- *Corresponding Author:
- Juan Lu
Department of Public Education, Hainan Vocational College of Political Science and Law, Haikou, Hainan 571100, China
E-mail: 672241343@qq.com
| This article was originally published in a special issue, “Advanced Targeted Therapies in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(1) Spl Issue “79-83” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To observe the clinical effect of NaoXueShu oral liquid in the treatment of hypertensive cerebral hemorrhage is the objective of the study. 85 neurosurgery inpatients from January 2020 to December 2021 were collected. 85 patients with hypertensive intracerebral hemorrhage were randomized into 43 patients in observation group and 42 patients in control group. The control group was only given conventional treatment and the observation group was given conventional treatment and the NaoXueShu oral liquid, 10 ml each time, using the oral or nasal feeding method, 3 times/d. The changes in the National institutes of health stroke scale score, Glasgow coma scale score, Barthel index and hematoma volume were compared between the two groups at admission and after 2 w and 4 w of treatment, so as to evaluate the effect of NaoXueShu oral liquid in the treatment of hypertensive cerebral hemorrhage. There was no significant difference in National institutes of health stroke scale score, Glasgow coma scale score, Barthel index and hematoma volume between the two groups at admission (p>0.05). At 2 w and 4 w, Glasgow coma scale score and Barthel index have significantly higher (p<0.05) National institutes of health stroke scale scores and hematoma volume of the observation group were significantly lower than the control group (p<0.05). The combination of NaoXueShu oral liquid with conventional treatment can improve the neurological deficit and improve the cure rate.
Keywords
Hypertensive cerebral hemorrhage, NaoXueShu oral liquid, neurological deficit, hematoma volume
Hypertensive cerebral hemorrhage occurs due to the formation of vascular wall, hyaline degeneration or fibrinoid degeneration and focal bleeding, ischemia and necrosis under the action of long-term hypertension. It is a spontaneous cerebral hemorrhage disease during emotional agitation or a sharp increase in blood pressure. It is characterized by high morbidity, high mortality rate and high disability rate[1]. Studies in Western countries have shown that cerebral hemorrhage accounts for 8 %-15 % of all strokes. Our country is as high as 21 %~48 %[2]. It seriously threatens the health of the middle-aged and elderly patients, and brings a heavy economic burden to the patient’s families and the society.
General data:
We selected neurosurgery patients from January 2020 to December 2021, 85 patients with hypertensive cerebral hemorrhage were admitted. All patients had first episode and were definitively diagnosed by cranial Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) examination. Inclusion criteria includes a clear history of hypertension; hematoma located in the brain lobes, basal ganglia, thalamic hemorrhage; hematoma volume<30 ml; Glasgow Coma Scale (GCS) scores≥7. Exclusion criteria includes patients with severe cardiac, hepatic or renal insufficiency or coagulation dysfunction; patients with a previous history of stroke and cerebral hemorrhage caused by cerebral trauma or cerebrovascular malformation. Among them, 46 males and 39 females were aged 45 y to 70 y old (53.2±5.6 y old). Patients were randomly divided into 43 patients in the observation group and 42 patients in the control group. Gender, age, amount of hematoma volume and site of bleeding were not significant (p>0.05), which is comparable. All patients or family members signed an informed consent form. It was also submitted to the hospital ethics committee for approval.
Materials and Methods
Methods:
The control group underwent conventional neurosurgery treatment, including dehydration, cranial pressure reduction, hemostasis, nutritional nerve, control of blood pressure, prevention of peptic ulcer and symptomatic supportive treatment. Cranial CT was reviewed on the 2nd d, 1st w, 2nd w and 4th w, to understand the intracranial hematoma absorption. The observation group started nasal feeding or oral NaoXueShu oral liquid on the 2nd d, 10 ml each time, 3 times/d, for 1 mo as a single course.
Observation indicators:
Observation indicators were calculated according to the needs of the subject and reference research[3]. The hematoma was measured at admission and at 2 w and 4 w after treatment, the National Institutes of Health Stroke Scale (NIHSS) score, GCS score and Barthel Index (BI), gender and age were recorded.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 19.0 software was used for analysis and processing, and the measurement data was measured by mean±Standard Deviation (SD) (x±s); t-test was used for comparison between and within groups; count data were analyzed by Chi square (χ2) test, p<0.05 was considered as statistically significant.
Results and Discussion
NIHSS score, hematoma volume, GCS score and BI score between the two groups were compared before treatment (p>0.05) and after treatment the GCS score and BI score of the observation group were significantly higher than those of the control group (p<0.05). The NIHSS score and hematoma volume were significantly lower than those in the control group (p<0.05) as shown in Table 1-Table 4.
| Group | N | Before the treatment | 2 w after treatment | 4 w after treatment |
|---|---|---|---|---|
| Control group | 42 | 16.12±4.56 | 14.59±1.35 | 11.13±2.68 |
| Observation group | 43 | 16.98±3.2 3 | 12.07±1.87 | 8.28±2.87 |
Note: Compared with the observation group, p<0.05
Table 1: The NIHSS Scores before and after Treatment were Compared between the Two Groups (x±s)
| Group | N | Before the treatment | 2 w after treatment | 4 w after treatment |
|---|---|---|---|---|
| Control group | 42 | 28.23±2.16 | 14.07±3.38 | 7.13±2.68 |
| Observation group | 43 | 29.16±1.94 | 11.36±2.35 | 5.24±1.36 |
Note: Compared with the observation group, p<0.05
Table 2: The Hematoma Volume Score before and after Treatment was Compared between the Two Groups (x±s)
| Group | N | Before the treatment | 2 w after treatment | 4 w after treatment |
|---|---|---|---|---|
| Control group | 42 | 9.00±3.42 | 11.00±2.32 | 13.00±3.72 |
| Observation group | 43 | 9.00±2.68 | 12.00±3.35 | 14.00±3.56 |
Note: Compared with the observation group, p<0.05
Table 3: The GCS Scores of the Two Groups before and after Treatment were Compared (x±s)
| Group | N | Before the treatment | 2 w after treatment | 4 w after treatment |
|---|---|---|---|---|
| Control group | 42 | 4.35±1.64 | 10.00±3.25 | 13.00±5.11 |
| Observation group | 43 | 3.26±1.35 | 12.00±4.36 | 14.00±4.65 |
Note: Compared with the observation group, p<0.05
Table 4: The BI Scores of the Two Groups before and after Treatment were Compared (x±s)
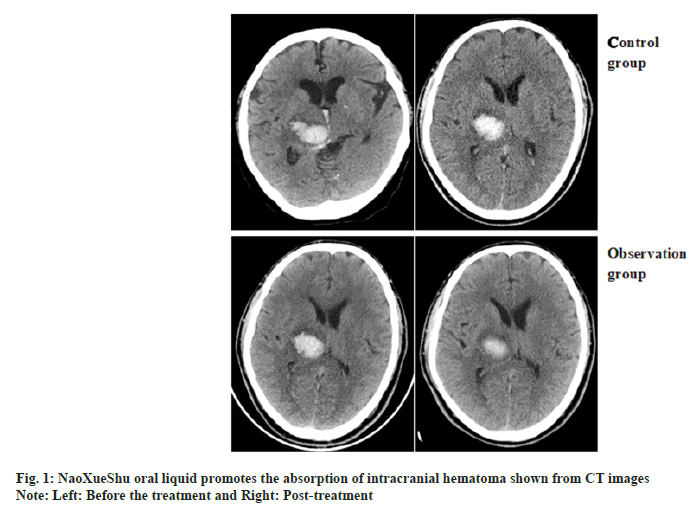
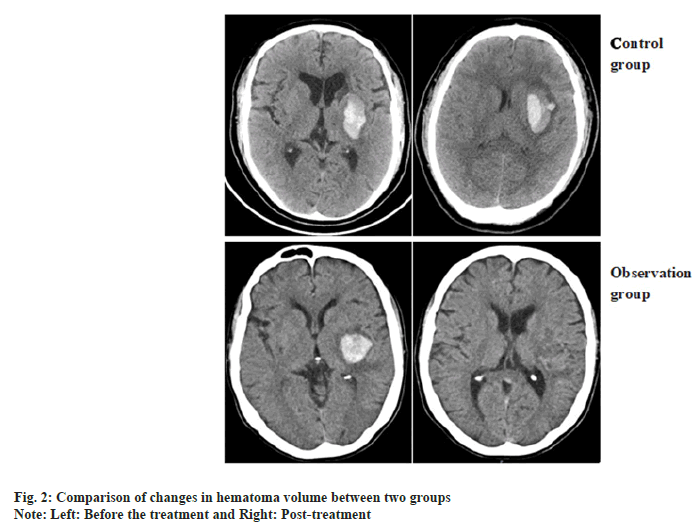
Comparison of changes in hematoma volume between two patients and the representative CT images of hematoma absorption progress from the patients who suffer from hypertensive cerebral hemorrhage with or without NaoXueShu treatment is shown in fig. 1 and fig. 2. NaoXueShu oral liquid promoted the hematoma absorption after 2 w of treatment.
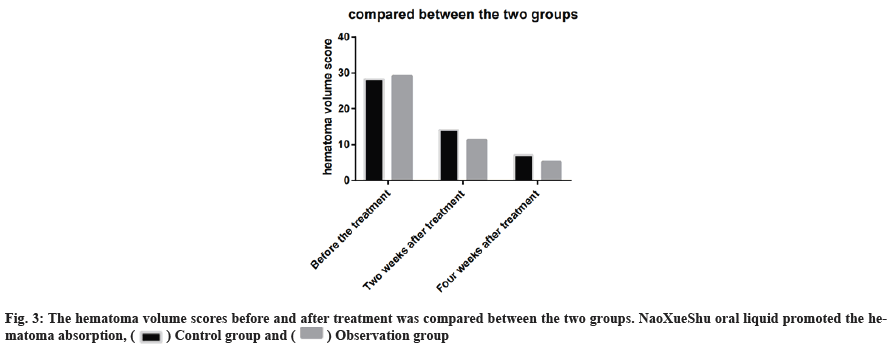
The hematoma volume scores before and after treatment was compared between the two groups. NaoXueShu oral liquid promoted the hematoma absorption (fig. 3).
The NIHSS scores were lower at the end of w 2 and w 4 in both groups, the levels were markedly reduced in the observation group than in the control group as shown in fig. 4.
As a common cardiovascular and cerebrovascular disease, hypertensive cerebral hemorrhage has a high mortality rate and disability rate, which has great social and family hazards[4] and the neurological deficit is the main cause and the main mechanism is as follows[5,6], compression of hematoma-destroy nerve tissue; disinterrupt the nerve conduction pathway; symptoms of hemiplegia, aphasia and other neurological symptoms; secondary brain injurycerebral edema, brain tissue ischemia and hypoxia, brain tissue hypoperfusion, blood brain barrer disruption, release of free radicals and inflammatory factors, this then causes cell degeneration, axonal injury; stress response after cerebral hemorrhage, body stress response after cerebral hemorrhage leads to water and electrolyte balance, acid-base balance, blood glucose metabolism and regulatory secretion disorder aggravate cerebral injury; others include brainstem injury and dehydration, cranial pressure reduction treatment cause effective circulating blood volume decline. NaoXueShu oral liquid is a Chinese patent medicine developed by Professor Xie Daozhen of Xiyuan Hospital of China Academy of Chinese Medical Sciences after years of clinical practice, based on the development of Buyang Huanwu Decoction in "Medicine Lin Gai Cuo" and Dahuang Zhechong soup in "Synopsis of Golden Chamber"[7]. Its main components are Astragalus, leech, stone calamus, Achyranthes bidentata and tetramethylpyrazine. Modern medical clinical research shows that the main components of NaoXueShu oral liquid, paeoniflorin, astragaloside, paeonol can reduce brain tissue edema, improve ischemia and hypoxia, and have the function of nutritional nerve tissue effect[8-10].
In this study, 85 patients with hypertensive cerebral hemorrhage were randomized for treatment. After 2 w and 4 w of treatment with NaoXueShu oral liquid, the GCS score and BI score were significantly higher than that of the control group (p<0.05), the NIHSS score and the amount of intracerebral hemorrhage hematoma were significantly lower than that of the control group (p<0.05). Niu et al.[11] explained routine minimally invasive evacuation of intracranial hematoma and NaoXueShu oral liquid were used for hypertensive cerebral hemorrhage. At 2 w and 4 w after treatment, the observation group was higher than that of the control group and the BI score was higher than that of the control group. Chen et al.[12] observed NaoXueShu oral liquid treatment of acute hypertension cerebral hemorrhage in 69 cases. The NIHSS scores decreased after 2 w of treatment. There was no significant difference. However, the NIHSS score after 4 w of treatment was significantly lower than that of the control group. The conventional treatment of combined NaoXueShu oral liquid, can improve neurological defect symptoms, improve the recovery and cure rate, reduce disability rate. Zhang et al.[13,14] has shown that on the basis of rat cerebral hemorrhage model, it was found that the oral solution intervention could inhibit the opening of blood-brain barrier, reduce the occurrence and development of brain tissue edema, reduce the degree of brain injury after cerebral hemorrhage, and improve the loss of nerve function and reduce the secondary brain damage after cerebral hemorrhage by inhibiting neuronal apoptosis. Liu et al.[15] in the study of hypertensive cerebral hemorrhage, this drug was found to effectively reduce serum Interleukin-6 (IL-6) and Tumor Necrosis Factor alpha (TNF-α), prevent the aggregation of inflammatory factors, prevent the occurrence of secondary injury of hypertensive cerebral hemorrhage and promote hematoma absorption, and improve the clinical prognosis.
NaoXueShu oral liquid combined with conventional internal medicine treatment can reduce hypertension intracerebral hemorrhage causes secondary injury, protects the damaged neurons, promotes hematoma absorption, improves the symptoms of neurological deficiency and improves the quality of life.
Conflict of interests:
The authors declared no conflict of interest.
References
- Gotoh S, Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, et al. Trends in the incidence and survival of intracerebral hemorrhage by its location in a Japanese community-The Hisayama study. Circ J 2014;78(2):403-9.
[Crossref] [Google scholar] [PubMed]
- Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 Update: A guideline from the American heart association/American stroke association stroke council, high blood pressure research council, and the quality of care and outcomes in research interdisciplinary working group: The American academy of neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 2007;116(16):e391-413.
- Wang JG, Li HJ. Risk factor for rehaemorrhagia after hypertension cerebral hemorrhage treatment by the frontal drainage cone. Chin J Stroke 2016;11(7):542-6.
- Yu J, Li J, Huang X. The Beijing version of the Montreal cognitive assessment as a brief screening tool for mild cognitive impairment: A community-based study. BMC Psychiatry 2012;12(1):1-8.
[Crossref] [Google scholar] [PubMed]
- Sun CH, Liao WL, Gao PL, Jiang WF, Pan WD. Advances in pathogenesis of secondary brain insults after hypertensive intracerebral hemorrhage and the prevention and treatment strategies of Western medicine and traditional Chinese medicine. J Neurol Neurorehabilit 2016;12(4):215-20.
- Wang MZ, Zhang L, Jiang WF, Liao WL, Dang CJ, Pan WD. Clinical therapeutic effect and mechanism study in the prevention and treatment of the second brain injury after hypertensive cerebral hemorrhage by benefiting qi, removing stasis and resolving phlegm. World J Integ Tradit West Med 2016;11(11):1544-7.
- Chang H, Chang LJ. NaoXueShu oral liquid combined with language training for cerebral stroke aphasia Clinical observation. J Integr Tradit Chin West Med Cardiovasc Cerebrovasc Dis 2012;8(11):952-3.
- Lei H, Liu ZQ, Du JJ. NaoXueShu oral liquid in patients with ischemic stroke effects of haemodynamics and neurological function. Chin J Pract Nerv Dis 2015;18(20):102-3.
- Miao WC, Zuo Z. Observation of NaoXueShu oral liquid in the treatment of hypertensive cerebral hemorrhage. Chin J Pract Nerv Dis 2014;17(14):102-3.
- Yang YL, Qi WQ, Lu XJ. Cerebral blood was measured simultaneously by HPLC MS/MS and the contents of the three components in the liquid. Chin J Clin Pract Med 2016;19(10):1284-7.
- Niu QD, Wang XM, Ye PP. Minimally invasive removal of intracranial hematoma combined with cerebral blood drainage and effect of fluid treatment in hypertensive cerebral hemorrhage. Chin Gen Med 2013;11(7):1048-9.
- Chen SJ, Wang HY, Zuo Y. Secondary nerve after NaoXueShu oral liquid treatment of cerebral hemorrhage and observation of the curative effect of functional impairment. J Integr Tradit Chin West Med Cardiovasc Cerebrovasc Dis 2016;14(2):199-202.
- Zhang YH, Liu CX, Zhou R. Rule of BBB opening in the rat ICH model and the experimental study of NaoXueShu oral liquid to treat cerebral oedema. J Integr Tradit Chin West Med Cardiovasc Cerebrovasc Dis 2016;14(14):1613-5.
- Ai X, Liu C. Neuroprotective effect of cerebral oral fluid in rats with acute cerebral infarction. J Integr Tradit Chin West Med Cardiovasc Cerebrovasc Dis 2015;13(11):1278-80.
- Liu Xiaoping. Experimental study and clinical effect evaluation of NaoXueShu oral liquid. China J Pharm Econ 2012;(6):128-30.
 ) Control group and (
) Control group and ( ) Observation group
) Observation group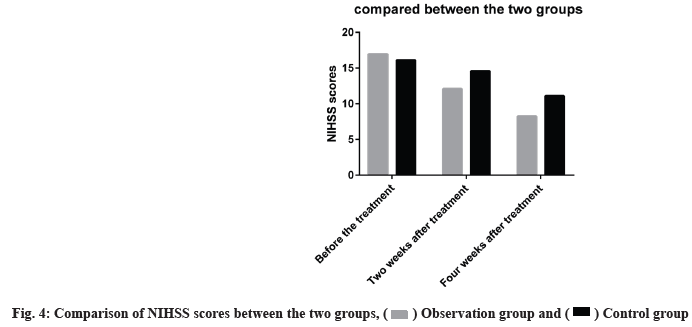
 ) Observation group and (
) Observation group and ( ) Control group
) Control group



