinical efficacy: Following 1 mo of treatment, an extensive assessment of treatment effectiveness was performed for both patient cohorts. A significant reduction in gingival swelling, absence of pus in the periodontal pockets, and a reduction in periodontal pocket depth of >2 mm, along with a marked improvement in tooth mobility, were considered as cured. A mild improvement in gingival swelling, absence of pus in the periodontal pockets, a reduction in periodontal pocket depth of >1 mm, and slight improvement in tooth mobility were considered as improvement. Failure to achieve the above criteria after treatment was classified as ineffectiveness.
Total effective rate=(Number of cured cases+number of improvement cases)/total number of cases×100 %
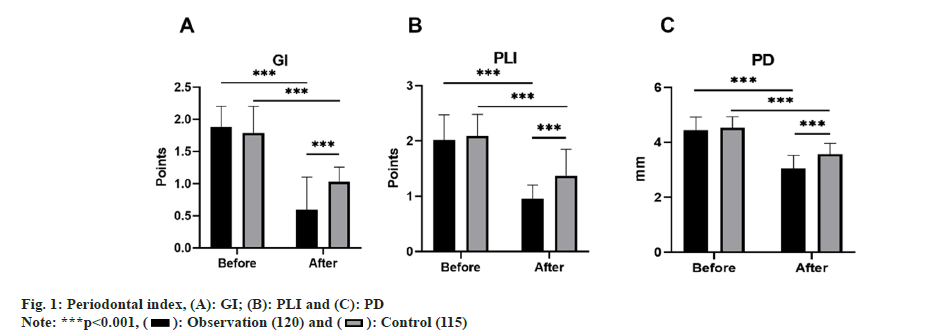
Periodontal index: Both groups of patients had their Gingival Index (GI), Plaque Index (PLI), and Probing Depth (PD) checked before and after 1 mo of treatment. GI is appraised on a scale from 0 to 3, accounting for the patients' gingival color, bleeding situation, and texture modifications, wherein a higher score indicates heightened gingival inflammation. PD is the depth from the gingival margin to the bottom of the periodontal pocket, measured using a periodontal probe. PLI is leveraged to gauge dental plaque thickness, with scoring ranging from 0 to 3, where greater scores indicate more pronounced levels of plaque.
Gingival crevicular fluid inflammatory factor index: Gingival crevicular fluid samples from both groups of patients were obtained before and after 1 mo of treatment, and the levels of Monocyte Chemotactic Protein-1 (MCP-1), Matrix Metalloproteinase-8 (MMP-8), and Interleukin-6 (IL-6) were determined using enzyme-linked immunosorbent assay.
Th17 and Treg cell levels: Both patient groups provided fasting venous blood samples (2 ml per sample) before and after 1 mo treatment, and the levels of Th17 and Treg cells were quantified using a FACSCalibur™ flow cytometer fabricated by BD in the United States.
Paradental micro ecological indicators: Prior to and following the treatment, patients were advised to gargle with mouthwash, followed by using a sterilized cotton ball to dry their mouths. Then, a sterilized paper point was placed in the periodontal pocket and crown-blind pocket for 30 s. The next step involved submerging the paper point in 1 ml of anaerobic transport fluid for analysis. Subsequently, the bacterial colony counts of Porphyromonas gingivalis (P. gingivalis), Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), Streptococcus mutans (S. mutans), and facultative anaerobic streptococci were logged.
Statistical analysis:
Utilizing the Statistical Package for the Social Sciences (SPSS) 25.0, the analysis was performed, comparing the measurement data, presented as mean±standard deviation, between the two groups via an independent sample t-test. Utilizing the Chisquare (χ2) test, the count data were analyzed. The criteria for establishing statistical significance were defined at a level of p<0.05.
Results and Discussion
The total treatment effectiveness rate in the observation group stood at 96.00 %, surpassing the 84.00 % in the control group, with a marked difference (p<0.05) as shown in Table 1.
| Group (n) | Cured | Improvement | Ineffectiveness | Overall effective rate |
|---|---|---|---|---|
| Observation (120) | 63 (52.50) | 53 (44.17) | 4 (3.33) | 116 (96.67) |
| Control (115) | 48 (41.74) | 49 (42.61) | 18 (15.65) | 97 (84.35) |
| χ2 | 10.502 | |||
| p | 0.001 |
Table 1: Curative Effect
Prior to treatment, there were no remarkable variances (p>0.05) between the two groups concerning GI, PLI, and PD. However, after 1 mo of treatment, the levels of these four indices in both groups depleted, with the observation group levels being inferior to those of the control group, and all contrasts were notable (p<0.05) as shown in fig. 1.
Prior to treatment, there were no meaningful divergences (p>0.05) between the two groups regarding gingival crevicular fluid MCP-1, MMP-8, and IL-6 levels. However, after 1 mo of treatment, the levels of these five indices in both groups decreased, with the observation group levels being lower than those of the control group, and all differences were statistically substantial (p<0.05) as shown in Table 2.
| Group (n) | MCP-1 (pg/ml) | MMP-8 (pg/ml) | IL-6 (pg/ml) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Observation (120) | 135.46±21.25 | 61.73±9.91* | 580.66±51.48 | 343.60±46.30* | 64.21±6.09 | 21.47±3.69* |
| Control (115) | 136.36±20.45 | 88.23±10.44* | 580.28±52.53 | 430.74±44.27* | 65.06±6.08 | 34.84±3.89* |
| t | 0.332 | 5.932 | -0.056 | 8.293 | 1.068 | 11.738 |
| p | 0.740 | 0.000 | 0.955 | 0.000 | 0.286 | 0.000 |
Note: (*) indicates noteworthy difference following treatment compared with prior to treatment
Table 2: Gingival Crevicular Fluid Inflammatory Factor Index (X̄±S, Points)
Prior to treatment, no notable disparities (p>0.05) were observed between the two groups in relation to Th17 and Treg cell levels. Nonetheless, after 1 mo of treatment, there was a decline in the levels of these two indices in both groups, with the observation group levels being lower than those of the control group, and all differences were remarkably significant (p<0.05) as shown in Table 3.
| Group (n) | Proportion of Th17 to CD4+ T cells (%) | Proportion of Treg in CD4+ T cells (%) | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Observation (120) | 2.67±0.52 | 1.31±0.28* | 5.34±0.92 | 4.23±0.80* |
| Control (115) | 2.65±0.50 | 1.73±0.34* | 5.37±0.90 | 4.87±0.94* |
| t | 0.252 | 10.387 | 0.256 | 5.594 |
| p | 0.801 | 0.000 | 0.798 | 0.000 |
Note: (*) indicates noteworthy difference following treatment compared with prior to treatment
Table 3: Th17 And Treg Cell Levels (X̄±S, Points)
Prior to treatment, differences in terms of the bacterial colony counts of P. gingivalis, A. actinomycetemcomitans, S. mutans, and facultative anaerobic streptococci did not display statistical significance (p>0.05) between the two groups. Nevertheless, following a month of treatment, the quantity of P. gingivalis, A. actinomycetemcomitans, and S. mutans experienced a reduction in both groups, with the observation group demonstrating lower counts than the control group, while the quantity of facultative anaerobic streptococci increased, and the observation group count surpassed that of the control group. These disparities were all statistically notable (p<0.05) as shown in Table 4
| Group (n) | Anaerobic bacillus melanin genes | Fusobacterium nucleatum | Peptostreptococcus | Streptococcus facultative aerobes | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Observation (120) | 5.53±0.77 | 0.98±0.18* | 3.84±0.53 | 0.55±0.52* | 2.71±0.46 | 0.93±0.26* | 9.58±1.01 | 33.52±3.65* |
| Control (115) | 5.52±0.78 | 1.65±0.48* | 3.91±0.41 | 0.97±0.18* | 2.76±0.43 | 1.20±0.40* | 9.57±1.04 | 22.04±2.76* |
| t | -0.115 | 14.271 | 1.146 | 8.145 | 0.831 | 6.222 | -0.136 | 8.371 |
| p | 0.908 | 0.000 | 0.253 | 0.000 | 0.407 | 0.000 | 0.892 | 0.000 |
Note: (*) indicates noteworthy difference following treatment compared with prior to treatment
Table 4: Peridental Microecological Indicators (X̄±S, Cfu/Ml)
Food impaction, inadequate restorations, and plaque are local stimuli that contribute to gingival inflammation. If not promptly removed, these factors could lead to the spread of inflammation to deeper tissues, resulting in collagen loss, epithelial growth towards the root, periodontal pocket formation, alveolar bone resorption, and the onset of chronic periodontitis[13,14]. Metronidazole, commonly prescribed for periodontitis, possesses antimicrobial and anti-amoebic properties and is categorized as a nitroimidazole drug. However, it often falls short in terms of short-term treatment efficacy, frequently requiring long-term medication, thereby elevating the risk of drug resistance and unsatisfactory treatment outcomes[15,16]. Containing aminomethyl propanol copolymer, concentrated glycerol, and other components, minocycline hydrochloride ointment effectively blocks bacterial protein synthesis, possessing a broad antibacterial range and demonstrating strong antibacterial effects against S. mutans, anaerobic bacteria, Actinomyces, Clostridium, and other strains. With its potent local sustained release antibacterial impact, fast onset, and extended duration, it yields exemplary overall results[17]. The research observed that the observation group, employing a combination of minocycline hydrochloride ointment and metronidazole, significantly outperformed the control group in terms of total treatment effectiveness.
GI, PLI, and PD are utilized as clinical indicators to assess the effectiveness of treating chronic periodontitis. Minocycline hydrochloride ointment not only displays antibacterial properties but also impedes bone resorption, encouraging the regeneration of periodontal tissues. Furthermore, it encourages the proliferation of fibroblasts in periodontal tissues, thus effectively bolstering gingival health and backing the recovery of periodontal tissues. Following 1 mo of treatment in this study, the GI, PLI, and PD indices in the observation group notably decreased and were lower than those of the control group. This finding illustrates that the combination of minocycline hydrochloride ointment and metronidazole can effectively ameliorate the symptoms of periodontitis, thereby facilitating the restoration of periodontal tissues.
Inflammation contributes to the development and progression of chronic periodontitis. MCP- 1 and IL-6 serve as inflammation indicators reflecting periodontal inflammation; an increase in their levels signifies the further development of inflammation and worsening of periodontitis[18,19]. Elevated MMP-8 expression, as a MMP, can worsen periodontal inflammation. This research found that the observation group experienced a notably more substantial reduction in the levels of gingival crevicular fluid MCP-1, MMP-8, and IL-6 compared to the control group, suggesting that the combination of minocycline hydrochloride ointment and metronidazole effectively suppresses inflammation and lowers the levels of inflammatory mediators.
Capable of synthesizing IL-17, Th17, a subset of Clusters of Differentiation 4 (CD4+) T cells, releases inflammatory factors and collaborates to induce a series of cascading inflammatory reactions, causing a deterioration of periodontal tissues. Treg cells can release anti-inflammatory factors, hinder the proliferation and differentiation of T cells, control the immune response of the body, and produce anti-inflammatory effects[20]. Following the treatment, the investigation revealed that the observation group displayed a more pronounced decline in Th17 and Treg cell levels compared to the control group. These results imply that the use of minocycline hydrochloride ointment combined with metronidazole might enhance chronic periodontitis by regulating the immune response and balancing the Th17/Treg ratio.
Mixed anaerobic bacterial infections are the main cause of the occurrence and development of chronic periodontitis. Anaerobic bacteria such as P. gingivalis, A. actinomycetemcomitans, S. mutans, and facultative anaerobic streptococci are the main pathogenic bacteria. Excessive anaerobic bacterial colonies present in the periodontal microecology impede the restoration of normal flora and ultimately deteriorate the condition of periodontitis. Minocycline hydrochloride ointment has good antibacterial effects, effectively killing anaerobic bacteria, and maintaining a balanced periodontal microecology. Following a month of treatment, the observation group presented a marked decrease in the bacterial colony counts of P. gingivalis, A. actinomycetemcomitans, and S. mutans, resulting in counts lower than those of the control group. An escalation in the bacterial colony count of facultative anaerobic streptococci was noted, with the observation group exceeding the control group, underscoring the ability of minocycline hydrochloride ointment in combination with metronidazole to regulate the periodontal microecology by reducing the level of harmful bacteria and augmenting that of beneficial bacteria.
In conclusion, our research results indicate that the utilization of minocycline hydrochloride ointment in conjunction with metronidazole for chronic periodontitis treatment is more effective in terms of efficacy and its impact on periodontal microecology in comparison to using metronidazole alone. This might be because of its antimicrobial, anti-inflammatory, and immunoregulatory qualities, and its capacity to regulate periodontal microecology. Nonetheless, our study is constrained by limitations such as a short research period and a small sample size. More comprehensive evaluations of the effectiveness and safety of this combination therapy would benefit from further studies with larger sample sizes and longer follow-up periods.
Conflict of interests:
The authors declared no conflict of interests.
References
- Kumar S. Evidence-based update on diagnosis and management of gingivitis and periodontitis. Dent Clin 2019;63(1):69-81.
[Crossref] [Google Scholar] [PubMed]
- Natto ZS, Abu Ahmad RH, Alsharif LT, Alrowithi HF, Alsini DA, Salih HA, et al. Chronic periodontitis case definitions and confounders in periodontal research: A systematic assessment. Biomed Res Int 2018;2018:457882.
[Crossref] [Google Scholar] [PubMed]
- Wang C, Wang L, Wang X, Cao Z. Beneficial effects of melatonin on periodontitis management: Far more than oral cavity. Int J Mol Sci 2022;23(23):14541.
[Crossref] [Google Scholar] [PubMed]
- Dannewitz B, Holtfreter B, Eickholz P. Periodontitis-therapy of a widespread disease. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2021;64(8):931-40.
[Crossref] [Google Scholar] [PubMed]
- Kwon T, Lamster IB, Levin L. Current concepts in the management of periodontitis. Int Dent J 2021;71(6):462-76.
[Crossref] [Google Scholar] [PubMed]
- Wu Y, Gu C, Tong X. Clinical efficacy of minocycline hydrochloride for the treatment of Peri-implant disease: A systematic review with meta-analysis of randomized controlled trials. J Oral Implantol 2023;49(3):245-52.
[Crossref] [Google Scholar] [PubMed]
- Sun YQ, Sun R, Zhao JH. The efficacy of minocycline hydrochloride ointment vs. iodoform gauze for alveolar osteitis: A prospective cohort study. BMC Oral Health 2022;22(1):448.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Deng J, Zhang T, Hua Y, Wang Y, Zhang Q, et al. A study on the use of phase transition lysozyme-loaded minocycline hydrochloride in the local treatment of chronic periodontitis. ACS Appl Bio Mater 2022;5(7):3146-57.
- Yuan Y, Xu J. Application analysis of minocycline hydrochloride ointment combined with tinidazole in the treatment of chronic periodontitis. Minerva Surg 2022;79(1):123-5.
[Crossref] [Google Scholar] [PubMed]
- Theodoro LH, Lopes AB, Nuernberg MA, Cláudio MM, Miessi DM, Alves ML, et al. Comparison of repeated applications of a PDT with amoxicillin and metronidazole in the treatment of chronic periodontitis: A short-term study. J Photochem Photobiol 2017;174:364-9.
[Crossref] [Google Scholar] [PubMed]
- Teughels W, Durukan A, Ozcelik O, Pauwels M, Quirynen M, Haytac MC. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo-controlled study. J Clin Periodontol 2013;40(11):1025-35.
[Crossref] [Google Scholar] [PubMed]
- Deng ZL, Szafrański SP, Jarek M, Bhuju S, Wagner-Döbler I. Dysbiosis in chronic periodontitis: Key microbial players and interactions with the human host. Sci Rep 2017;7(1):3703.
[Crossref] [Google Scholar] [PubMed]
- Billings M, Holtfreter B, Papapanou PN, Mitnik GL, Kocher T, Dye BA. Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J Clin Periodontol 2018;45(1):S130-48.
[Crossref] [Google Scholar] [PubMed]
- Invernici MM, Salvador SL, Silva PH, Soares MS, Casarin R, Palioto DB, et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J Clin Periodontol 2018;45(10):1198-210.
[Crossref] [Google Scholar] [PubMed]
- Dingsdag SA, Hunter N. Metronidazole: An update on metabolism, structure-cytotoxicity and resistance mechanisms. J Antimicrob Chemother 2018;73(2):265-79.
[Crossref] [Google Scholar] [PubMed]
- Gómez-Sandoval JR, Robles-Cervantes JA, Hernández-González SO, Espinel-Bermudez MC, Mariaud-Schmidt R, Martínez-Rodríguez V, et al. Efficacy of clindamycin compared with amoxicillin-metronidazole after a 7-day regimen in the treatment of periodontitis in patients with diabetes: A randomized clinical trial. BMJ Open Diabetes Res Care 2020;8(1):e000665.
[Crossref] [Google Scholar] [PubMed]
- Tan OL, Safii SH, Razali M. Clinical efficacy of repeated applications of local drug delivery and adjunctive agents in nonsurgical periodontal therapy: A systematic review. Antibiotics 2021;10(10):1178.
[Crossref] [Google Scholar] [PubMed]
- Almeida-da-Silva CL, Alpagot T, Zhu Y, Lee SS, Roberts BP, Hung SC, et al. Chlamydia pneumoniae is present in the dental plaque of periodontitis patients and stimulates an inflammatory response in gingival epithelial cells. Microbial Cell 2019;6(4):197-208.
[Crossref] [Google Scholar] [PubMed]
- Sun L, Girnary M, Wang L, Jiao Y, Zeng E, Mercer K, et al. IL-10 dampens an IL-17 mediated periodontitis-associated inflammatory network. J Immunol 2020;204(8):2177-91.
[Crossref] [Google Scholar] [PubMed]
- Li X, Tang L, Lin YF, Xie GF. Role of vitamin C in wound healing after dental implant surgery in patients treated with bone grafts and patients with chronic periodontitis. Clin Implant Dent Relat Res 2018;20(5):793-8.
[Crossref] [Google Scholar] [PubMed]
 ): Observation (120) and (
): Observation (120) and ( ): Control (115)
): Control (115)



