- *Corresponding Author:
- Ling Zheng
Department of Pharmacy,
Zhuji People’s Hospital, Zhuji,
Zhejiang Province 311800,
China
E-mail: chenchaolintcm@126.com
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3) Spl Issue “267-276” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To figure out the pathway by which epigallocatechin gallate restrains angiogenesis and glycolysis in colorectal cancer through the circular ribonucleic acid (actin gamma 2)/microRNA-370-5p axis. Detection of circular actin gamma 2 and microRNA-370-5p in colorectal cancer patient tissues and cells and vascular endothelial growth factor A in colorectal cancer cells was conducted. Cell viability and proliferation, angiogenesis, glucose and lactate of colorectal cancer cells were examined. Circular actin gamma 2 and microRNA-370-5p's combination link was verified. Epigallocatechin gallate restrained cell viability, angiogenesis and glycolysis in colorectal cancer cells. In tissues and cells of colorectal cancer patients, circular actin gamma 2 was elevated but microRNA-370-5p was reduced. Elevation of circular actin gamma 2 or repression of microRNA-370-5p motivated vascular endothelial growth factor A, cell proliferation, angiogenesis and glycolysis in colorectal cancer cells. Meanwhile, it was demonstrated that circular actin gamma 2 competitively combined with microRNA-370-5p. MicroRNA-370-5p inhibitor turned around the repressive effect of epigallocatechin gallate and knockdown of circular actin gamma 2 on colorectal cancer progression. Circular actin gamma 2 competitively combines with microRNA-370-5p to control colorectal cancer progression and epigallocatechin gallate restrains colorectal cancer angiogenesis and glycolysis through the circular actin gamma 2/microRNA-370-5p pathway.
Keywords
Epigallocatechin gallate, colorectal cancer, circular actin gamma 2, microRNA-370-5p, angiogenesis, glycolysis
Colorectal Cancer (CRC) is an extremely familiar malignant tumor worldwide. With its elevated morbidity and mortality, it creates a huge threat to human health and life[1]. Surgery and chemotherapy are still the major approaches of cancer cure nowadays. However, CRC patients’ prognosis is still unpleasing, nearly 1/3rd of patients relapse after surgery and the survival rate is also worrying[2]. Current chemotherapeutic drugs for CRC’s cure cover 5-Fluorouracil (5-FU)[3], capecitabine[4], irinotecan (Camptosar)[5] and oxaliplatin[6], etc., although these drugs are very effective in CRC’s cure, they are accompanied by plentiful side effects that cannot be ignored, because they will indiscriminately attack cells in the body and numerous normal cells will be killed, so patients feel very pain during the cure process[7]. Therefore, it is imperative to develop novel CRC therapeutic drugs with fewer side effects.
Epigallocatechin-3-Gallate (EGCG), an active compound of green tea with anticancer efficacy, has been clarified to refrain tumor growth and enhance drug sensitivity in diversified cancers[8]. For example, in Breast Cancer (BC), EGCG refrains cell proliferation and differentiation, induces apoptosis and targets angiogenesis by depressing Vascular Endothelial Growth Factor (VEGF) transcription[9]. In bladder cancer, EGCG enhances the sensitivity of bladder cancer cells to the chemotherapeutic drug doxorubicin[10]. Meanwhile, EGCG enhances the sensitivity of CRC cells to the cancer chemotherapeutic drug 5-FU by restraining the Glucose Regulated Protein 78 (GRP78)/Nuclear Factor Kappa B (NF-κB)/micro Ribonucleic Acid (miR)-155-5p/Multidrug Resistance Protein 1 (MDR1) pathway[11]. EGCG can also restrain CRC cell proliferation[12]. However, it is unknown whether EGCG affects CRC angiogenesis and glycolytic processes. Circular RNA (circRNA) is a covalently closed continuous non-coding RNA without 5' cap or 3' poly (a) tail vs. linear RNA, circRNA is more stable and more resistant to Ribonuclease (RNase)[13]. Recently, more and more studies have clarified deregulated circRNA takes on a crucial role in diversified cancers including CRC[14]. For example, circular Protein Tyrosine Kinase 2 (circPTK2) is elevated in CRC tissues and elevation of circPTK2 motivates tumor growth and metastasis of CRC[15]. CircDDX17 is clearly reduced in CRC tissues and performs as a CRC tumor suppressor[16]. Circular Actin Gamma 2 (circACTG2) is a newly discovered circRNA that has been testified to be differentially expressed in CRC[17]. However, its function in CRC and the latent mechanisms remain unclear. The purpose of this study was to figure out EGCG's functions in CRC.
Materials and Methods
Sample collection:
Thirty-four pairs of human CRC tissues and matched healthy colorectal tissues were gathered in Traditional Chinese Medical Hospital of Zhuji. The experiment was authorized and permitted by the Ethics Committee of Traditional Chinese Medical Hospital of Zhuji. The clinical staging of each liver cancer was classified in the light of criteria developed by the International Union for Cancer Control (UICC), and cancer and para-cancerous tissue samples were tested by a trained pathologist. All patients included in the research offered signed informed consent for the use of their clinical specimens in this study. Tissues were stored at -80° until use.
Cell screening and culture:
The normal colonic epithelial cell line Fetal Human Cells (FHC), four CRC cell lines, HT29, HCT116, SW480 and SW620 were gained from American Type Culture Collection (ATCC) (Manassas, Virginia, United States of America (USA)). Dulbecco’s modified eagle medium replenished with 10 % Fetal Bovine Serum (FBS) was employed as the cell culture medium.
EGCG (purity ≥98 %) was bought from sigma (St. Louis, MO, USA), dissolved in distilled water at a concentration of 100 mmol/l and stored as a stock solution at -80°. Seeding of SW620 cells cultured to the logarithmic growth phase in 6-well plates at 1×105 cells per well, serum starving[18] and then treatment with different concentrations of EGCG (control, 50 μM, 100 μM) were implemented.
Cell transfection:
Seeding of untreated or EGCG-treated SW620 cells was into 6-well plates (2×105 cells/well) and incubation was conducted. Obeying the plan provided by the manufacturer, Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was employed to transfect 50 nM miR-370-5p mimic/inhibitor, mimic/inhibitor Negative Control (NC), circACTG2 short hairpin RNA for knockdown circACTG2 (sh-circACTG2), circACTG2 elevation plasmid (oe-circACTG2) for elevating circACTG2, and the corresponding sh/oe-NC (all GeneChem, Shanghai, China). Cells were replaced with complete medium after transfection. MiR-370-5p mimic/inhibitor and mimic/inhibitor-NC were constructed by Sangon Biotech Co., Ltd.
RNA extraction and real-time quantitative Polymerase Chain Reaction (PCR):
Extraction of total RNA from SW620 cells applying TRIzol (Invitrogen) was conducted and then circACTG2 was analyzed employing the high capacity complementary Deoxyribonucleic Acid (cDNA) reverse transcription kit (Applied Biosystems, Carlsbad, CA, USA). Reverse transcription of circACTG2 into cDNA by random hexamers; miR-370-5p was analyzed by TaqMan miRNA (Applied Biosystems), and reverse transcription of miR-370-5p into cDNA by oligonucleotide (dT) 18 primer was implemented. Reverse Transcription quantitative PCR (RT-qPCR) was performed applying SYBR Green qPCR Mix (Invitrogen) and linked primers (GenePharma, Shanghai, China). Normalization of the relative expression of circACTG2 was to Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) and the expression of miR-370-5p was normalized to the internal reference gene U6. The PCR was conducted. The dissolution program was designed. Calculation of the fold change in gene expression was by comparing the threshold cycle (Ct) method using equation 2-ΔΔCt. The primer sequence was manifested in Table 1[19].
| Name | Primer sequence (5'-3') |
|---|---|
| miR-370-5p | F: ACACTCCAGCTGGGCAGGTCACGTCTCTGC; |
| R: CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGTAACTGC | |
| U6 snRNA | F: CTCGCTTCGGCAGCACA; |
| R: AACGCTTCACGAATTTGCGT | |
| GAPDH | F: GACAGTCAGCCGCATCTTCT; |
| R: GCGCCCAATACGACCAAATC | |
| circACTG2 | F: ACCTCACGGACTACCTCATG; |
| R: CCAGAGCCATTGTCACACAC |
Table 1: Primer Design
Determination of cell viability and cell proliferation by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl-2H-Tetrazolium Bromide (MTT) assay:
The viability of SW620 cells treated with EGCG at 0, 24, 48, and 72 h or the proliferation of SW620 cells at 0, 24, 48 and 72 h after transfection was tested via MTT assay. Cells were cultured in 96-well plates (5×104 cells/well). After cell adherence, 10 μl MTT was replenished to the fresh medium to replace the old medium.
The medium was discarded after incubation with MTT-covering medium and then Dimethyl Sulfoxide (DMSO) (10a0 μl) was supplemented to each well. After that, measurement of absorbance was conducted at 490 nm. The repression rate was calculated as:
(Optical Density (OD) of the control group-OD of experimental group/OD of control group)×100 %
Human Umbilical Vein Endothelial Cell (HUVEC) tubule formation assay:
To examine angiogenic capacity, HUVECs (ATCC) were seeded at 2×104 cells per well in 96-well plates covered with Matrigel (Corning). Then, suspended SW620 cells were supplemented into the wells and culture was conducted. Capillaries were quantified under a 100× microscope, with measuring the total length of intact tubule structures. Experiments were repeated 3 times per treatment.
Western Blot (WB) detection of protein levels:
Extraction of total protein from transfected SW620 cells was conducted applying radio-immunoprecipitation assay lysis buffer covering protease inhibitor mixture. Quantification of proteins was by bicinchoninic acid protein detection kit (Pierce, KeyGEN BioTECH, Jiangsu, China), then separation by Sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferring to polyvinylidene fluoride membrane (Millipore, Darmstadt, Germany) were implemented. Blocking membranes with 5 % nonfat milk and incubation with primary antibodies anti-VEGFA (ab1316) and anti-GAPDH (ab8245, Abcam), and horseradish peroxidase-conjugated secondary antibodies (1:10 000; Cell Signaling Technology (CST), Danvers, MA, USA) were implemented. Visualization of signals was implemented applying enhanced chemiluminescence substrate (Millipore, USA) for normalization with endogenous GAPDH.
Glucose uptake and lactate production assay:
In strict accordance with the instructions, detection of the cellular glucose and lactate was executed applying a glucose assay kit (Abcam, Cambridge, United Kingdom) and a lactate assay kit (Abcam), respectively. EGCG-treated or transfected SW620 cells were cultivated in 6-well plates (3×104 cells/well). Then, the supernatant was harvested. Glucose uptake and lactate production were analyzed by testing absorbance at 450 nm or 570 nm applying a microplate reader (Thermo Fisher). Results were clarified as repression percent vs. untreated controls (SW620 cells).
The luciferase activity assay:
The latent binding sites of circACTG2 and miR-370-5p were forecast applying the biological prediction website http://starbase.sysu.edu.cn/. The Wild-Type (WT) and Mutant (MUT) plasmids of circACTG2 were constructed by Geneseed Co., Ltd. (Guangzhou, China) and named circACTG2-WT/MUT, respectively. Co-transfection of the constructed plasmids was into SW620 cells with miR-370-5p mimic or NC and TurboFect™ reagent (Thermo Fisher, Waltham, MA, USA) in the light of the manufacturer's instructions. Then test of the luciferase activity was by Dual-Lucy Assay Kit (Solarbio, Beijing, China).
Statistical analysis:
Data were analyzed and plotted by GraphPad Prism 7 (GraphPad, La Jolla, CA, USA). Results are clarified as mean±standard deviation. All differences between two independent groups were assessed by one-way analysis of variance. p<0.05 emphasized obvious statistical meaning.
Results and Discussion
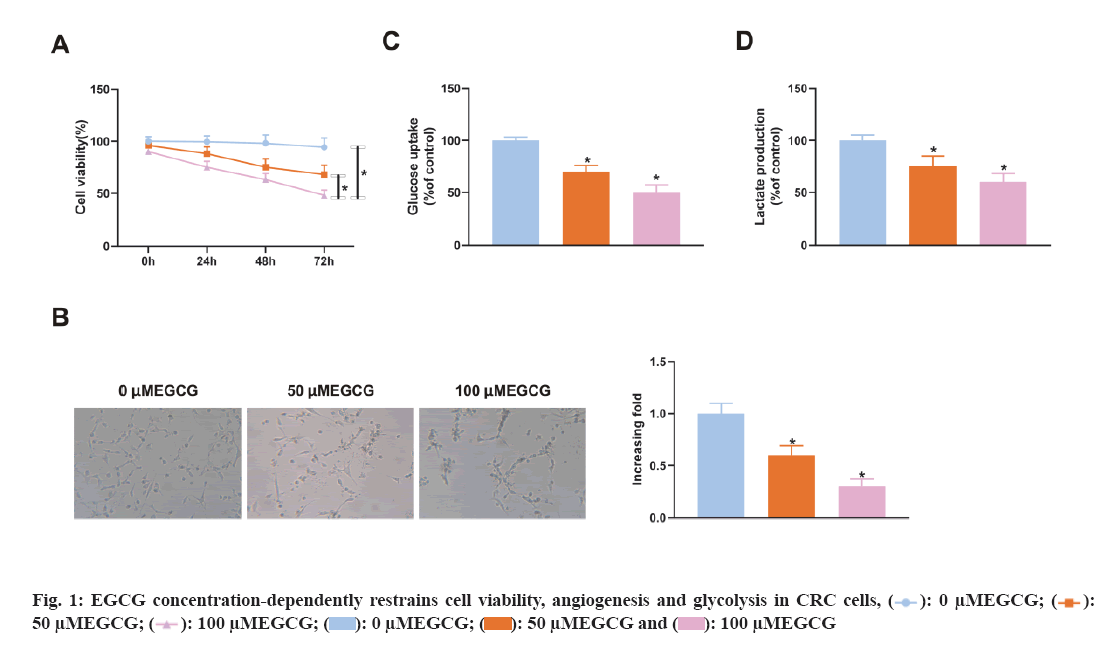
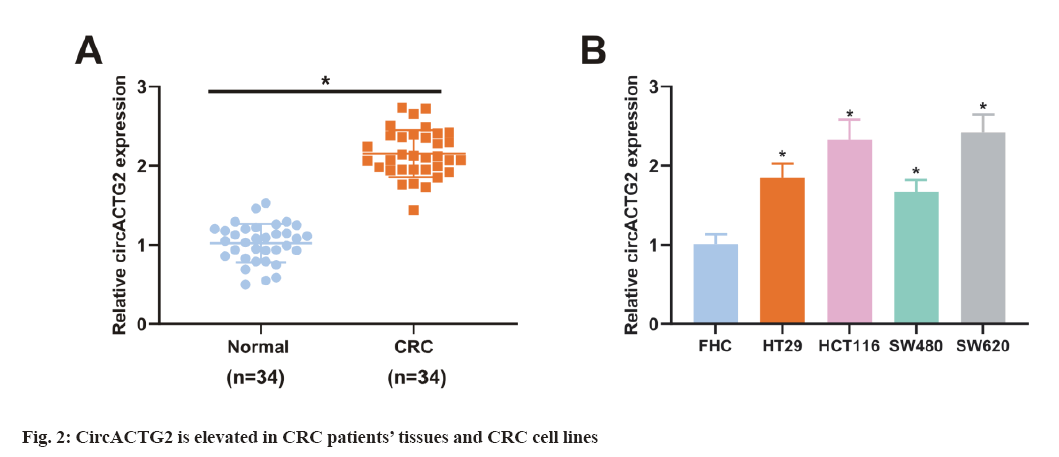
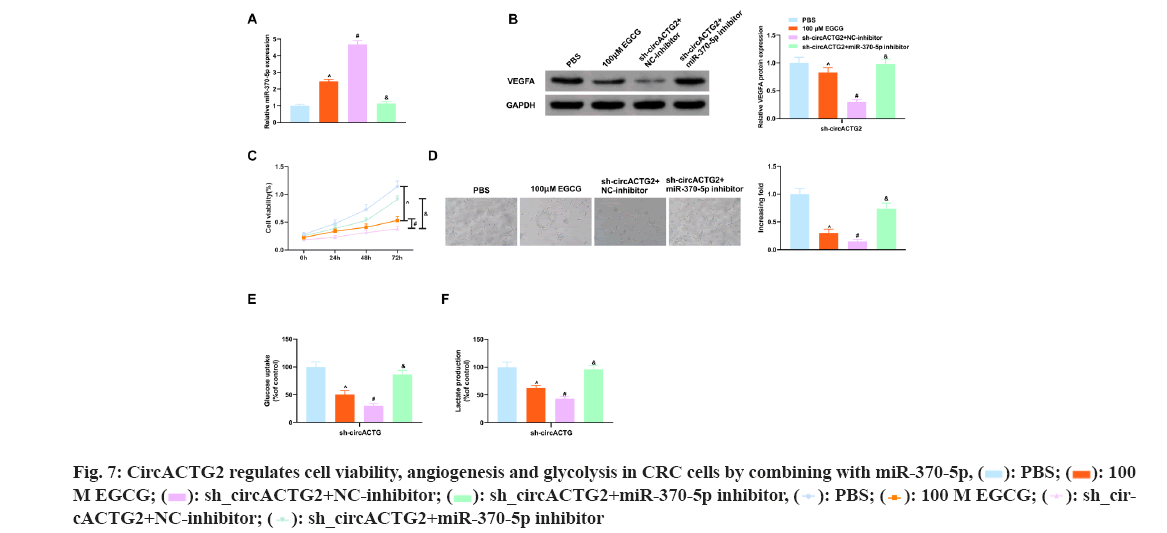
To figure out EGCG's functions in CRC tissues, SW620 cells were introduced with different concentrations of EGCG (control, 50 μM, 100 μM). As illustrated in fig. 1A, EGCG concentration-dependently declined SW620 cell viability. Subsequently, it was performed the HUVEC tubule formation assay, co-cultured EGCG-treated SW620 cells with HUVEC cells and tested the angiogenesis ability of HUVEC cells. As clarified in fig. 1B, SW620 cells after EGCG treatment clearly restrained the angiogenesis of HUVEC cells. Finally, it was further examined EGCG's impact on glucose uptake and lactate production in SW620 cells. As illustrated in fig. 1C-fig. 1D, EGCG concentration-dependently reduced glucose uptake and lactate production in SW620 cells. The above results exhibited EGCG effectively restrained cell viability, angiogenesis and glycolysis of CRC cells. To figure out the expression difference of circACTG2 in CRC patient tissues and healthy issues, circACTG2 was tested in 34 pairs of human CRC tissues and matched healthy colorectal tissues. It came out circACTG2 was clearly elevated in CRC patient tissues vs. healthy colorectal tissues (p<0.05) (fig. 2A). CircACTG2 was also clearly elevated in four CRC cell lines, HT29, HCT116, SW480 and SW620, vs. the normal colon epithelial cell line FHC (p<0.05) (fig. 2B).
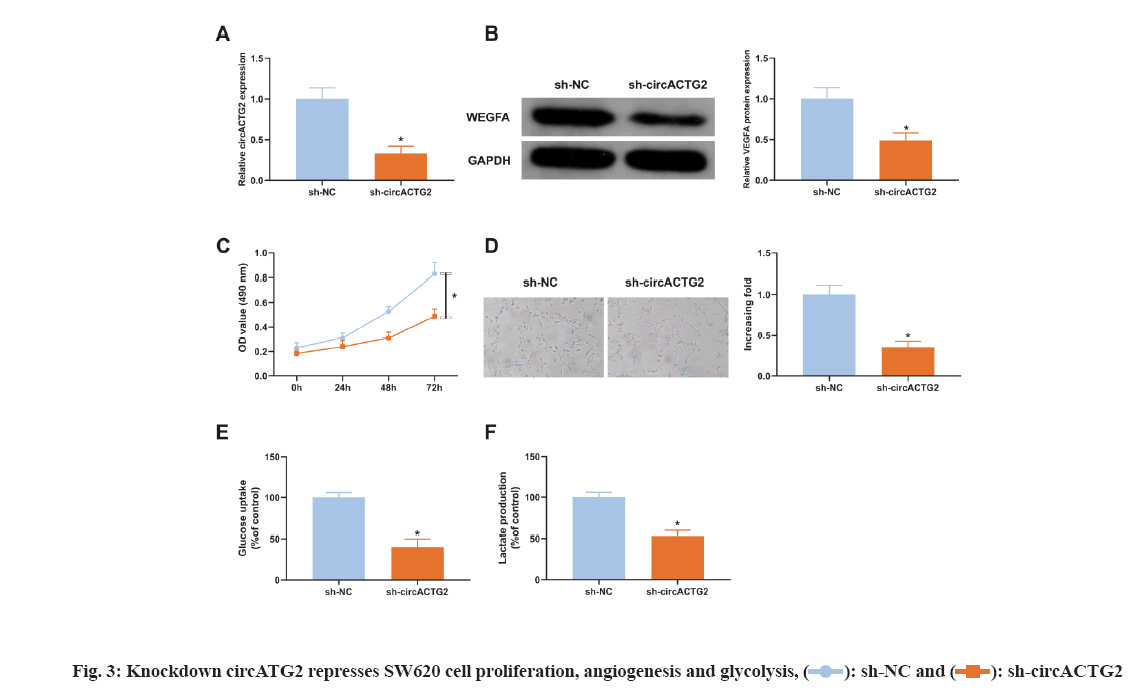
Considering the elevation of circACTG2 in CRC tissues, it was constructed sh-circACTG2 to knock down circACTG2 and then transfected with the corresponding NC (sh-NC) into SW620 cells respectively for research. It turned out that SW620 cells transfected with sh-NC, circACTG2 in SW620 cells transfected with sh-circACTG2 was clearly reduced (p<0.05) (fig. 3A). Meanwhile, VEGFA in SW620 cells transfected with sh-circACTG2 was memorably declined (p<0.05) (fig. 3B). Subsequently, it was detected SW620 cells’ proliferation and found that knockdown circACTG2 refrained SW620 cells’ proliferation (fig. 3C). Then, HUVEC tubule formation test was conducted. SW620 cells transfected with sh-NC/sh-CirCACTG2 were cultured with HUVEC cells. The results clarified the number of angiogenesis of HUVEC cells cultured with SW620 cells transfected with sh-circACTG2 was reduced (fig. 3D). Finally, the results of glucose uptake and lactate production assay illustrated knockdown circACTG2 refrained glucose uptake and lactate production in SW620 cells (fig. 3E-fig. 3F). These results indicated knockdown circACTG2 refrained cell proliferation, angiogenesis and glycolysis in CRC cells.
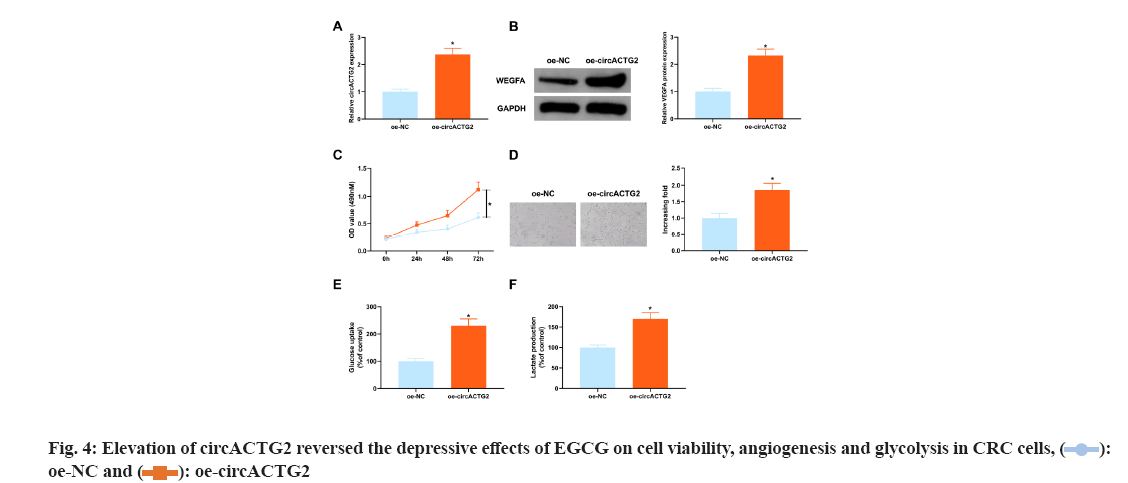
The above experimental results clarified the depressive effect of 100 μM concentration of EGCG on SW620 cells was the clearest, so it was selected for subsequent experiments. In view of the depressive effect of knockdown circACTG2 on CRC progression, circACTG2-elevating plasmid (oe-circACTG2) and its NC (oe-NC) were introduced into SW620 cells treated with EGCG at a concentration of 100 μM to further figure out the effect of circACTG2 on EGCG in CRC progression. It came out elevation of circACTG2 turned around the depressive effect of EGCG on circACTG2 and circACTG2 in SW620 cells was clearly elevated (p<0.05) (fig. 4A). Meanwhile, enhancement of circACTG2 clearly motivated VEGFA (p<0.05) (fig. 4B). Moreover, elevation of circACTG2 accelerated CRC cell proliferation (fig. 4C). Next, it was conducted HUVEC tubule formation test, oe-NC/oe-circATG2-transfected and EGCG-treated SW620 cells were cultured with HUVEC cells, and the results conveyed, the number of angiogenesis of HUVEC cells cultured with SW620 cells transfected with oe-circACTG2 was clearly elevated (p<0.05) (fig. 4D). It was further figured out the effect of circACTG2 on glucose uptake and lactate production in EGCG-treated CRC cells, and discovered that elevation of circACTG2 turned around the effect of EGCG and enhanced glucose uptake and lactate production (fig. 4E-fig. 4F). All in all, elevation of circACTG2 attenuated EGCG's impacts on cell viability, angiogenesis and glycolysis in CRC cells.
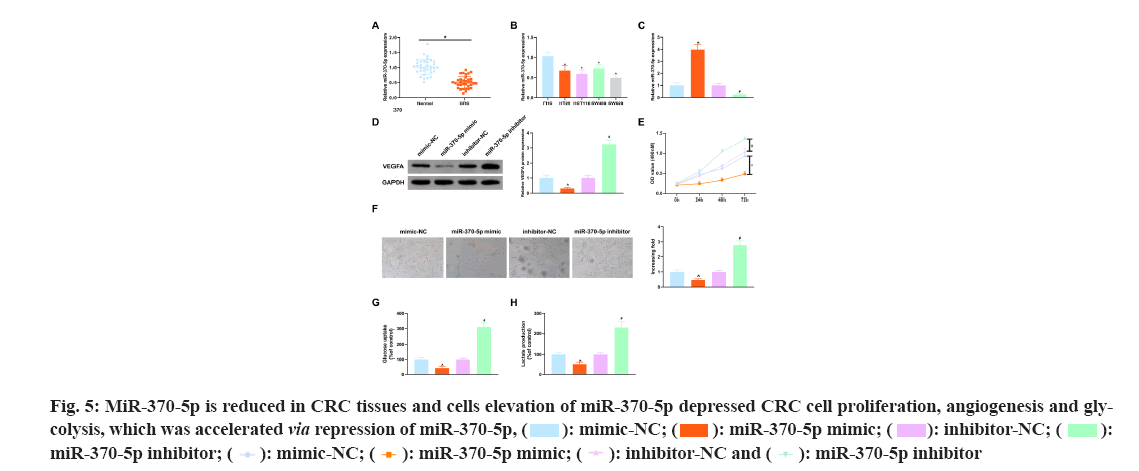
To figure out whether miR-370-5p is differentially expressed in CRC tissues and healthy tissues, miR-370-5p was tested in 34 pairs of human CRC tissues and matched healthy colorectal tissues, and it turned out, miR-370-5p was reduced in CRC patient tissues vs. healthy colorectal tissues (p<0.05) (fig. 5A). MiR-370-5p in four CRC cell lines HT29, HCT116, SW480 and SW620 was also memorably declined (p<0.05) vs. the normal colon epithelial cell line FHC (p<0.05) (fig. 5B).
To further figure out miR-370-5p's functions in CRC tissues, miR-370-5p mimic and miR-370-5p inhibitor and corresponding NCs (mimic-NC and inhibitor-NC) were introduced, respectively into SW620 cells not treated with EGCG. MiR-370-5p was tested in SW620 cells after transfection, as clarified in fig. 5C, miR-370-5p in SW620 cells transfected with miR-370-5p mimic was clearly elevated vs. the mimic- NC (p<0.05), and vs. the inhibitor-NC, miR-370-5p in SW620 cells after transfection with miR-370-5p inhibitor was clearly reduced (p<0.05). It came out elevation of miR-370-5p restrained VEGFA (fig. 5D). Then the proliferation of SW620 cells after transfection was tested, and the results clarified elevation of miR-370-5p clearly restrained the proliferation of SW620 cells (fig. 5E). Next, the SW620 cells transfected with the above substances were cultured with HUVEC cells and as illustrated in fig. 5F, elevation of miR-370-5p refrained HUVEC cell angiogenesis, but repression of miR-370-5p accelerated that. Finally, analysis of the effects of the above substances on glucose uptake and lactate production in SW620 cells and as clarified in fig. 5G-fig. 5H, elevation of miR-370-5p clearly refrained glucose uptake and lactate production in SW620 cells. All in all, elevation of miR-370-5p refrained CRC cell proliferation, angiogenesis and glycolysis.
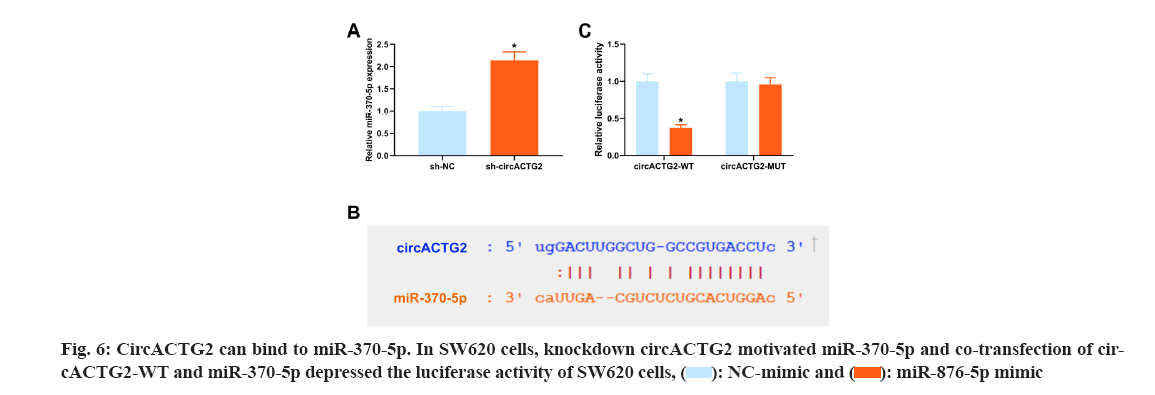
CircRNAs usually competitively adsorb downstream miRNAs and mediate target protein to influence disease progression[20]. To reveal the regulatory mechanism of EGCG in the CRC process, it was co-transfected circACTG2-WT/circACTG2-MUT with miR-370-5p mimics into EGCG-treated SW620 cells. Next, the miRNAs bound by circACTG2 were figured out. A former study clarifies targeting RASAL2 by miR-370-5p in CRC restrains Yes-Associated Protein (YAP) signaling to depress cell proliferation and invasion in CRC[21]. In the research, it was discovered that knockdown circACTG2 elevated miR-370-5p (fig. 6A). Hence, it was speculated that miR-370-5p was the downstream miRNA of circACTG2. The binding site of circACTG2 and miR-370-5p was discovered through the biological prediction website http://starbase.sysu.edu.cn/ (fig. 6B). To further verify the link between circACTG2 and miR-370-5p, it was conducted the luciferase activity assay, and it turned out the luciferase activity of SW620 cells co-transfected with circACTG2-WT and miR-370-5p mimics was clearly reduced. However, luciferase activity of SW620 cells co-transfected with circACTG2-MUT and NC-mimic did not change memorably (fig. 6C).
The above experimental results have shown EGCG restrained cell viability, angiogenesis and glycolysis in SW620 cells, while knockdown circACTG2 further restrained CRC progression, whereas repressing miR-370-5p did the opposite. So EGCG-treated SW620 cells were co-transfected with sh-circACTG2 and miR-370-5p inhibitor. As shown in the fig. 7 below, miR-370-5p inhibitor reversed the negative effects caused by EGCG and knockdown of circACTG2, and restored cell viability, angiogenesis, and glycolysis in SW620 cells. In conclusion, EGCG refrained CRC angiogenesis and glycolysis through the circACTG2/miR-370-5p pathway.
CRC remains an extremely familiar cancer, and since most patients are diagnosed late and the tumor has spread to distant sites, the mortality and recurrence rate of CRC remains high[22]. Therefore, there is an urgent need to develop new treatments for CRC. EGCG is the most biologically active polyphenol in green tea and takes on multiple roles in cancer prevention and treatment[10]. A previous study clarifies EGCG induces CRC cell apoptosis by inducing endoplasmic reticulum stress through the Protein Kinase RNA-Like Endoplasmic Reticulum Kinase (PERK)/phosphorylated eukaryotic translation Initiation Factor 2 Alpha (p-eIF2α)/Activating Transcription Factor 4 (ATF4) and Inositol-Requiring Enzyme 1 α (IRE1α) pathways[23]. However, the latent molecular mechanism by which EGCG refrains CRC has not been fully elucidated. In the present study, it was found that EGCG concentration-dependently refrained CRC cell viability and angiogenesis, and reduced cellular glucose uptake and lactate production. This effect is performed by targeting the circACTG2/miR-370-5p pathway.
Previous studies have shown EGCG can enhance chemotherapy-induced cytotoxicity by targeting the Extracellular-Signal-Regulated Kinase (ERK) pathway in diversified cancer cell lines[24] and can participate in the cancer cell cycle development by activating/inhibiting several signaling pathways or regulating non-coding RNAs[25]. For example, EGCG depresses the growth and tumorigenicity of BC cells by down regulating miR-25[26]. EGCG represses Epidermal Growth Factor Receptor (EGFR)/Insulin-like Growth Factor Receptor 1 (IGFR1) activation through Mitogen-Activated Protein Kinase (MEK)/ERK1/2 and Phosphatidylinositol 3-Kinase (PI3K)/Protein Kinase B (AKT) pathways to control CRC cell proliferation and apoptosis[27]. In this study, to further reveal the mechanism by which EGCG repressed CRC progression, CRC cells were treated with EGCG at 0 μM, 50 μM, and 100 μM, and it turned out EGCG concentration-dependently refrained CRC cell viability in vitro, which is consistent with those of previous studies[27].
Furthermore, it was found that EGCG clearly repressed cellular angiogenesis and glycolysis. Although the study demonstrated EGCG could effectively inhibit CRC cell viability in vitro, in vivo animal experiments were not conducted to further verify the effect of EGCG on CRC tumor growth. A former study clarifies although EGCG was effective against CRC cells in vitro and did not affect the growth of normal colon epithelial cells, the in vivo effect of EGCG was much lower than in vitro, and ECGC itself was very sensitive, and had poor stability, and its stereoscopic conformation will change with the alternation of concentration, which is the biggest problem to be overcome in the use of EGCG in the cure of CRC in vivo, so new strategies need to be studied to improve the stability of ECGC[9]. Interestingly, in the present study, it was found that EGCG treatment impacted circACTG2, which is the first observation of the effect of EGCG on circRNA in CRC.
Elevated studies have shown circRNA plays a momentous role in the occurrence and progression of CRC[28]. A previous study clarifies circACTG2 is deregulated in CRC and may be involved in CRC progression[17]. However, its function in CRC is unclear. In the present study, it was found that circACTG2 was clearly up regulated in CRC patient tissues and cells, which suggest that circACTG2, could serve as a novel biomarker for CRC. Next, it was explored the role of circACTG2 in CRC. It was found that repression of circACTG2 clearly repressed CRC cell proliferation, declined VEGFA, and angiogenesis, glucose uptake, and lactate production. Furthermore, it was found that elevation of circACTG2 reversed the effects of EGCG and motivated CRC cell proliferation, VEGFA, angiogenesis and glycolysis. These results further confirmed circACTG2 is a downstream molecule of EGCG in CRC. Recent studies have found circRNAs are usually involved in regulating the function of downstream genes by adsorbing microRNAs[29]. For example, circZFR controls CRC cell proliferation and migration through the miR-532-3p/Forkhead Box O4 (FOXO4) axis[30]. Therefore, the downstream targets of circACTG2 were further explored. The negative regulation of miR-370-5p by circACTG2 was confirmed by bioinformatics analysis and the luciferase activity assay.
MiR-370-5p is a newly identified miRNA that has been shown to take on an important role in a variety of cancers, including ovarian cancer[31], BC[32] and hepatocellular carcinoma[33], etc. In addition, Zhang[34] et al. report miR-370-5p is down regulated in CRC tissues and cell lines, and elevation of miR-370-5p represses CRC cell proliferation and invasion by regulating Enhancer of Zeste 1 Polycomb Repressive Complex 2 Subunit (EZH1). In this study, it was also found that miR-370-5p was reduced in CRC patient tissues and cells, elevation of miR-370-5p depressed CRC cell proliferation, angiogenesis and glycolysis, while reduction of miR-370-5p clearly turned around the inhibitory effects of EGCG and circACTG2 on CRC cell proliferation, angiogenesis and glycolysis. These results suggest that EGCG depresses CRC malignant progression by targeting the circACTG2/miR-370-5p axis. It is hoped that the target of miR-370-5p can be further explored in future studies, which will further explain the molecular mechanism of EGCG action.
In conclusion, the study demonstrates EGCG concentration-dependently depresses CRC progression and its anticancer effect was achieved by targeting the circACTG2/miR-370-5p axis to depress CRC cell angiogenesis and glycolysis. The results of this study further confirm the role of EGCG in the treatment of CRC, providing a theoretical basis for EGCG as a new anticancer drug for CRC and a new potential target for CRC treatment.
Authors’ contributions:
Chaolin Chen and Bixia Xuan have contributed equally to this work.
Conflict of interests:
The authors declared
References
- Yin TF, Zhao DY, Zhou YC, Wang QQ, Yao SK. Identification of the circRNA-miRNA-mRNA regulatory network and its prognostic effect in colorectal cancer. World J Clin Cases 2021;9(18):4520-41.
[Crossref] [Google Scholar] [Pub Med]
- Fan X, Wang Y, Jiang T, Liu T, Jin Y, Du K, et al. B-Myb accelerates colorectal cancer progression through reciprocal feed-forward transactivation of E2F2. Oncogene 2021;40(37):5613-25.
[Crossref] [Google Scholar] [Pub Med]
- Nikravesh H, Khodayar MJ, Behmanesh B, Mahdavinia M, Teimoori A, Alboghobeish S, et al. The combined effect of dichloroacetate and 3-bromopyruvate on glucose metabolism in colorectal cancer cell line, HT-29; the mitochondrial pathway apoptosis. BMC Cancer 2021;21(1):903.
- Su J, Dai B, Yuan W, Wang G, Zhang Z, Li Z, et al. The influence of PD-L1 genetic variation on the prognosis of R0 resection colorectal cancer patients received capecitabine-based adjuvant chemotherapy: A long-term follow-up, real-world retrospective study. Cancer Chemother Pharmacol 2020;85(5):969-78.
[Crossref] [Google Scholar] [Pub Med]
- Augustine T, Maitra R, Zhang J, Nayak J, Goel S. Sensitization of colorectal cancer to irinotecan therapy by PARP inhibitor rucaparib. Invest New Drugs 2019;37(5):948-60.
[Crossref] [Google Scholar] [Pub Med]
- Fohlen A, Bordji K, Assenat E, Gongora C, Bazille C, Boulonnais J, et al. Anticancer drugs for intra-arterial treatment of colorectal cancer liver metastases: In vitro screening after short exposure time. Pharmaceuticals 2021;14(7):639.
- Li M, Sun X, Yao H, Chen W, Zhang F, Gao S, et al. Genomic methylation variations predict the susceptibility of six chemotherapy related adverse effects and cancer development for Chinese colorectal cancer patients. Toxicol Appl Pharmacol 2021;427:115657.
[Crossref] [Google Scholar] [Pub Med]
- Almatroodi SA, Almatroudi A, Khan AA, Alhumaydhi FA, Alsahli MA, Rahmani AH. Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea and its role in the therapy of various types of cancer. Molecules 2020;25(14):3146.
[Crossref] [Google Scholar] [Pub Med]
- Romano A, Martel F. The role of EGCG in breast cancer prevention and therapy. Mini Rev Med Chem 2021;21(7):883-98.
[Crossref] [Google Scholar] [Pub Med]
- Luo KW, Zhu XH, Zhao T, Zhong J, Gao HC, Luo XL, et al. EGCG enhanced the anti-tumor effect of doxorubicine in bladder cancer via NF-κB/MDM2/p53 pathway. Front Cell Dev Biol 2020;8:606123.
[Crossref] [Google Scholar] [Pub Med]
- La X, Zhang L, Li Z, Li H, Yang Y. (−)-Epigallocatechin Gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-κB/miR-155-5p/MDR1 pathway. J Agric Food Chem 2019;67(9):2510-8.
[Crossref] [Google Scholar] [Pub Med]
- Zhang Z, Zhang S, Yang J, Yi P, Xu P, Yi M, et al. Integrated transcriptomic and metabolomic analyses to characterize the anti-cancer effects of (−)-epigallocatechin-3-gallate in human colon cancer cells. Toxicol Appl Pharmacol 2020;401:115100.
[Crossref] [Google Scholar] [Pub Med]
- Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, et al. Circular intronic long noncoding RNAs. Mol Cell 2013 Sep 26;51(6):792-806.
[Crossref] [Google Scholar] [Pub Med]
- Fu P, Lin L, Zhou H, Zhao S, Jie Z. Circular RNA circEGFR regulates tumor progression via the miR-106a-5p/DDX5 axis in colorectal cancer. Braz J Med Biol Res 2021;54(8):e10940.
[Crossref] [Google Scholar] [Pub Med]
- Yang H, Li X, Meng Q, Sun H, Wu S, Hu W, et al. CircPTK2 (hsa_circ_0005273) as a novel therapeutic target for metastatic colorectal cancer. Mol Cancer 2020;19(1):13.
[Crossref] [Google Scholar] [Pub Med]
- Li XN, Wang ZJ, Ye CX, Zhao BC, Li ZL, Yang Y. RNA sequencing reveals the expression profiles of circRNA and indicates that circDDX17 acts as a tumor suppressor in colorectal cancer. J Exp Clin Cancer Res 2018;37(1):325.
[Crossref] [Google Scholar] [Pub Med]
- Bhuyan R, Bagchi A. Prediction of the differentially expressed circRNAs to decipher their roles in the onset of human colorectal cancers. Gene 2020;762:145035.
[Crossref] [Gpoogle Scholar] [Pub Med]
- Sakamoto Y, Terashita N, Muraguchi T, Fukusato T, Kubota S. Effects of epigallocatechin-3-gallate (EGCG) on A549 lung cancer tumor growth and angiogenesis. Biosci Biotechnol Biochem 2013;77(9):1799-803.
[Crossref] [Google Scholar] [Pub Med]
- Zhao H, Zheng Y, You J, Xiong J, Ying S, Xie L, et al. Tumor suppressor role of miR?876?5p in gastric cancer. Oncol Lett 2020;20(2):1281-7.
[Crossref] [Google Scholar] [Pub Med]
- Yu F, Lin Y, Ai MM, Tan GJ, Huang JL, Zou ZR. Knockdown of circular RNA hsa_circ_PVT1 inhibited laryngeal cancer progression via preventing wnt4/β-catenin signaling pathway activation. Front Cell Dev Biol 2021;9:890.
- Ren L, Zhang Z, Feng Y, Luo M, Hao Z. MicroRNA?876?5p represses the cell proliferation and invasion of colorectal cancer through suppressing YAP signalling via targeting RASAL2. Clin Exp Pharmacol Physiol 2020;47(5):867-76.
[Crossref] [Google Scholar] [Pub Med]
- Wang X, Tao G, Huang D, Liang S, Zheng D. Circular RNA NOX4 promotes the development of colorectal cancer via the microRNA?485?5p/CKS1B axis. Oncol Rep 2020;44(5):2009-20.
[Crossref] [Google Scholar] [Pub Med]
- Md Nesran ZN, Shafie NH, Ishak AH, Mohd Esa N, Ismail A, Md Tohid SF. Induction of endoplasmic reticulum stress pathway by green tea epigallocatechin-3-gallate (EGCG) in colorectal cancer cells: Activation of PERK/p-eIF2α/ATF4 and IRE1α. BioMed Res Int 2019;2019:3480569.
[Crossref] [Google Scholar] [Pub Med]
- Wei R, Wirkus J, Yang Z, Machuca J, Esparza Y, Mackenzie GG. EGCG sensitizes chemotherapeutic-induced cytotoxicity by targeting the ERK pathway in multiple cancer cell lines. Arch Biochem Biophy 2020;692:108546.
[Crossref] [Google Scholar] [Pub Med]
- Yang L, Zhang W, Chopra S, Kaur D, Wang H, Li M, et al. The epigenetic modification of epigallocatechin gallate (EGCG) on cancer. Curr Drug Targets 2020;21(11):1099-104.
[Crossref] [Google Scholar] [Pub Med]
- Zan L, Chen Q, Zhang L, Li X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 2019;10(1):374-82.
[Crossref] [Google Scholar] [Pub Med]
- Zhu W, Li MC, Wang FR, Mackenzie GG, Oteiza PI. The inhibitory effect of ECG and EGCG dimeric procyanidins on colorectal cancer cells growth is associated with their actions at lipid rafts and the inhibition of the epidermal growth factor receptor signaling. Biochem Pharmacol 2020;175:113923.
[Crossref] [Google Scholar] [Pub Med]
- Zeng Y, Xu Y, Shu R, Sun L, Tian Y, Shi C, et al. Altered expression profiles of circular RNA in colorectal cancer tissues from patients with lung metastasis. Int J Mol Med 2017;40(6):1818-28.
[Crossref] [Google Scholar] [Pub Med]
- Liu K, Mou Y, Shi X, Liu T, Chen Z, Zuo X. Circular RNA 100146 promotes colorectal cancer progression by the microRNA 149/HMGA2 axis. Mol Cell Biol 2021;41(2):e00445-20.
[Crossref] [Google Scholar] [Pub Med]
- Bian L, Zhi X, Ma L, Zhang J, Chen P, Sun S, et al. Hsa_circRNA_103809 regulated the cell proliferation and migration in colorectal cancer via miR-532–3p/FOXO4 axis. Biochem Biophys Res Commun 2018;505(2):346-52.
[Crossref] [Google Scholar] [Pub Med]
- Wang K, Zhu G, Bao S, Chen S. Long non-coding RNA LINC00511 mediates the effects of ESR1 on proliferation and invasion of ovarian cancer through miR-424-5p and miR-370-5p. Cancer Manag Res 2019;11:10807-19.
[Crossref] [Google Scholar] [Pub Med]
- Sang K, Yi T, Huang X, Pan C, Zhou J, Yu L. MiR-370-5p inhibits the progression of breast cancer via targeting LUC7L3. J Recept Signal Transduct Res 2020;41(5):442-50.
[Crossref] [Google Scholar] [Pub Med]
- Zhang L, Liu Y, Tao H, Zhu H, Pan Y, Li P, et al. Circular RNA circUBE2J2 acts as the sponge of microRNA-370-5P to suppress hepatocellular carcinoma progression. Cell Death Dis 2021;12(11):1985.
[Crossref] [Google Scholar] [Pub Med]
- Zhang Y, Li L, Lu KX, Yu LB, Meng J, Liu CY. LncRNA SNHG3 is responsible for the deterioration of colorectal carcinoma through regulating the miR-370-5p/EZH1 axis. Eur Rev Med Pharmacol Sci 2021;25(19):6131-7.
[Crossref] [Google Scholar] [Pub Med]




 M EGCG;
M EGCG;