- *Corresponding Author:
- Yulong Tang
Department of Pediatrics, The Second Peoples’ Hospital of Nantong, Nantong, Jiangsu Province 226002, China
E-mail: xrzs20081010@163.com
| This article was originally published in a special issue, “Recent Progression in Pharmacological and Health Sciences” |
| Indian J Pharm Sci 2024:86(2) Spl Issue “222-227” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study seeks to assess the efficacy of terbutaline in conjunction with erythromycin in managing Mycoplasma pneumoniae bronchitis. Between September 2022 and August 2023, we chose 87 children diagnosed with Mycoplasma pneumoniae bronchitis, which were admitted to our hospital and subsequently split into an observation group consisting of 45 cases and a control group with 42 cases through random selection. Routine treatments, such as antitussives and antipyretics, were administered to both groups. Meanwhile, the control group was treated with intravenous erythromycin, and the observation group received terbutaline inhalation treatment as an adjunct. The evaluation of the clinical efficacy of both groups involved a scrutiny of various parameters, including the period of symptom resolution, inflammatory markers, immune function indicators, and lung function markers. Comparatively, the observation group exhibited a much higher efficacy, with a total effective rate of 91.11 %, in contrast to the control group (71.43 %, p<0.05). Subsequent to the treatment, the levels of inflammatory markers (high-sensitivity C-reactive protein, tumor necrosis factor-alpha and procalcitonin) in the observation group saw a substantial decline compared to pre-treatment levels, and were lower than those in the control group (p<0.05). For immune function indicators, the observation group displayed considerably higher cluster of differentiation 4+ cell counts and cluster of differentiation 4+/cluster of differentiation 8+ ratios, as well as notably lower cluster of differentiation 8+ cell counts in comparison to the control group (p<0.05). A reduced duration of symptom resolution and disappearance of lung sounds was found in the observation group as opposed to the control group. Furthermore, following the treatment, the lung function markers (forced expiratory volume 1/forced vital capacity %) demonstrated notable improvement in the observation group as opposed to the control group (p<0.05). Terbutaline in conjunction with erythromycin exhibits good efficacy in managing Mycoplasma pneumoniae bronchitis in children. This comprehensive treatment can considerably ameliorate symptoms, decrease inflammation, modulate immune function, and enhance lung function. As a result, it merits clinical endorsement.
Keywords
Terbutaline, erythromycin, Mycoplasma pneumoniae, bronchitis, tracheobronchitis
Bronchitis has a heightened occurrence in infants, primarily attributable to elements such as cold climate, reduced immune function, bronchial spasms leading to ischemia, and exposure to air pollution and particulate matter, which render children more vulnerable to the condition[1,2]. The primary clinical manifestations of bronchitis encompass cough, sputum production, fever, abnormal lung auscultation, and wheezing.
Mycoplasma pneumoniae (M. pneumoniae) primarily impacts children and adolescents, serving as a prevalent pathogen that spreads via droplet transmission, causing M. pneumoniae or tracheobronchitis[3]. Bronchitis, frequently observed in children, is a respiratory infection distinguished by symptoms like nasal congestion, clear rhinorrhea, sore throat, and hoarseness. Failure to seek treatment may result in the progression to chronic bronchitis, leading to increased complexities in treatment, prolonged disease duration, and substantial impact on the life quality of affected children [4].
Erythromycin is presently widely employed in pediatric clinical environments to treat M. pneumoniae infection[5]. Nevertheless, the diminishing sensitivity of M. pneumoniae to the drug and the emergence of drug resistance have led to a gradual limitation in the effectiveness of erythromycin. Additional drug combinations are frequently required to enhance treatment effectiveness. Terbutaline, a bronchodilator primarily composed of terbutaline sulfate, selectively stimulates Beta (β)-2 receptors in the airways. Its capacity to alleviate bronchial smooth muscles and restrain the release of inflammatory mediators makes it suitable for addressing diverse pulmonary ailments[6,7]. Nevertheless, there is insufficient research available on the utilization of terbutaline alongside erythromycin in addressing M. pneumoniae bronchitis.
As a result, this study seeks to observe the therapeutic efficacy of terbutaline in combination with erythromycin in managing M. pneumoniae bronchitis. It intends to explore the impact of this combination therapy on clinical symptoms and the microbiological outcomes, with the goal of providing more effective treatment options and clinical guidance.
Materials and Methods
General information:
During the period spanning from September 2022 to August 2023, we chose 87 children diagnosed with M. pneumoniae bronchitis, who were admitted to our hospital and subsequently split into an observation group consisting of 45 cases and a control group with 42 cases through random selection. Comprising 25 males and 20 females, the observation group had an age range of 5 y-12 y, with an average age of (7.14±1.54) y, and average disease duration of (5.18±0.53) d. The control group included 22 males and 20 females, aged 5 y-13 y, with an average age of (7.34±1.36) y, and a disease duration ranging from 3 d-12 d, with an average of (5.36±0.66) d. No substantial distinctions were found in fundamental characteristics, such as age, gender, and disease period between the two groups (p>0.05), indicating their similarity.
Inclusion criteria: Children diagnosed with M. pneumoniae infection bronchitis through X-ray examination and pathogenic detection and aged between 5 and 13, and obtained informed consent from the guardian and signed an informed consent form.
Exclusion criteria: Patients with severe pneumonia; children with congenital digestive system diseases, genetic metabolic diseases, sepsis, congenital heart disease; patients with asthma, pulmonary tuberculosis, and significant organ diseases such as heart, brain, lung, liver, and kidney and individuals allergic or hypersensitive to the medications employed in this study.
Treatment methods:
Standard treatments including antitussives and antipyretics were administered to both groups. Furthermore, on the 1st d of hospitalization, the control group underwent a 5 d course of intravenous erythromycin administration (Hunan Kelun Pharmaceutical Co., Ltd., Batch No: H43020028) at a dosage of 10 mg/kg/d administered once daily. In conjunction with the control group’s routine, the study group was administered additional terbutaline inhalation treatment. The terbutaline was amalgamated with 2 ml of normal saline to produce a final concentration of 5 mg/ml, and the resultant blend was inhaled twice a day for 15 min per session, over a 5 d period.
Observation indicators and evaluation criteria:
Clinical efficacy: An evaluation of the clinical efficacy between the two groups was undertaken. The criteria for efficacy determination were as follows; complete disappearance of clinical symptoms and normal imaging examination after treatment indicated cure; notable enhancement in clinical symptoms and imaging examinations approaching normalcy indicated marked improvement; partial improvement in clinical symptoms and imaging examination indicated improvement and failure to meet the above criteria after treatment indicated ineffectiveness.
Inflammatory markers and T lymphocyte subsets: A comparison of inflammatory markers and T lymphocyte subsets was undertaken in the two groups. Blood samples were drawn from both groups, with each sample amounting to 5 ml before and after treatment. The isolated serum was refrigerated and reserved for analysis, and the levels of highly sensitive Tumor Necrosis Factor-Alpha (TNF-α), Procalcitonin (PCT), and high sensitivity C-Reactive Protein (hs-CRP) were gauged via the Enzyme-Linked Immunosorbent Assay (ELISA). T lymphocyte subsets in both groups were assessed using flow cytometry, encompassing Cluster of Differentiation (CD) 8+ and CD4+, and calculating the CD4+/CD8+ ratio.
Symptom resolution time: The time to resolution of symptoms between the two groups was compared, encompassing the duration of cough and sputum disappearance, defervescence, and the clearance of lung sounds.
Lung function: Comparative measurement of lung function in both groups was conducted before and after treatment. This included the assessment of the Forced Expiratory Volume 1 (FEV1) and the FEV1/ Forced Vital Capacity (FVC) using a spirometer. A higher value signified superior lung function.
Statistical analysis:
Utilizing the Statistical Package for the Social Sciences (SPSS) 25.0 statistical software, the analysis was performed. By utilizing an independent sample t-test, a comparison between the two groups was performed using the measurement data presented as mean±standard deviation. Utilizing the Chi-square (χ2) test, the count data were analyzed. The criteria for establishing statistical significance were defined at a level of p<0.05.
Results and Discussion
Following treatment, the observation group exhibited a total effective rate of 91.11 %, notably surpassing the 71.43 % in the control group, signifying a notable difference (p<0.05), as indicated in Table 1.
| Group (n) | Cured | Marked improvement | Improvement | Ineffectiveness | Overall effective rate |
|---|---|---|---|---|---|
| Observation (45) | 11 (24.44) | 16 (35.56) | 14 (31.11) | 4 (8.89) | 41 (91.11) |
| Control (42) | 8 (19.05) | 14 (33.33) | 8 (19.05) | 12 (28.57) | 30 (71.43) |
| χ2 | 5.607 | ||||
| p | 0.018 |
Table 1: Curative Effect
At the outset of the treatment, no notable disparities in the levels of hs-CRP, PCT, and TNF-α between the two groups was observed (p>0.05). Yet, posttreatment, the levels of hs-CRP, PCT, and TNF-α substantially decreased in both groups compared to pre-treatment levels, and the observation group demonstrated lower levels than the control group, documenting a remarkable difference (p<0.05) as shown in Table 2.
| Group (n) | hs-CRP (mg/l) | PCT (ng/ml) | TNF-α (pg/ml) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Observation (45) | 7.57±0.39 | 1.91±0.15* | 8.30±0.43 | 0.39±0.13* | 10.36±0.60 | 5.49±0.37* |
| Control (42) | 7.41±0.45 | 3.47±0.34* | 8.26±0.65 | 1.85±0.23* | 10.47±0.58 | 8.15±0.50* |
| t | -1.732 | 16.397 | -0.303 | 36.484 | 0.843 | 28.254 |
| p | 0.087 | 0.000 | 0.763 | 0.000 | 0.402 | 0.000 |
Note: (*): Indicates noteworthy difference following treatment compared with prior to treatment
Table 2: Inflammatory Cytokine Levels
Prior to treatment, no marked discrepancies in the levels of CD4+, CD8+, and CD4+/CD8+ was identified between the two groups (p>0.05). Subsequent to treatment, there was an enhancement in the immune function of both groups, with elevated CD4+ and CD4+/CD8+ levels, as well as reduced CD8+ levels in the observation group relative to the control group, demonstrating a notable difference (p<0.05), as indicated in Table 3.
| Group (n) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Observation (45) | 47.76±3.39 | 41.11±4.78 | 21.82±5.33 | 26.81±4.00 | 2.38±0.63 | 1.51±0.27 |
| Control (42) | 46.80±3.42 | 44.17±4.12 | 21.85±4.84 | 23.41±3.32 | 2.33±0.51 | 1.88±0.36 |
| t | -1.308 | 3.188 | -0.026 | -4.309 | -0.392 | 5.584 |
| p | 0.194 | 0.002 | 0.979 | 0.000 | 0.696 | 0.000 |
Note: (*): Indicates noteworthy difference following treatment compared with prior to treatment
Table 3: T Lymphocyte Subsets
The observation group exhibited earlier times for defervescence (2.64±0.57), resolution of cough (4.56±1.10), and disappearance of lung sounds (4.04±0.82) compared to the control group (3.24±0.73, 5.36±1.01, and 5.26±0.89 respectively), indicating a remarkable difference (p<0.05) as shown in fig. 1.
No notable differences in FEV1 and FEV1/FVC % between the two groups was found prior to treatment (p>0.05). Subsequently, following treatment, there was an increase in both FEV1 and FEV1/FVC % in both groups, with the observation group demonstrating a higher level of improvement as opposed to the control group (p<0.05) as shown in Table 4.
| Group (n) | FEV1 (L) | FEV1/FVC | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Observation (45) | 1.52±0.29 | 2.16±0.48* | 34.98±7.60 | 51.20±11.62* |
| Control (42) | 1.40±0.35 | 1.83±0.42* | 34.26±8.21 | 39.09±13.19* |
| t | -1.731 | -3.372 | -0.424 | -4.552 |
| p | 0.087 | 0.001 | 0.672 | 0.000 |
Note: (*): Indicates noteworthy difference after treatment compared with before treatment
Table 4: Pulmonary Function Index
Airway hyper responsiveness, a pathophysiological change present in bronchitis, is associated with type III hypersensitivity reactions and T-cell mediated immune responses[8]. Currently, the primary strategy for managing this condition in clinical practice is comprehensive treatment. Addressing acute bronchitis in children involves key interventions such as antibacterial, anti-inflammatory, expectorant, and cough suppressive methods, along with improving lung ventilation. Erythromycin contributes significantly to the treatment of bronchitis by virtue of its diverse mechanisms of action, which involve anti-inflammatory, bronchodilatory, and immunoregulatory effects[9,10]. Terbutaline, a stable selective α2 adrenergic receptor agonist, works by binding to α2 adrenergic receptors, thereby elevating cellular cyclic adenosine monophosphate levels. As a result, it effectively relaxes bronchial smooth muscles, clears secretions in the trachea and bronchi, and relieves bronchospasm. As a result, it is frequently utilized in the treatment of bronchial asthma, bronchitis, and cough-variant asthma[11]. The study was conducted to look into the therapeutic efficacy of combining terbutaline with erythromycin for the treatment of M. pneumoniae bronchitis, and to carry out comparative analysis of clinical efficacy, inflammatory markers and T lymphocyte subgroups, as well as the resolution time of symptoms and lung function in the two groups of children.
At the outset, the findings brought to light that the total effective rate in the observation group (91.11 %) was substantially higher than in the control group (71.43 %), indicating a notable difference. This implies that the combination of terbutaline with erythromycin in treating M. pneumoniae bronchitis is remarkably more effective than treatment with erythromycin alone. This aligns with previous research, signifying the favorable impact of terbutaline, a bronchodilator, in effectively relaxing bronchial smooth muscles and reducing airway resistance, thereby alleviating symptoms in children[12].
Following an infection, there is a marked rise in the serum levels of hs-CRP, PCT, and TNF-α, which can be employed as evaluation markers for appraising the treatment of acute bronchitis[13]. The study assessed and compared the levels of inflammatory markers and T lymphocyte subgroups. The findings indicated that prior to treatment; no notable distinctions existed in the levels of inflammatory markers (hs- CRP, PCT, and TNF-α) between the two groups. Nevertheless, subsequent to the treatment, the levels of these inflammatory markers were notably lower in the observation group compared to pre-treatment levels, and were inferior to those in the control group, with a remarkable difference. This suggests that the combined treatment of terbutaline with erythromycin effectively suppresses the inflammatory response, leading to an improved relief of inflammatory symptoms.
T-lymphocyte subsets are instrumental in modulating immune function, wherein CD4+ and CD8+ are classifications of T lymphocytes. CD4+ fosters antibody production and the differentiation of B lymphocytes, thereby diminishing the body’s immune response, while CD8+ exerts a cytotoxic impact on antigen-expressing target cells, and is vital in combating viral infections, acutely rejecting allogeneic transplants, and eradicating tumor cells. It assists the body in removing pathogens, and thus, the ratio of the levels of the two subsets objectively mirrors the body’s immune function[14,15]. In this study, the combined treatment with terbutaline and erythromycin enhanced the immune function of children. The observation group exhibited remarkably higher levels of CD4+ and CD4+/CD8+, with a notably lower level of CD8+ compared to the control group, demonstrating a notable distinction. Symptom improvement and resolution occurred earlier in the observation group than in the control group, and there was also a marked improvement in lung function markers (FEV1, FEV1/FVC %). FEV1 and FEV1/FVC % directly indicate children’s respiratory function[16]. This further underscores the effectiveness of terbutaline combined with erythromycin in managing M. pneumoniae bronchitis, as it effectively relieves inflammatory symptoms and improves immune and lung function. In summary, the combined administration of terbutaline with erythromycin proves to be significantly effective in managing bronchitis caused by M. pneumoniae. Not only does this combined treatment effectively alleviate inflammatory symptoms, but it also improves immune function and lung function. Nonetheless, even with the clinical importance of this study’s findings, there remains a need for additional large-scale, multi-center studies to validate its effectiveness and safety. This will further enhance treatment strategies and furnish vital guidance to clinicians in the management of bronchitis caused by M. pneumoniae.
Conflict of interests:
The authors declared no conflict of interests.
References
- Wang R, Zhang Y, Huang Y. Clinical efficacy of Tanreqing injection in the treatment of bronchiolitis in children and its effect on serum inflammatory factors. Drug Eval Study 2018;41(2):4.
- Liu X, Hu Y, Chen H. Efficacy and inflammatory markers of montelukast sodium in the treatment of bronchiolitis caused by respiratory syncytial virus infection in children. Chin J Hosp Epidemiol 2018;28(2):276-9.
- Wang Z. Analysis of the efficacy of azithromycin combined with transfer factor oral liquid in the treatment of allergic cough induced by Mycoplasma pneumoniae infection in children. Chin Med Clin 2019;19(7):3.
- Wang Z, He X, Sheng W, Zhang G, Luo J. Analysis of correlation between atmospheric PM2.5 and outpatient visits in children with bronchitis. Zhejiang Prev Med 2018:1092-4-8.
- He M. Application of dynamic monitoring of urinary micro-protein in erythromycin treatment of children with Mycoplasma pneumoniae infection. Electron J Mod Med Health Res 2020;4(18):3.
- Ye F. Clinical observation of budesonide and azithromycin combined with terbutaline in the treatment of acute bronchitis in children. Chin Pharm 2017;12(10):3.
- Li Z. Study on the clinical effect of Xiaoer Fei Ke granule combined with cyclic ester erythromycin in the treatment of acute bronchitis in children. Clin Med Eng 2021;28(5):639-40.
- Wang We. Pediatrics. The 8th ed. Pediatrics; 2013.
- Zhang J. Analysis of the efficacy and adverse reactions of azithromycin lactose in the treatment of bronchitis in children. Med Profession 2021;9:1.
- Wang J. To investigate the clinical effect of low-dose erythromycin in the treatment of bronchiolitis in children. Gansu Sci Technol 2022;5:38.
- Zhao L, Zhao H, Zhi L. Therapeutic effect of Shufeng Jiedu capsule combined with terbutaline on acute infantile bronchitis and its effect on IL-4, PCT, hs-CRP level. Chin J Tradit Chin Med 2020.
- Han L. Efficacy of erythromycin, budesonide combined with terbutaline in the treatment of asthmatic bronchitis. Mod Drug Appl China 2010;21:2.
- Zhang L, Shan Hui. To analyze the clinical effect of azithromycin lactose in the treatment of bronchitis in children. China Med Guide 2020;18(18):3.
- Yang F. Effect of Fanfushu combined with nebulized budesonide on T lymphocytes in children with acute attack of bronchial asthma. Lab Med Clin 2017;14(11):1570-2.
- Wang Y, Cui N, Zhuge Y. Effect of budesonide aerosol inhalation on serum IL-4, IFN- γ, TNF- α and T lymphocyte subsets in children with bronchiolitis. Adv Mod Biomed 2017;17(32):6271-4.
- Wen W, Liu Y, Li Z. Effect of Yinma Jiedu granule on pulmonary function and expression of inflammatory factors in children with phlegm-heat obstructing lung syndrome of acute bronchitis. China Med Innov 2022;19(35):5.
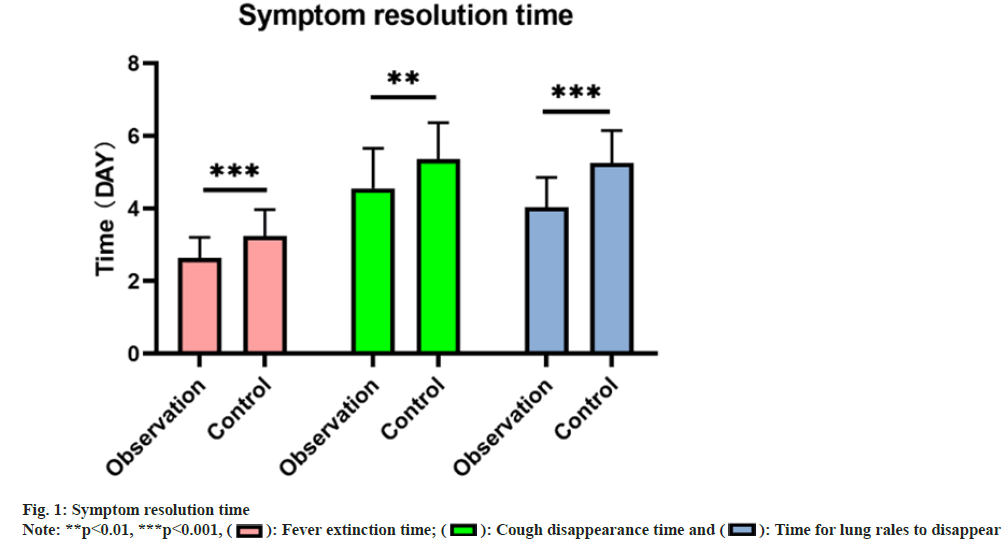
 : Time for lung rales to disappear
: Time for lung rales to disappear



