- *Corresponding Author:
- W. Zhu
Department of Gastroenterology, Pediatric Gastrointestinal Surgery, Sinopharm Dongfeng General Hospital, Hubei University of Medicine, Shiyan, Hubei 442000, China
E-mail: zhukcty089407@163.com
| This article was originally published in a special issue, “Advanced Targeted Therapies in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(1) Spl Issue “72-78” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To evaluate the efficacy of vonoprazan fumarate tablets in refractory reflux esophagitis and to provide new insights into the clinical management of refractory reflux esophagitis. A total of 60 refractory reflux esophagitis E patients meeting the inclusion criteria were recruited and assigned via random number table method to receive either conventional treatments (control group) or vonoprazan fumarate plus conventional treatments (treatment group), with 30 cases in each group. Traditional Chinese medicine decoction was administered for adjuvant therapy. Outcome measures included clinical symptom score, gastroesophageal reflux disease healthrelated quality of life scores, esophageal mucosal damage, serum inflammatory factors, serum calcitonin gene related peptide, 5-hydroxytryptamine levels and relapse. Vonoprazan fumarate resulted in milder esophageal mucosal damage and symptoms, and a lower incidence of recurrence vs. conventional treatment alone (p<0.05). Patients with vonoprazan fumarate showed higher quality of life and lower inflammatory factor levels vs. those without (p<0.05). No documented safety events were found during the administration of vonoprazan fumarate. Vonoprazan fumarate tablets are effective in acid suppression, reducing serum calcitonin gene related peptide and 5-hydroxytryptamine levels in refractory reflux esophagitis patients, alleviating the inflammatory response and promoting esophageal mucosal healing with a high safety profile. Vonoprazan fumarate tablets supplemented with traditional Chinese medicine decoction offers a viable option for the treatment of refractory reflux esophagitis. Further studies are required prior to clinical promotion.
Keywords
Vonoprazan fumarate, refractory reflux esophagitis, gastroesophageal reflux disease, traditional Chinese medicine decoction
Reflux Esophagitis (RE) is a common clinical disease in gastroenterology and belongs to Gastroesophageal Reflux Disease (GERD). It is a lesion of the esophageal mucosa caused by acidic gastric juice or bile reflux, which may cause erosion, inflammation and ulceration in severe cases. The main clinical symptoms of Refractory Reflux Esophagitis (RRE) are retrosternal pain, acid reflux, heartburn and dysphagia[1]. Studies of epidemiological data show that patient with RE accounts for approximately 50 %-70 % of patients with GERD. Untimely treatment may result in a burning sensation in the stomach, retrosternal pain and dysphagia in patients with RE, thereby seriously compromising the quality of life. RE is also a clinically intractable chronic disease due to its recurrent nature[2].
Currently, it is considered that patients with RE without significant mitigation in symptoms after 8 w or more of standard Proton Pump Inhibitors (PPI) therapy can be diagnosed with RRE[3,4]. Currently, the clinical treatment of RRE is still based on oral medications with doubled doses of PPI accompanied by increased gastrointestinal prokinetics. The main drugs are mucosal protective agents and gastrointestinal prokinetics, such as mosapride, omeprazole and domperidone[5,6]. Nonetheless, RRE patients after current proper management are still predisposed to disease relapse. The management of RRE is a recognized medical conundrum in the medical community[6]. The presence of RE is caused by the synergy of multiple factors, among which the relative imbalance between defensive and aggressive factors, i.e., the weakening of protective factors and the enhancement of aggressive factors at the esophagus, is an established basic mechanism. PPI have partially improved the prognosis of the disease, but disease progression into RRE is still reported in some cases. Esophageal mucosa is an important basis for gastroscopic grading and its damage severely aggravates RE[7,8].
Potassium ion-competitive acid blockers are novel acid-suppressing drugs that exert long-lasting acid-suppressive effects by reversibly binding Hydrogen Potassium ATPase (H+/K+-ATP) and continuously blocking H+/K+-ATPase synthesis. Vonoprazan fumarate tablets are novel potassium competitive acid blockers, alkaline compounds with first dose full potency and sustained inhibition of gastric acid secretion and are potential alternatives to PPIs for the treatment of acid-related diseases[9]. Vonoprazan fumarate has a higher pka value (9.3) than PPI, can be immediately ionized in an acidic environment, rapidly aggregates in acidic secretory tubules and has almost the same pharmacokinetic activity under neutral (pH=7.5) and weak acidic conditions (pH=6.5). Moreover, it also has strong resistance to acid catabolism[10].
Based on the typical reflux and heartburn discomfort, RRE can be classified as "esophageal disease" in Traditional Chinese Medicine (TCM). A consensus was reached in 2017 by TCM experts in spleen and stomach diseases, which summarized the pathogenesis of this disease into three major features; rebellion, heat and depression[11,12]. Thus, Puling Lianxia decoction was used for adjuvant therapy. In recent years, the role of visceral esophageal hypersensitivity in the pathogenesis of RRE has received increasing attention and studies have indicated that this may be one of the main reasons for the poor outcome of PPI therapy. Calcitonin Gene-Related Peptide (CGRP) and 5-Hydroxytryptamine (5-HT) all play important roles in visceral sensitization in RRE patients. To this end, the present study was undertaken to evaluate the efficacy of vonoprazan fumarate tablets in RRE.
Materials and Methods
Participants:
A total of 60 RRE patients meeting the inclusion criteria were recruited and assigned via random number table method to receive either conventional treatments (control group) or vonoprazan fumarate plus conventional treatments (treatment group), with 30 cases in each group. All patients provided written informed consent. The study protocol was approved by the hospital ethics committee and all processes conformed to the declaration of Helsinki ethical guidelines for clinical research. The patient characteristics between the two groups were comparable (p>0.05) as shown in Table 1.
|
Treatment | Control | t/χ2 | p |
|---|---|---|---|---|
| n | 30 | 30 | ||
| Sex (male, female) | 20/10 | 17/13 | 0.191 | 0.662 |
| Age (years) | 27-66 | 25-65 | 0.801 | 0.426 |
| 50.33±12.20 | 50.73±3.15 | |||
| Duration of disease (months) | 44.83±26.75 | 41.74±24.53 | 0.555 | 0.58 |
| Body Mass Index (BMI) | 24.5±3.5 | 24.0±3.6 | 0.306 | 0.76 |
| LA classification | 0.335 | 0.36 | ||
| A | 14 | 15 | ||
| B | 8 | 9 | ||
| C | 5 | 4 | ||
| D | 3 | 2 |
Table 1: Patient Characteristics
Diagnostic criteria:
Western medical diagnostic criteria: Referring to the Chinese consensus on the treatment of GERD with integrative Chinese and western medicine (2017)[13], with typical GERD symptoms, such as significant heartburn, acid reflux and retrosternal burning pain; treatment with standard doses of PPI (twice daily) for 8 w continuously as prescribed by the doctor, without effective improvement of symptoms and/or endoscopic failure to heal the broken esophagus; GerdQ scale score ≥8; endoscopy confirmed the presence of RE and 24 h impedance-pH monitoring confirmed the presence of reflux.
Endoscopic diagnostic criteria: With reference to the Los Angeles (LA) classification developed by the LA congress of gastroenterology (1994)[14]. Grade A has one or more esophageal mucosal ruptures with a long diameter less than 0.5 cm; grade B has one or more esophageal mucosal ruptures with a long diameter greater than 0.5 cm, but without fused lesions; grade C has mucosal ruptures with fusion, with an extent less than 75 % of the circumference of the esophagus and grade D has mucosal ruptures with fusion, with an extent greater than 75 % of the circumference of the esophagus. L-A, L-B, L-C and L-D were used to indicate the severity of LA grading, respectively.
Inclusion and exclusion criteria:
Inclusion criteria: Aged 18 y-65 y old, regardless of sex; who met the above diagnostic criteria; who voluntarily signed the informed consent form; with negative results for Helicobacter pylori; with good cooperation.
Exclusion criteria: Who refused to sign the informed consent; pregnant or lactating women; with cardiovascular diseases, diabetes, kidney disease and other serious diseases requiring long-term medication; with psychiatric diseases and long-term antidepressants; with positive Helicobacter pylori; with previous allergies to TCM; who were participating in other clinical trial studies.
Intervention method:
The patients in the two groups were given symptomatic treatments such as acid suppression, mucosal repair, motivation and bile adsorption. Patients received 40 mg of Esomeprazole (produced by AstraZeneca Pharmaceutical Co., Ltd., State Drug Quotient J2008003320) orally 0.5 h before meals, twice daily, 5 mg of mosapride citrate tablets (produced by lunanbetter pharmaceutical Co., Ltd., state drug quotient H19990317) orally, thrice daily, 0.5 g of magnesium aluminum carbonate (produced by Bayer healthcare Co., Ltd., state pharmacopoeia ZP H20100556) 0.5 h after meals, thrice daily and 10 ml of Kangfu Xin oral liquid (produced by Sichuan good doctor Panxi Pharmaceutical Co., Ltd., state pharmacopoeia Z51021834) orally, thrice daily.
Patients in the treatment group additionally received 20 mg of vonoprazan fumarate tablets (Tianjin Wutian Pharmaceutical Co., Ltd., State Pharmacopoeia J20200011) daily[15].
Puling Lianxia decoction was administered to all patients. 15 g of Rhizoma Acori Tatarinowii, 12 g of Curcuma Radix, 10 g of Herba eupatorii, 9 g of Coptidis Rhizoma, 2 g of Evodiae fructus, 10 g of Gardeniae fructus, 15 g of Poria, 9 g of Pinelliae Rhizoma, 9 g of Magnoliae officinalis Cortex, 9 g of Alismatis Rhizoma, 12 g of Bupleuri Radix, 12 g of Aurantii fructus, 10 g of Paeoniae Radix Alba, 9 g of Chuanxiong Rhizoma, 15 g of Cyperi Rhizoma, 15 g of scorched medicated leaven and 15 g of raw oysters were decocted with water to obtain 200 ml of filtrate and administered with half in the morning and half in the evening at 2 h after meals. The duration of treatment was 1 mo.
Outcome measures:
Clinical symptom scores: The clinical symptoms of patients in each group before and after treatment were evaluated by a 4-point scale. The main symptoms of acid reflux, retrosternal pain, heartburn and heartburn were scored as 0, 2, 4 and 6 according to none, mild, moderate and severe respectively, with higher scores indicating more severe symptoms.
Quality of life: The GERD Health-Related Quality of Life Scale (GERD-HRQL) consists of 10 questions. According to the questions asked, no symptoms, symptoms but not bothersome, symptoms are bothersome but do not occur daily, symptoms are bothersome and occur daily, symptoms are bothersome and interfere with daily activities and symptoms prevent daily activities, correspond to scores of 0, 1, 2, 3, 4 and 5, respectively. The scores ranged from 0 to 50.
Esophageal mucosal injury: The degree was graded according to LA classification. Grade A has one or more esophageal mucosal ruptures with a long diameter less than 0.5 cm; grade B has one or more esophageal mucosal ruptures with a long diameter greater than 0.5 cm, but without fused lesions; grade C has mucosal ruptures with fusion, with an extent less than 75 % of the circumference of the esophagus and grade D has mucosal ruptures with fusion, with an extent greater than 75 % of the circumference of the esophagus.
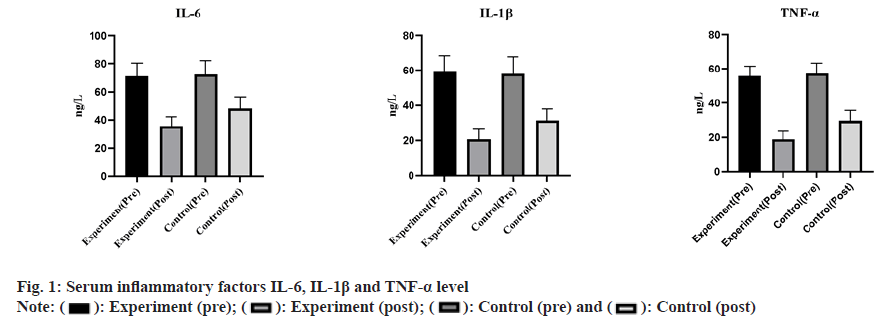
Serum inflammatory factors: 4 ml of elbow fasting venous blood was collected and centrifuged (time 15 min, radius 8 cm, speed 3500 r/min) to obtain the serum. Serum inflammatory factors (Interleukin (IL)-6, IL-1 beta (β), Tumor Necrosis Factor Alpha (TNF-α)) levels were determined by enzyme-linked immunosorbent assay, using the kit of Jingmei Biological Engineering Co.
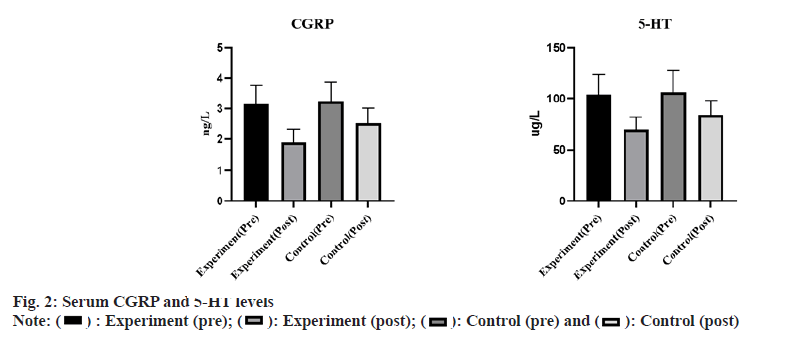
Serum CGRP, 5-HT levels: Serum CGRP and 5-HT were determined before and after treatment by enzymelinked immunosorbent assay.
Recurrence: Patients were followed up for 4 w and the recurrence rates of the two groups were compared.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 23.0 statistical software was used to process the data. The count data were expressed as n (%) and tested with the Chi-square (χ2) test. The measurement data were expressed as mean±Standard Deviation (SD); an independent sample t-test was used for intergroup comparison and paired sample t-test was used for intra-group comparison. A difference in statistical significance was indicated by p<0.05.
Results and Discussion
There was no statistically significant difference between the scores of acid reflux, retrosternal pain, burning pain in the stomach and epigastrium and heartburn symptoms before treatment in the two groups (p>0.05). After treatment, all scores were significantly decreased in both groups, with lower scores in the treatment group than in the control group (p<0.05) as shown in Table 2.
| Group | n | Time point | Acid reflux | Pain behind the sternum | Stomach burning pain | Heartburn |
|---|---|---|---|---|---|---|
| Treatment | 30 | Before treatment | 3.12±0.42 | 2.83±0.43 | 3.54±0.53 | 3.63±0.45 |
| 30 | After treatment | 1.74±0.24*# | 1.51±0.14*# | 1.47±0.15*# | 1.33±0.22*# | |
| Control | 30 | Before treatment | 3.15±0.44 | 2.85±0.44 | 3.56±0.55 | 3.59±0.43 |
| 30 | After treatment | 1.92±0.22* | 1.58±0.16* | 1.55±0.17* | 1.54±0.21 |
Note: (*): p<0.05 when compared with before treatment and (#): p<0.05 when compared with the control group
Table 2: Clinical Symptom Scores (x±s)
After treatment, GERD-HRQL scores were all significantly reduced, with lower scores in the treatment group than in the control group (p<0.05) as shown in Table 3.
| Group | n | GERD-HRQL scores | χ2/t | p | |
|---|---|---|---|---|---|
| Before treatment | After treatment | ||||
| Treatment | 30 | 34.23±7.60 | 24.02±7.57* | 301 | 0 |
| Control | 30 | 33.63±6.91 | 28.60±6.85* | 151 | 0 |
Note: (*): p<0.05 when compared with before treatment
Table 3: GERD-HRQL Scores (x±s)
The difference in the degree of esophageal mucosal injury between the two groups before treatment was not statistically significant (p>0.05). After treatment, the treatment group showed better alleviation of esophageal mucosal damage than the control group (p<0.05) as shown in Table 4.
| Group | n | Time point | Normal | A | B | C | D |
|---|---|---|---|---|---|---|---|
| Treatment | 30 | Before treatment | 0 | 15 | 9 | 4 | 2 |
| 30 | After treatment | 23 | 5 | 1 | 1 | 0 | |
| Control | 30 | Before treatment | 0 | 14 | 8 | 5 | 3 |
| 30 | After treatment | 16 | 8 | 4 | 1 | 1 |
Table 4: Esophageal Mucosal Damage (%)
The differences in serum IL-6, IL-1β and TNF-α levels between the two groups before treatment did not come up to the statistical standard (p>0.05). Significantly reduced serum IL-6, IL-1β and TNF-α levels were observed in the treatment group (p<0.05) as shown in fig. 1.
There was no statistically significant difference in serum CGRP and 5-HT levels between the two groups before treatment (p>0.05). After treatment, serum CGRP and 5-HT levels in the 2 groups were significantly decreased and the treatment group showed lower outcomes (p<0.05) as shown in fig. 2.
After 4 w of follow-up of patients in each group, no recurrence was found in the treatment group and 4 cases showed recurrence in the control group, with a recurrence rate of 13.33 % (χ2=4.166, p=0.041<0.05).
RE refers to symptoms and complications such as heartburn caused by reflux of gastric and/or duodenal contents, especially gastric acid from gastric juice, into the esophagus, often accompanied by endoscopic breakdown of the esophageal mucosa and it is a form of GERD[6]. With the application of PPIs, there has been a breakthrough in the cure rate of RE. However, it was clinically found that 4 %-12 % of patients had no effective symptom relief after 8 w of continuous treatment with standard-dose PPI[16]. In China, RE without significant improvement in symptoms after 8 w of treatment with standard doses of PPI is defined as RRE based on the 2013 guidelines for the diagnosis and management of GERD. The incidence of RRE is increasing year by year and it has become one of the more difficult diseases in gastroenterology[17]. Vonoprazan fumarate tablets are potassium ioncompetitive acid blockers with first-dose full and long-lasting inhibition of gastric acid secretion and are a potential alternative for the treatment of acidrelated diseases PPI[18].
The disease of RRE is located in the stomach and esophagus and is closely related to the spleen and liver, and the key to the pathogenesis is phlegm obstruction and heat stagnation. The discomfort in the stomach and epigastric region of RRE patients impedes the normal operation of the gastric qi and the stagnation of qi leads to poor blood flow and failure of qi and blood to reach the stomach, resulting in the loss of spleen health[19]. Puling Lianxia decoction is derived from "Banxia Houpu decoction", "Zuojin Pill" and "Chaihu Shugan” powder. Banxia Houpu decoction has the effect of lowering rebelliousness, resolving phlegm and promoting the flow of Qi. This formula contains five herbs, namely, Poria, Pinelliae Rhizoma, Magnoliae officinalis Cortex, Perillae Folium and ginger, which are often used in the treatment of GERD caused by the interconnection of phlegm and qi. Zuojin pill clears liver fire, subdues rebelliousness and stops vomiting and is commonly used to treat acid swallowing and bitter mouth caused by stomach heat. Chaihu Shugan powder includes seven herbs, Bupleuri Radix, Chuanxiong Rhizoma, Cyperi Rhizoma, Aurantii Fructus, Paeoniae Radix Alba, orange peel and licorice, which dredges the liver and moves qi, regulates qi and relieves pain, and is commonly used clinically for the treatment of gastric discomfort caused by liver qi stagnation [14].
In this study, Puling Lianxia decoction+vonoprazan fumarate tablets resulted in a low incidence of recurrence and lower GERD-HRQL scores, indicating a good treatment efficiency and high safety profile. The reason may be that when H ions located in the gastric wall cells are transported by H+-K+-ATPase into the tubular space across the apical membrane of the acrosome cells, an equal amount of K ions are also transported in the opposite direction into the cytoplasm of the acrosome cells and this whole process of ion transport is ion tropically balanced. Regulated by the extracellular K ion concentration, studies on the molecular mechanism of H+-K+-ATPase have led to a new class of antisecretory agents, namely Potassium Competitive Acid Blockers (P-CAB), which competitively inhibits H+-K+-ATPase activity with K ions, thereby elevating the pH in the stomach. Vonoprazan fumarate is a P-CAB that is developed to produce biological effects orally for the treatment and prevention of acid-related diseases[20]. As a new member of the P-CAB family, the safety profile of vonoprazan fumarate is comparable to that of PPIs, with most of the mild adverse reactions such as diarrhea, dyspepsia, headache and abdominal pain. During the treatment within 8 w, there were no abnormal changes in the liver function of the patients and no significant abnormal changes were observed in the electrocardiogram results before and after the medication[21].
Studies have confirmed the important role of inflammatory chemokine’s and IL in the occurrence and development of RRE. The results of the present study showed significantly reduced levels of IL-6, IL-8 and TNF-α in patients receiving vonoprazan fumarate tablets vs. those without, suggesting that vonoprazan fumarate tablets effectively alleviate clinical symptoms, promote esophageal mucosal healing and reduce inflammation in the gastric body and sinus of RRE patients. However, whether vonoprazan fumarate tablets have anti-inflammatory effects by non-acid-dependent mechanisms remains to be verified[22].
It has been suggested that the poor outcome of PPI treatment is also associated with visceral hypersensitivity. It was found that CGRP expression was significantly increased in the esophageal mucosa of patients with CGRP and negatively correlated with patient’s nociceptive threshold. Capsaicin receptors are highly expressed in the esophageal tissue of RRE patients and acid exposure stimulates the release of CGRP from esophageal nerve endings, thus increasing the sensitivity of the patient's esophageal mucosa to acid or mechanical stimuli[23]. Therefore, CGRP is closely related to visceral sensitivity in patients with RRE. The results of the current study showed lower CGRP and 5-HT levels in the treatment group than in the control group, indicating that vonoprazan fumarate tablets provided more improvement than PPI on visceral hypersensitivity in RRE patients. However, the pathogenesis of RRE is complex, and factors such as poor patient compliance with medication, non-acid reflux, and esophageal hypersensitivity can all contribute to the persistence of GERD symptoms.
This study has the following limitations such as; this study was a single-center study with a small sample size due to limitations in research funding, research time and other objective factors. No cellular molecular experiments have been conducted and the mechanism of action of Chinese herbal medicine for the treatment of RRE requires further investigation. The SF-36 scale can be applied jointly when studying the effect of treatments on the quality of survival of RRE patients. Future multicenter studies will be performed to provide more reliable data. Network pharmacology studies can be performed to identify the targets of action of the active ingredients of TCM and to investigate their possible mechanisms of action. Follow-up can be extended appropriately with additional monitoring of the gastroscopic esophageal mucosa.
Vonoprazan fumarate tablets are effective in acid suppression, reducing serum CGRP and 5-HT levels in RRE patients, alleviating the inflammatory response and promoting esophageal mucosal healing with a high safety profile. Vonoprazan fumarate tablets supplemented with TCM decoction offer a viable option for the treatment of RRE. Further studies are required prior to clinical promotion.
Authors’ contributions:
Li Yang and Shan Sun have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Ochiai Y, Iizuka T, Kikuchi D, Hoteya S. Tu1178 efficacy of vonoprazan for refractory reflux esophagitis after esophagectomy. Gastrointest Endosc 2018;87(6):AB556-7.
- Kinoshita Y, Hongo M, Kusano M, Furuhata Y, Miyagishi H, Ikeuchi S. Therapeutic response to twice-daily rabeprazole on health-related quality of life and symptoms in patients with refractory reflux esophagitis: A multicenter observational study. Intern Med 2017;56(10):1131-9.
[Crossref] [Google Scholar] [PubMed]
- Shirai Y, Kawami N, Iwakiri K, Kuwana M. Use of vonoprazan, a novel potassium-competitive acid blocker, for the treatment of proton pump inhibitor-refractory reflux esophagitis in patients with systemic sclerosis. J Scleroderma Relat Disord 2023;7(1):57-61.
[Crossref] [Google Scholar] [PubMed]
- Ochiai Y, Iizuka T, Hoshihara Y, Suzuki Y, Hayasaka J, Nomura K, et al. Efficacy of vonoprazan for refractory reflux esophagitis after esophagectomy. Dig Dis 2021;39(6):569-76.
- Orbelo DM, Enders FT, Romero Y, Francis DL, Achem SR, Dabade TS, et al. Once-daily omeprazole/sodium bicarbonate heals severe refractory reflux esophagitis with morning or nighttime dosing. Dig Dis Sci 2015;60(1):146-62.
[Crossref] [Google Scholar] [PubMed]
- Frazzoni M, Bertani H, Manta R, Mirante VG, Frazzoni L, Conigliaro R, et al. Impairment of chemical clearance is relevant to the pathogenesis of refractory reflux oesophagitis. Dig Liver Dis 2014;46(7):596-602.
[Crossref] [Google Scholar] [PubMed]
- Kahrilas PJ, Persson T, Denison H, Wernersson B, Hughes N, Howden CW. Healing of refractory reflux oesophagitis–An ongoing unmet clinical need; authors' reply. Alimen Pharmacol Ther 2014;40(8):989.
- Hunt RH, Yuan Y, Scarpignato C. Healing of refractory reflux oesophagitis–An ongoing unmet clinical need. Alimen Pharmacol Ther 2014;40(8):987-9.
- Sugano K. Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: Safety and clinical evidence to date. Ther Adv Gastroenterol 2018;11:1756283X-745776.
[Crossref] [Google Scholar] [PubMed]
- Echizen H. The first-in-class potassium-competitive acid blocker, vonoprazan fumarate: Pharmacokinetic and pharmacodynamic considerations. Clin Pharmacokinet 2016;55(4):409-18.
[Crossref] [Google Scholar] [PubMed]
- Li J. Observation on the efficacy of adding tonic Chinese and Yiqi soup as an adjunct to the treatment of reflux esophagitis. J Pract Chin Med 2022;38(7):1165-7.
- Zhang J, Hao H, Ren S. Hao Hailong's experience in treating reflux esophagitis with the Xin Kai bitter lowering and harmonizing method. Hubei J Tradit Chin Med 2022;44(6):28-30.
- Wan J. Clinical observation of 40 cases of refractory reflux esophagitis treated with the Heshui method. Hubei Univ Tradit Chin Med; 2018.
- Hao X. Clinical observation of Puling Lianxia Decoction combined with western medicine in the treatment of refractory reflux esophagitis. Hebei Coll Tradit Chin Med; 2020.
- Yamashita H, Kanamori A, Kano C, Hashimura H, Matsumoto K, Tsujimae M, et al. The effects of switching to vonoprazan, a novel potassium-competitive acid blocker, on gastric acidity and reflux patterns in patients with erosive esophagitis refractory to proton pump inhibitors. Digestion 2017;96(1):52-9.
[Crossref] [Google Scholar] [PubMed]
- Kahrilas PJ, Persson T, Denison H, Wernersson B, Hughes N, Howden CW. Predictors of either rapid healing or refractory reflux oesophagitis during treatment with potent acid suppression. Aliment Pharmacol Ther 2014;40(6):648-56.
[Crossref] [Google Scholar] [PubMed]
- Frazzoni M, Bertani H, Manta R, Mirante VG, Frazzoni L, Conigliaro R, et al. Impairment of chemical clearance is relevant to the pathogenesis of refractory reflux oesophagitis. Dig Liver Dis 2014;46(7):596-602.
[Crossref] [Google Scholar] [PubMed]
- Kanamori A, Tominaga K, Masuyama H, Ishikawa M, Masuyama S, Kondo M, et al. Size reduction of gastric fundic gland polyposis by de-escalation of acid-suppressive therapy. DEN Open 2023;3(1):e135.
[Crossref] [Google Scholar] [PubMed]
- Zhu F, Li Y, Zhang P. Effectiveness of Yi qi and gangrene removal tang in the treatment of reflux esophagitis and its effect on the changes of gastrointestinal motility, interleukin-17 (IL-17) and interleukin-23 (IL-23) in patients. Chin J Tradit Chin Med 2022:1-6.
- Marabotto E, Ziola S, Savarino V, Giannini EG, Furnari M, Bodini G, et al. Vonoprazan fumarate for the treatment of gastric ulcers: A short review on emerging data. Clin Exp Gastroenterol 2020;13:99-104.
[Crossref] [Google Scholar] [PubMed]
- Martinucci I, Blandizzi C, Bodini G, Marabotto E, Savarino V, Marchi S, et al. Vonoprazan fumarate for the management of acid-related diseases. Expert Opin Pharmacother 2017;18(11):1145-52.
[Crossref] [Google Scholar] [PubMed]
- Ota K, Takeuchi T, Kojima Y, Kawaguchi S, Iwatsubo T, Hakoda A, et al. Administration of a standard dose of vonoprazan fumarate delays gastric emptying in Japanese healthy adults: A prospective clinical trial. J Gastroenterol 2021;56(8):722-31.
[Crossref] [Google Scholar] [PubMed]
- Mei T, Noguchi H, Suetsugu K, Hisadome Y, Kaku K, Okabe Y, et al. Effects of concomitant administration of vonoprazan fumarate on the tacrolimus blood concentration in kidney transplant recipients. Biol Pharm Bull 2020;43(10):1600-3.
[Crossref] [Google Scholar] [PubMed]
 : Experiment (pre);
: Experiment (pre);  : Experiment (post);
: Experiment (post);  : Control (pre) and
: Control (pre) and  : Control (post)
: Control (post)