- *Corresponding Author:
- D. H. Yan
Department of Medical Science and Technology,
Suzhou Chien-Shiung Institute of Technology,
Suzhou,
Taicang 215411,
P. R. China
E-mail: danhong_yan@163.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “123-130” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In order to study the performance of doxorubicin nanomedicine on gastric cancer cells, the prepared mesoporous silica nanoparticle samples and the doxorubicin hydrochloride solution samples of different concentrations were synthesized and prepared to concentrations of 0.01 mg/ml and 0.03 mg/ml, 0.05 mg/ml, 0.07 mg/ml and 0.09 mg/ml respectively. Doxorubicin hydrochloride-loaded mesoporous silica nanoparticles samples were applied to the cultured gastric cancer cells. Through transmission electron microscope, specific surface area and pore size distribution analyzer, ultraviolet spectrophotometer and other instruments, test the morphology, surface area, pore volume and average pore size, drug release performance and adsorption of mesoporous silica nanoparticles samples loaded with doxorubicin hydrochloride- desorption performance, drug loading, drug loading and encapsulation rate, and gastric cancer cell survival rate, etc., so as to conduct in vitro research and analysis of doxorubicin drugs for gastric cancer cells. The test results show that mesoporous silica nanoparticles samples are suitable for drug delivery, with high drug loading and encapsulation rate and drug controlled release effect as the acidity of the buffer increases, it is beneficial to the release of drugs in the slightly acidic environment of the tumor site; mesoporous silica nanoparticles, the drug loading of the sample after three times of ultrafiltration is about 55 % and the encapsulation efficiency is above 90 %; as the sample concentration increases, the survival rate of gastric cancer cells gradually decreases. This shows that doxorubicin nanomedicine can be used to inhibit gastric cancer cells and can assist in the treatment of gastric cancer.
Keywords
Gastric cancer cell, doxorubicin nanomedicine, anticancer drugs, encapsulation rate, mesoporous silica nanoparticles
Cancer has become a very important issue in the medical field in China. Gastric cancer is one of the common emergent cancers. It can be prevented by reducing the prevalence of risk factors and improving the effectiveness of clinical treatment [1]. Cancer treatment means it includes radiotherapy, chemotherapy and so on. In order to further improve the treatment effect and survival treatment of patients, immunotherapy is gradually derived. However, most of the chemotherapeutic drugs have certain cytotoxicity, which can damage normal cells in human body. Therefore, the controlled release system of drugs is used to achieve targeted release of drugs in the lesions, so as to improve the efficacy and reduce the side effects of cytotoxicity. At present, nanomaterials have been used as the carrier of drug controlled release system, using nanomaterials to load anticancer drugs.
The synthesis methods and conditions of nanocarriers are relatively simple, mild and controllable, and the residual toxicity is low. Therefore, nanocarriers are widely used in anti-tumor drug carriers [2]. There are many kinds of nanocarriers. Take Mesoporous Silica Nanoparticles (MSNs) as an example. Due to their high drug loading efficiency, excellent biocompatibility and stability, they can effectively improve the controlled release efficiency of drugs by increasing the acidity of buffer. Therefore, it has attracted a great deal of attention and research from biomedical researchers in the fields of loading, targeted delivery and controlled release of anti-tumor drugs [3]. In order to verify it’s in vitro antitumor experimental efficacy of gastric cancer cells, the experimental study was carried out. The results showed that the controlled release of nano anticancer drugs had a certain inhibitory effect on gastric cancer cells.
Materials and Methods
Instrument:
Heat collector stirrer, 524G (Shanghai Meiyingpu Instrument Manufacturing Co., Ltd.); oil bath, GY- 100L (Henan Gongyi Ruide Instrument Equipment Co., Ltd.); CNC ultrasonic cleaner, JM-23D-40 (Shenzhen Alliance Cleaning Equipment Co., Ltd.); transmission electron microscope, YS039 (Shanghai Cangmao Industrial Co., Ltd.); zeta potentiometer, zetasizer nano (OnStar New Carbon Material Changzhou Co., Ltd.); specific surface area and pore size distribution analyzer, ASAP 2425 (MAC Instruments); multifunctional biochemical incubator, SPM-258 (Beijing Century Sunshine Technology Co., Ltd.) [4].
Main reagents, consumables and drugs:
Triethanolamine, Jinan Yuanxiang Chemical Co., Ltd.; cetyltrimethylammonium bromide, Shandong Suitai Biotechnology Co., Ltd.; ethyl orthosilicate, Guangzhou Shuangtao Fine Chemical Industry; anhydrous ethanol, analytical pure, Jinan Rongguang Chemical Co., Ltd.; hydrochloric acid, Tai 'an Senmao Chemical Co., Ltd.; 3-aminopropyl triethoxysilane, Wuhan Huaxiang Science and Technology Biology Co., Ltd.; Methoxy Polyethylene Glycol Succinimide ester (MPEG-SC), 5 kDa, Guangzhou Carbonic Water Technology Co., Ltd.; Tris(hydroxymethyl)aminomethane Hydrochloride (Tris-Hcl) buffer, Shanghai Guchen Biotechnology Co., Ltd.; Fetal Bovine Serum (FBS), Shanghai Bohu Biotechnology Co., Ltd.; Dulbecco's Modified Eagle Medium (DMEM) (100 mg/ml), Nanjing Shenghang Biotechnology Co., Ltd.; trypsin, Nanning Pangbo Biological Engineering Co., Ltd.; frozen protective agent dimethyl sulfoxide, Guangzhou Aiside Intelligent Technology Co., Ltd.; doxorubicin hydrochloride, analytical purity, purity >98 %, Beijing Huafong Lianbo Technology Co., Ltd [5]; human gastric cancer cell line was provided by a hospital.
Sample preparation:
MSNs sample preparation: 10 % cetyltrimethyl ammonium chloride solution is prepared. 1.0 g cetyltrimethylammonium bromide and 9.0 g deionized water were used to complete the preparation of the solution.
10 % triethanolamine solution is prepared. 1.0 g triethanolamine and 9.0 g deionized water were used to complete the preparation of the solution.
The mixture of above two solutions was added to the round-bottom flask and placed in a constant temperature oil bath at a certain temperature for magnetic stirring. After 25 min at 310 rpm, ethyl orthosilicate was added drop by drop at a frequency of 3 s/drop [6]. In order to ensure uniform nucleation and uniform growth of particles, it is necessary to add slowly and stir at an appropriate speed at the initial stage of the reaction. When the reaction solution gradually becomes milky white from clarification, the dripping is finished and the stirring is continued for 0.5 h, 1 h, 2 h, 3 h and 4 h, so that the temperature is cooled to room temperature. A little ethanol was inserted into the cooled solution to generate floccule and the products were collected by centrifugation at 13 000 rpm. The precipitation was cleaned with ethanol for three times to completely remove the surfactant as far as possible [7]. In order to further remove the surfactant completely, the cleaned MSNs were dispersed in ethanol solution of hydrochloric acid by ultrasound and treated with reflux extraction at 85° for 8 h. After reflux extraction, the solution was centrifuged and the precipitation was dispersed in water by ultrasound. The particle size and zeta potential were detected by nano particle size and zeta potentiometer and the particle morphology and specific surface area were observed by transmission electron microscope and specific surface area analyzer. They were placed in a vacuum drying oven at 65° for 12 h to obtain MSNs.
Doxorubicin hydrochloride sample preparation:
A certain amount of doxorubicin hydrochloride was accurately put into ultra-pure water and dissolved to prepare mother liquor with a certain concentration [8]. In order to obtain the standard solution of doxorubicin hydrochloride with different concentrations, the mother liquor was successively diluted with ultra-pure water and prepared with 0.01 mg/ml, 0.03 mg/ml, 0.05 mg/ml, 0.07 mg/ml and 0.09 mg/ml of Adriamycin hydrochloride solution, respectively.
Doxorubicin hydrochloride loading is as follows. Put 6.5 mg of MSNs samples into 10 ml covered centrifugation tube and weigh them in parallel, suck 4 ml of 0.09 mg/ml doxorubicin hydrochloride solution into 6 centrifugation tubes and wrap it in a tin foil, shake at 160 r/min for 70 h, so that doxorubicin hydrochloride can be fully loaded onto MSNs samples. After 70 h, the centrifuge tube was taken out and the red solution was centrifuged at a speed of 13000 r/min for 12 min. The supernatant was sucked into the 15 ml pointed centrifuge sample was washed with ultra-pure water for several times until the supernatant solution became colorless and transparent. All the supernatant was stored in a pointed centrifugal tube and the volume of supernatant was counted. The absorbance value was measured by ultraviolet spectrophotometer. The obtained samples loaded with doxorubicin hydrochloride were placed in a vacuum drying oven at 48° for 48 h and then ground to obtain MSNs samples loaded with doxorubicin hydrochloride for standby [9]. MSNs loaded with doxorubicin hydrochloride at 0.01, 0.03, 0.05 and 0.07 mg/ml could be obtained by the same method.
Gastric cancer cells:
Cell culture: Human gastric cancer cell lines were cultured in DMEM supplemented with 10 % FBS and 100 mg/ml double antibody in a constant temperature incubator containing 5 % Carbon Dioxide (CO2) at 37°.
Recovery: Place cells removed from the liquid nitrogen tank into a thermos and soak them with liquid nitrogen. The medium was preheated by 37° water bath [10]. The cell freeze tube was thawed quickly in a 37° water bath and the 3 ml culture medium was centrifuged at 1000 rpm for 4 min to remove the supernatant. Under the condition that bubbles were avoided, the cells were blown evenly to a single dispersion with a pipette and then the cells were added to the culture bottle and gently shaken [11]. It was placed in a constant temperature incubator containing 5 % CO2 at 37°.
Passage: Passage treatment was performed when the cells adhered to the wall and almost covered the surface of the flask. The medium and 0.25 % trypsin were heated in a 37° water bath. The cells were cleaned with 5 ml FBS. 1 ml membrane protease was added for digestion until cells slipped and 2.5 ml medium was added to terminate digestion [12]. The supernatant was removed by centrifugation and 3 ml medium was added to blow evenly. The medium was placed in a constant temperature incubator containing 5 % CO2 at 37° for cultivation.
Cryopreserved: Cell cryopreservation fluid was prepared. FBS 90 %, cryopreservation protectant 10 %, fusion formation. The cryo-storage tube was placed in the refrigerator at -20° for 2 h and then placed in the refrigerator at -80° overnight. Finally, it was removed and quickly put into the liquid nitrogen tank for storage [13].
Cell survival rate calculation: The cells in logarithmic growth phase were taken out and counted with a blood counting board. Each well was inoculated with 3×103 pieces in 96-well plates. In order to reduce liquid evaporation, FBS was used to seal the surrounding fluid and incubate for 12 h until the cells adhered to the wall. The culture fluid was removed and cleaned with FBS [14]. A certain concentration gradient of Polyethylene Glycol (PEG) modified MSNs was prepared, which were 0, 6.25, 25, 100, 400 μm/ml and each concentration was set with 6 complex holes. 100 μl medium containing PEG modified MSNs was added to each well, 100 μl fresh medium and the culture was continued for 48 h. The holes without cell suspension were used as zero adjusting holes and the holes with cell suspension without PEG modification were used as blank control [15]. After 48 h, remove the medium and add Cell Counting Kit-8 (CCK-8) solution to each well. In order to avoid any influence on Optical Density (OD) value, pay attention to not producing bubbles when adding. After addition, the cells were placed in a constant temperature incubator at 37° for 1 h and the absorbance at OD 450 nm was measured by a microplate reader and the cell survival rate was calculated.
Results and Discussion
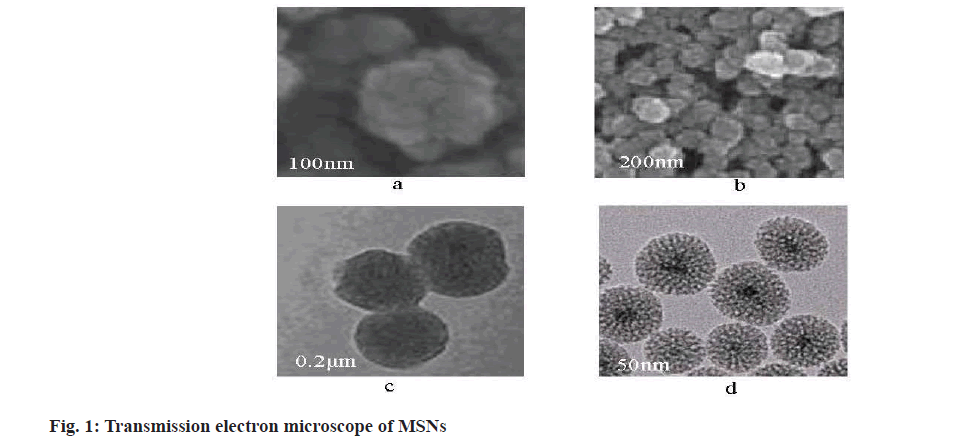
The optimum synthesis conditions of MSNs were 10 ml in 10 wt % hexadecyltrimethylammonium bromide solution, 0.4 ml in 10 wt % triethanolamine solution and 0.75 ml in ethyl orthosilicate solution at 95°. The structure of synthesized MSNs under different magnifications of transmission electron microscope was shown in fig. 1.
It can be seen from fig. 1, the synthesized MSNs particles are spherical, with uniform overall distribution, uniform particle size and good dispersion. There are regular mesopores in the particles, which can be used for drug loading experiments.
The 100 mg MSNs sample was measured by specific surface area and pore size distribution analyzer. The specific surface area, pore volume and average pore size of the samples were calculated at 310° and 5 h. The calculation results are shown in Table 1.
| Test material | Specific surface area/(m2/g) | Pore volume/(cm3/g) | Average pore size/(nm) |
|---|---|---|---|
| MSNs | 436.07 | 0.83 | 3.14 |
Table 1: Calculation Results of Surface Area, Pore Volume and Average Pore Diameter
According to the results in Table 1, MSNs has a large specific surface area and the average mesoporous aperture is about 3 nm. Good specific surface area gives samples great advantages in drug loading and is suitable for drug delivery.
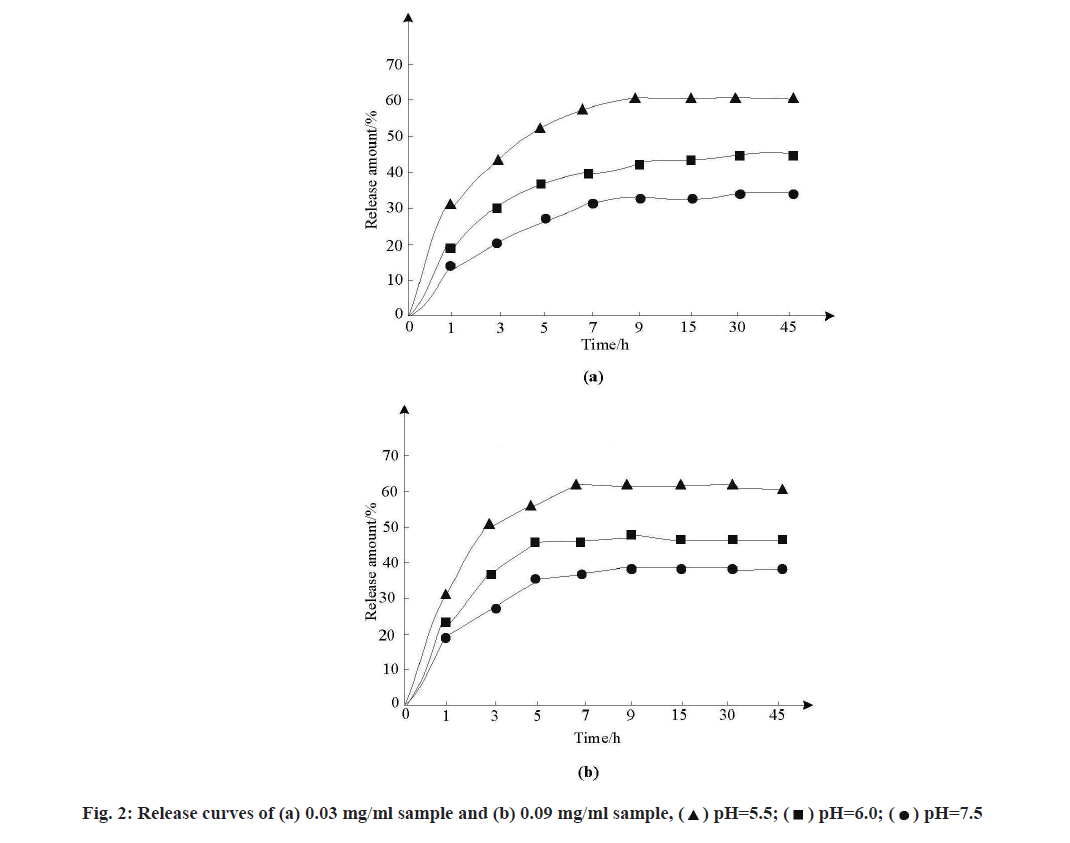
As the standard curves of anticancer drugs in different simulated release solutions were different, the standard curves of MSNs samples loaded with Adriamycin hydrochloride in solutions of three pH values were tested. MSNs samples loaded with doxorubicin hydrochloride at concentrations of 0.03 mg/ml and 0.09 mg/ml were dispersed into FBS at pH 5.5, pH 6.0 and pH 7.5, respectively. After corresponding treatment, the release amount of samples was calculated and the release curve was drawn, as shown in fig. 2.
As can be seen from fig. 2, the release conditions of samples with the same concentration in the three buffers were different and sudden release appeared at the initial stage of release, which was conducive to the drug’s effect in the initial stage and the release amount gradually stabilized with the extension of time. The release amount of FBS buffer with pH 7.5 was the least, about 28 %~33 %. The release of FBS in pH 6.0 buffer increased to 43 %~48 % and the release of FBS in pH 5.5 buffer increased to 53 %~58 %, indicating that with the gradual increase of acidity of buffer, the release of drugs in the slightly acidic environment of tumor site.
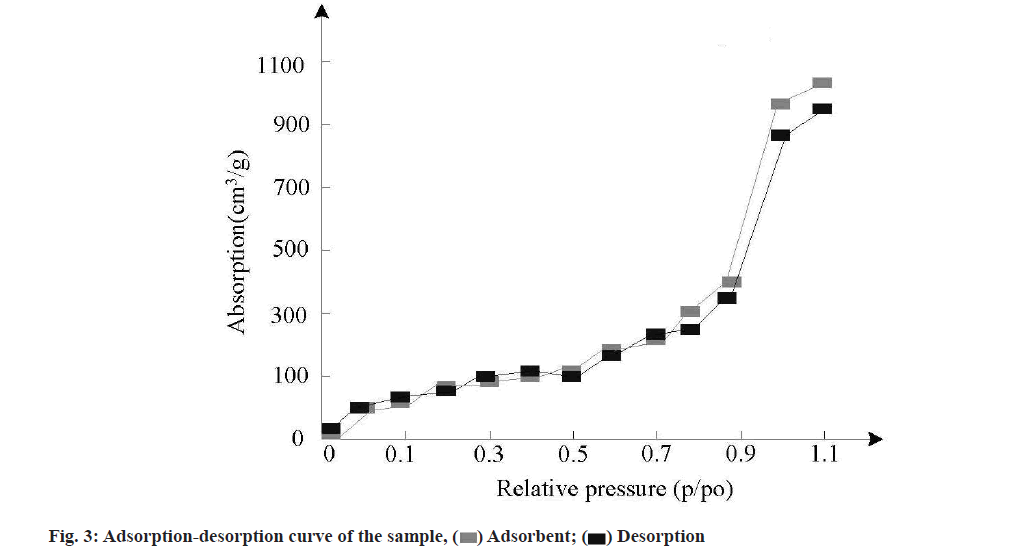
Adsorption-desorption test results were characterized. Nano-carrier materials have many physical and chemical properties, such as specific surface area, pore volume, pore size, etc., which will directly affect the drug loading capacity and release capacity greatly. In order to test the feasibility of MSNs samples as antitumor drug carriers, a certain amount of MSNs samples were placed in nitrogen medium and degassed for 7 h at 100°. After that, nitrogen adsorption and desorption were conducted to test the nitrogen adsorptiondesorption performance of the samples. The test results are shown in fig. 3.
As can be seen from fig. 3, the nitrogen adsorption curve and desorption curve of the self-assembled structure of the sample do not coincide, resulting in a hysteresis ring. Compared with the International Union of Pure and Applied Chemistry, this kind of curve belongs to type IV adsorption-desorption curve of porous materials. Due to the mesoporous structure on the surface of the sample, the hysteresis ring is generated, so the sample has a good controlled release effect.
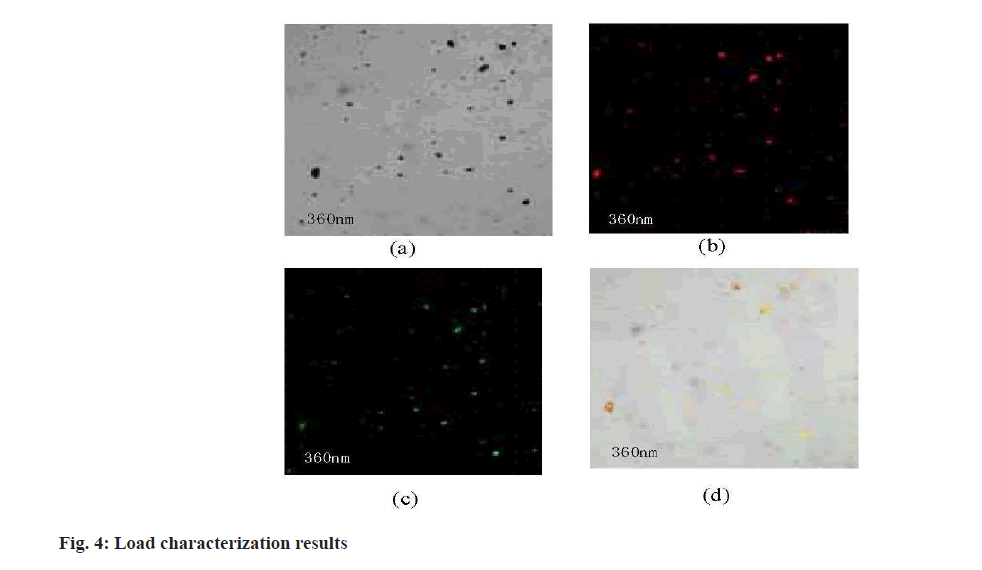
In order to judge that MSNs samples could load Adriamycin hydrochloride, the loading rate and loading capacity of Adriamycin hydrochloride were 56.9 % and 0.015 mg/ml, respectively, according to the absorbance measured by ultraviolet spectrophotometer corresponding to its standard curve. The load of MSNs samples was characterized by confocal laser scanning. The result is shown in fig. 4.
Fig. 4 (a) is the confocal laser microscope under bright field after loading; fig. 4 (b) is the confocal laser microscopy of spontaneous red fluorescence in loaded samples; fig. 4 (c) is the confocal laser microscopy of spontaneous green fluorescence of the sample after loading; fig. 4 (d) is the confocal laser microscopy of fig. 4 (a), fig. 4 (b) and fig. 4 (c) after the combination of the three. Observation of confocal laser microscopy showed that the MSNs sample was successfully loaded with doxorubicin hydrochloride.
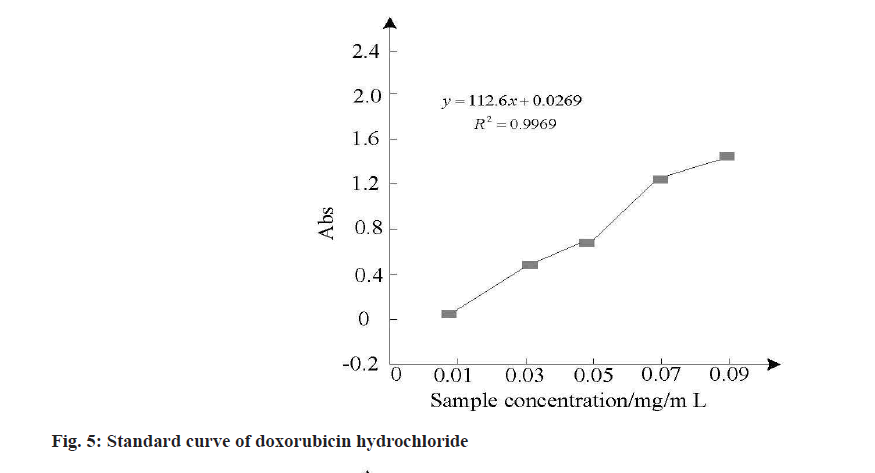
To judge the effect of the MSNs samples in the drug loading, the need to obtain the absorbance of different concentration of hydrochloric acid doxorubicin and MSNs samples of the drug, to complete the absorbance of acquisition and drug loadings calculation, the calculation formula by using ultraviolet spectrophotometer and drug loadings, and draw the hydrochloric acid doxorubicin standard curve, the result is shown in fig. 5 and are shown in Table 2.
| Sample | Ultrafiltration times | Drug (%) | Entrapment efficiency |
|---|---|---|---|
| MSNs samples loaded with doxorubicin hydrochloride | 1 | 61.4 | 93.2 |
| 2 | 57.3 | ||
| 3 | 52.8 |
Table 2: Drug Loading of MSNS Samples
The formula for calculating the drug load of MSNs samples is as follow. Drug loading=Input drug quality-Free drug quality/Loading drug quality+Input nanomaterial quality×100 %
The analysis of fig. 5 and Table 2 shows that drug load is strongly correlated with ultrafiltration centrifugation times and drug load will be affected by centrifugation times. Due to the low centrifugation times, free drugs cannot be separated from the carrier, resulting in high drug load and encapsulation rate. Therefore, the drug load and encapsulation rate were calculated after three times of ultrafiltration centrifugation. The calculation results in Table 2 showed that MSNs samples had higher drug loading and encapsulation rate for doxorubicin hydrochloride. After three times of ultrafiltration, the drug loading was about 55 % and the encapsulation rate was above 90 %.
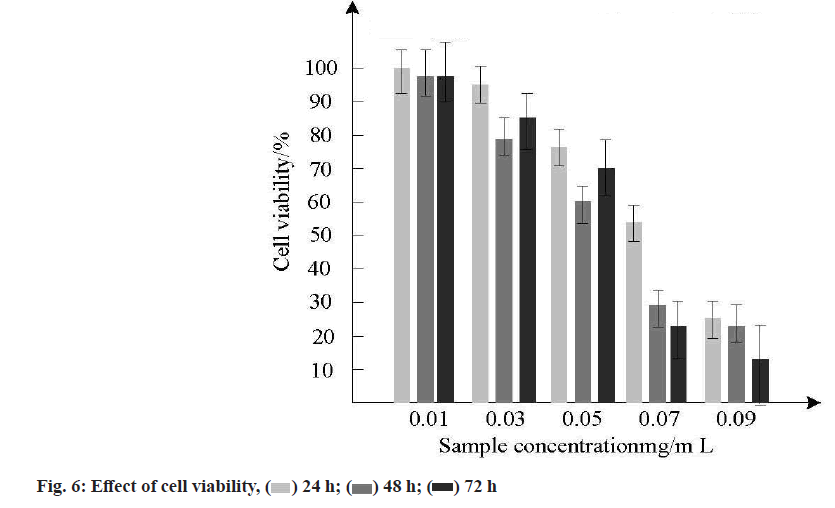
In order to study the effect of different incubation times (24 h, 48 h, 72 h) on the survival rate of gastric cancer cells, the survival rate of five MSNs samples loaded with doxorubicin hydrochloride at different concentrations was calculated by cell survival. Multiple tests were conducted and the survival rate was calculated as the average value for analysis. The error of multiple tests was small and the results were shown in fig. 6.
As shown in fig. 6, when the concentration of MSNs loaded with doxorubicin hydrochloride increased from 0.01 mg/ml to 0.09 mg/ml, the inhibitory effect on gastric cancer cells was concentration-dependent and the higher the concentration, the lower the survival rate of gastric cancer cells, indicating that nano-controlled release anticancer drugs can inhibit gastric cancer cells, which can be used as a basis for treatment of gastric cancer.
In this paper, the in vitro performance of nano-controlled release anticancer drugs in gastric cancer cells was studied. Taking silicon dioxide nanoparticles (MSNs) as an example, MSNs samples loaded with doxorubicin hydrochloride at different concentrations were prepared for relevant experiments. The experimental results showed that: the drug release performance of the sample was tested and the results showed that the MSNs sample prepared could promote the drug release in the slightly acidic environment of the tumor site with the gradual increase of acidity of the buffer; the adsorptiondesorption test results and load characterization results were observed through the relevant test of drug load on the samples. It was known that the average mesopores diameter of MSNs samples was about 3 nm, so the loading of doxorubicin hydrochloride was successfully completed; by calculating the drug loading capacity and encapsulation rate of MSNs samples, the results showed that MSNs samples had a certain sustained release effect because of their large drug loading capacity and high encapsulation rate; by monitoring gastric cancer cell survival, judge when MSNs sample concentration is higher, the survival rate of gastric cancer cells is lower, this is because the MSNs having long cycle, can improve the stability of the drug in the body, take the drugs through the high permeability often Direct- Current (DC) effect to the body more tumor site, thus effectively inhibit the growth of gastric cancer cells. The next step is to use the nanodelivery system in vivo for other cancers.
Acknowledgements:
This work was supported by the Natural Science Fund for Colleges and Universities in Jiangsu Province; Study on gene-modified mesenchymal stem cells combined with fibroin/hydroxyapatite bone materials, (No. 19KJB310017).
Conflict of interests:
The authors declared no conflict of interest.
References
- Yao X, Liu H, Xu Y, Chen Y, Tian W. Targeted and controlled release of HNTs coupling SPOP antibody loaded anticancer drugs. J Funct Mater 2018:49(1):1121-6.
- Xiang-Zi L, Ping-Jing H, Zhen-Duo Z, Guo-Xing Z, Xiao-Ping S, Min W, et al. Release mechanisms and properties of pH-responsive drug nanocarriers. Chinese J Inorg Chem 2018;34(8):1399-412.
- He L, Ji Y, Zhou YZ, Lu SJ, Ding BY, Zhang J. Research on the mechanism and influencing factors of cationic nanocarriers in the delivery of antitumor drugs. China Pharm 2019;30(13):1859-63.
- Dai LL, Liu JJ, Zhou J, Luo Z, Cai KY. Construction of responsive nanodrug delivery system and research on tumor therapy. J Med Biomech 2019;34(S1):27-8.
- Geng J, Liu YM, Luo HY. Isolation and characterization of side population cells from gastric cancer BGC-823 cells. Chin J Clin Pharmacol 2019;35(9):873-6.
- Xin L, Yang WF, Zhang HT, Li YF. Preparation of folic acid/chitosan-Prdx6 shRNA nanoparticles and its anti-carcinoma effect on gastric cancer cell proliferation. China Biotechnol 2017;37(1):7-13.
- Luo X, Peng X, Chen CG, Hou YJ, WU YS, Wang YL. Using folic acid-modified polyethylenimine-SPIO nanoparticles for PD-L1 siRNA delivery to target gastric cancer. Chin J Pathophysiol 2017;33(12):2179-87.
- Zhang YH, Zhang Q, Zhang AM, Pan SJ, Hong L, He JH. Multifunctional magnetic nanocapsules for enhanced targeted magnetic resonance imaging and photodynamic therapy in vivo. J Pract Med 2019;35(24):3752-9.
[Crossref] [Google Scholar] [PubMed]
- Zeng CL. Expression of stem cell markers CD133, CD44v6, PSCA and Oct-4 in gastric carcinoma and their clinical significance. Chin J Cell Stem Cell 2017;7(6):334-8.
- Li W, Zhao YF, Cao YY, Hu PJ, Li XZ. Fabrication and properties of novel nanorods drug carriers. Chem Ind Eng Prog 2017;36(9):3436-46.
[Crossref]
- Feng SN, Zhang HJ, Gao XD, Nakanishi HD. Erythrocyte membrane biomimetic boron nitride nanospheres as carrier for the delivery of antitumor drug. J Food Sci Biotechnol 2019;38(9):72-7.
- Duan CQ, Li SZ, Wang XR, Lu T. Nano-carrier nuclear targeted delivery of anti-tumor drugs: Research advances. J Int Pharm Res 2018;45(12):899-904.
- Gao ZW, Zheng J, Lou JS, Song DS, Yao L, Ding TL, et al. Analysis of clinical efficacy and prognosis of DC-CIK cellular immunotherapy in with gastric cancer. Chin J Cancer Biother 2017;24(2):174-9.
- Han XD, Jiang XJ, Guo LJ. Study on the relationship and mechanism between Helicobacter pylori and multidrug resistance in gastric cancer cells. Diet Health 2019;6(37):6-7.
- Li ZZ, Wang ZJ, Zhao LL, Zheng ZL, Zhang RP. Preparation and in vitro drug release properties of 5-fluorouracil/gelatin nanocomposite. Fine Chem 2018;35(6):1037-40.