- *Corresponding Author:
- L. Deb
Sri Krupa Institute of Pharmaceutical Science, Velkatta, Kondapak, Medak-502 277, India
E-mail: lokeshdeb.ibsd@nic.in
| Date of Submission | 09 August 2012 |
| Date of Revision | 12 February 2013 |
| Date of Acceptance | 17 February 2013 |
| Indian J Pharm Sci 2013;75(2):156-161 |
Abstract
To rationalize scientifically the traditional claim on use of Wedelia biflora (Linn.) D. C. for the treatment of wounds and infections, the present study was designed to evaluate the antimicrobial and wound healing activity of ethanol extract of leaves of W. biflora. In in vitro assays the test extract was subjected to antimicrobial activity by agar well-diffusion method and minimum inhibitory concentration method in different microbial strains. Wound healing activity of the test extract was studied by excision wound model and incision wound model in Wistar albino rats. In excision wound model, 97.90% wound healing was recorded in 10% w/w extract treated group on 16 th days of postsurgery, whereas only 58.50% was observed in control group. In incision model, higher breaking strength, high hydroxyl proline content and histopathological study in extract treated groups revealed higher collagen redeposition than the control group. The agar well-diffusion evaluation and minimum inhibitory concentration established antimicrobial efficacy of ethanol extracts of W. biflora. These observations established the traditional claim and therapeutic activity of W. biflora and it could be a potent wound healing candidate for use in future.
Keywords
Antimicrobial activity, excision wound, incision wound, Wedelia biflora
Wounds are inevitable incident of life, they often occur as a result of physical injury, chemical injury and microbial infections. Healing of wounds is a complex process in which the skin (or another organ‑tissue) repairs itself after injury, once the protective barrier is broken; the normal (physiologic) process of wound healing is immediately set in motion [1]. Though, the healing process takes place by naturally, an infection may seriously delay this process [2]. However, sometimes the degree of wounds crosses beyond its natural healing capacities, and also there is a chance of microbial infection around the wounded tissues, which requires a number of drugs ranging from simple nonexpensive analgesics to complex and expensive chemotherapeutic agents [3]. In modern biomedical area, development for the management of wound healing is an expensive program for the peoples of developed countries.
Several drugs obtained from natural sources are known to increase the healing and repair process of different types of infected wounds [1]. Some of these natural drugs already been screened scientifically for their therapeutic efficacy to repair wounds in different pharmacological models, but many of the traditionally used herbs and herbal formulations remains unexplored for their usefulness against infections and wounds.
Wedelia biflora (Linn.) D.C. belongs to a family compositae, is an ancient weed found in Eastern and Western sea coasts in India and other Southern Asia. Ethnomedically, the leaves of the plant is used by traditional healers and local peoples of Tamil Nadu, India for dressing of wounds, treatment of ulcers, sore throat, varicose of veins, skin diseases, headache and fever [4]. Roots are used to check vaginal discharge and stomach ache. The flowers are said to be violent purgative [5]. The pounded leaves are used for preparing poultice for treating cuts, ulcer, sore, and varicose veins. A decoction of the roots and leaves are prescribed for stomach ache. The leaves are also credited with diuretic properties [6]. Various reports reveals that this plant contains useful antifungal phytoconstituents such as 3′‑formyl‑2′,4′,6′‑tri hydroxydihydrochalcone [7], veratrylidenehydrazide, 3,3′‑di‑O‑methylquercetin, 2,7‑dihydroxy‑3 (3t′‑ methoxy‑4′‑hydroxy)‑5‑methoxyisoflavone and 3′,7‑di‑O‑methylquercetin [8]. With this background, the present study was undertaken to emphasise the effect of leaves of W. biflora against experimentally induced wounds in Wistar rats and antimicrobial property against a wide range of microorganisms.
Materials and Methods
The leaves of W. biflora were collected from Potheri village, Kancheepuram district, Tamil Nadu, India in the month of December 2009. The plant was authenticated at National Institute of Herbal Science, Plant Anatomy Research Centre (PARC), Tambaram, Chennai, Tamil Nadu, India. A voucher specimen was deposited in the Department museum under specimen number PARC/2009/190/WB.
Ethanol (absolute) and petroleum ether (60‑80°) were procured from Merck Ltd, Mumbai, India. Nutrient agar was procured from Hi‑Media, Mumbai, India. Hard paraffin, cetostearyl alcohol, wool fat and sodium carbonate were procured from SD Fine Chemicals, Mumbai, India.
Animals and microorganisms
Male Wister rats weighing 180‑200 g were obtained from Veterinary Science and Research Institute, Madhavaram, Chennai. The animals were housed in standard individual metal cages and maintained at 22±1° with an alternate 12 h light‑dark cycle. Food and water were provided ad libitum. All the experiments on animals were conducted after obtaining permission from Institutional Animal Ethical Committee of SRM University, Chennai (IAEC/ SRM/51/2009). Bacterial culture (Bacillus subtilis, Staphylococcus aureus, Escherichia coli and Klebsiella pneumoniae) and fungal culture (Candida albicans and Aspergillus niger) were procured from Microbial Type Culture Collection, Chandigarh, India.
Extraction, sample preparation and phytochemical analysis
Around 300 g of fresh leaves of W. biflora was clean; shade dried and reduces into coarse powder in a wearing blender. The powder material was extracted three times with petroleum ether (60‑80º) to remove wax and extracted three times with absolute ethanol (99.5%) as solvent in 1:4 (w/v) ratios at temperature 60±5º in a cycle of 48 h each on soxhlet apparatus. The ethanol extracts were concentrated in Rotavapour (Ratavac, Germany) at reduced pressure below 40º.
Extract ointment was prepared according to Indian Pharmacopoeia. The simple ointment base I.P. containing wool fat (5%), hard paraffin (5%), cetostearyl alcohol (5%), white soft paraffin (80%) and W. biflora extracts (5% and 10% in two different set). These ingredients were mixed and heated with gentle stirring until homogenous ointment is formed.
The preliminary phytochemical screening were carried out for alkaloids (Draggendorff ’s test), flavonoids (Shinoda’s reaction), saponins (Frothing test), tannins (5% alcoholic ferric chloride), terpenoids (2,4‑dinitro phenyl hydrazine), glycosides (Fehling’s test), steroids (Libermann’s Burchard test) and anthraquinone (Borntrager’s test) [9].
Antimicrobial activity by agar well‑diffusion method
Antimicrobial screening test was performed as per earlier report [10] and subject to availability of the organism in the laboratory, by using B. subtilis, S. aureus, E. coli, K. pneumoniae, C. albicans and A. niger microbial strains. In brief, 30 mg of crude ethanol extracts of leaves of W. biflora was dissolved in 1 ml of ethanol and filtered through 0.2 μm nylon membrane filter for further use. Culture media was prepared using 40 g/l nutrient agar and were autoclaved at 121º, 15 psi for 15 min. A volume of 20 ml of agar was transfer on petridish and allowed to solidify. Each petridish were divided into four sectors and in each sector 4 mm bore was made using a sterile borer. Each bore is filled with 50 μl of test compound or ethanol as control followed by 10 μl of inoculums and incubated for 24 h at 37o for bacteria and 72 h at 28o for fungi. Results of the antimicrobial screening were recorded as the average diameter of zone of inhibition (ZI) surrounding the wells containing the test solution in compared to control [10,11]. Chloramphenicol and amphotericin B were used as standards for bacteria and fungi, respectively.
Determination of minimum inhibitory concentration
Minimum inhibitory concentration (MIC) assay was performed by using nutrient broth containing 0.05% phenol red and supplemented with 10% glucose (NBGP). All the test compounds including standard drugs were initially dissolved in dimethylsulphoxide (DMSO) and the solution obtained was added to NBGP to a final concentration of 5000 μg/ml for each crude extract. This was serially diluted to obtain a concentration ranging from 1.22 to 5000 μg/ml. 100 μl of each concentration was added to a well (96 well microtitre plate) containing 95 μl of NBGP and 5 μl of standard inoculums, the appropriate concentration of inoculums is 2×104 to 105 CFU/ml. The negative control well consists of 195 μl of NBGP and 5 μl of standard inoculums. The plate was covered with a sterile plate sealer, then agitated to mix the contents of the well using shaker and incubated at 37° for 24 h. The assay was repeated twice and microbial growth was determined by observing the change of colour in the wells (red when there is no growth and yellow when there is a growth. The lowest concentration showing no colour change in the well was considered as MIC [12].
Excision wound model
Animals were kept under light ether anaesthesia throughout the surgical procedures. An impression of 500 mm2 was made after leaving at least 5 mm space from the ears. The skin of the impressed area was excised carefully to the complete thickness and a wound of 500 mm2 was produced. Haemostasis was achieved by application of normal saline solution. Ointment base I.P. as control, 0.2% w/w nitrofurazone ointment as standard, and 5% and 10% w/w ointment of W. biflora extract in group I‑IV, respectively were applied topically once in a day, until the wound was completely healed. The physical attributes of wound healing, i.e. wound closure (contraction); epithelisation and scar features were recorded. The wound contraction was studied by tracing the raw wound area on transparent paper in every alternative day from 0 to 20 days. The criterion for complete epithelisation was fixed as a formation of the scar with absence of raw wound area. The wound area was measured planimetrically by the help of mm2 scale graph paper [13,14].
Incision wound model
Four groups of animal containing six in each group were taken. The animals were anaesthetised under light ether anaesthesia. One paravertebral straight incisions of 5 cm length was made including the cutaneous muscle of the depilated back of each rat. Incisions were made at least 1 cm apart to the vertebral column. The wounds were closed with sutures at equidistant points of 1 cm apart by silk thread of zero grades with the help of a straight round‑bodied needle [11]. Wounds were cleaned with 70% alcohol soaked cotton swabs. The animals were treated with the ointment base, nitrofurazone ointment, and extracts in ointment form topically. The sutures were removed after 8 days. The wound breaking strength, mucopolysaccharide, collagen and DNA were estimated by adapting standard procedures [15‑17].
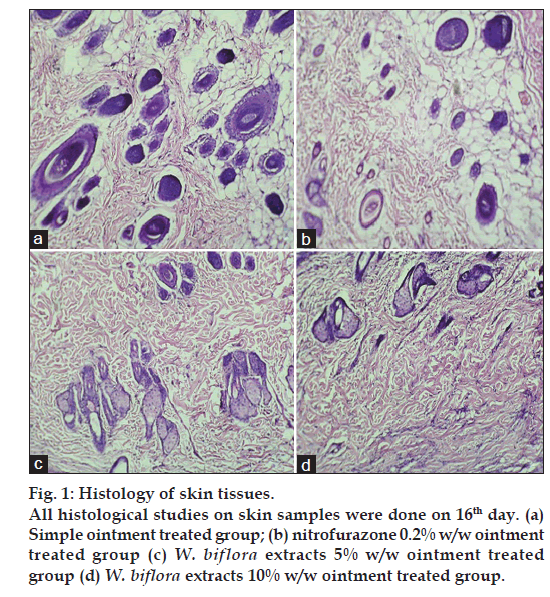
Histopathological studies
Skin specimens from treated and untreated rats were collected in 10% buffered formalin. After the usual processing, 5 μm thick sections were cut and stained with Haematoxylin and Eosin [18]. Sections were qualitatively assessed under the light microscope and graded with respect to keratinisation and scar formation.
DPPH radical scavenging activity
The free radical‑scavenging activity of ethanol extract of W. biflora was measured in terms of hydrogen donating or radical‑scavenging ability using the stable radical DPPH (2,2-diphenyl-1-picrylhydrazyl, Hi‑media, Mumbai, India). A solution of DPPH (0.1 mM) in ethanol was prepared in ethanol was prepared and 1.0 ml of this solution was added to 3.0 ml of all the extracts solution in water at different concentrations (10‑100 μg/ml). Thirty minutes later, the absorbance was measured at 517 nm [10,11]. Lower absorbance of the reaction mixture indicates higher free radical‑scavenging activity. Rutin (Ozone, Mumbai, India) was used as a standard antioxidant. The results were expressed as IC50 (inhibitory concentration 50) value i.e., concentration of samples exhibited 50% inhibition of DPPH radicals [19].
Nitric oxide scavenging activity
Nitric oxide scavenging activity of ethanol extract of W. biflora was determined by using Griess reagent (1% sulphonilamide, 2% phosphoric acid and 1% naphthylethylenediamine dihydrochloride). Reaction mixture containing 3 ml of sodium nitroprusside (10 mmol) in phosphate buffer saline (pH‑6.8) and test extract 100‑500 μg/ml were incubated at 25° for 150 min for study. Control is also prepared by using 3 ml of sodium nitroprusside in ethanol which is use as solvent to dissolve the extract and allowed for incubation. After incubation, 0.5 ml of Griess reagent was added and the absorbance was measured at 546 nm using UV/Vis spectrophotometer. The results were expressed as IC50 value i.e., concentration of samples exhibited 50% inhibition of nitric oxide radicals [20].
Statistical analysis
The results are expressed as mean±SEM, (N=6). Statistical significance was determined by using one‑way ANOVA followed by Dunnett test to identify the differences between treated groups and control. The data were considered significant at P<0.05. The analysis was performed by Graph pad Prism 3 software, La Jolla, USA.
Results and Discussion
Fresh 300 g of leaves of W. biflora on ethanol (99.5%) yielded 20.63% w/w extract. Preliminary phytochemical screening of ethanol extract of W. biflora confirmed the presence of alkaloids, flavonoids, tannins, terpenoids, proteins, carbohydrates and coumarins.
In infected wounds bacteria or other microorganisms have colonised, causing either an interruption in wound healing or deterioration of the wound. Most wounds are typically contaminated by bacteria. However, infected wounds result when the body’s immune defences are overwhelmed. Staphylococci and streptococci are the most common pathogenic organisms in community acquired superficial wounds. Moreover, different pathogenic organisms, which cause various wound infections after surgery may vary to the different anatomical site of surgery [21]. In our present study, the growth of test organism was inhibited by ethanol extract of W. biflora with higher range of ZI 11.3‑21.6 mm. Whereas, bacterial strain Klebsiella pneumonias was found as more sensitive and B. subtilis was found less sensitive. In case of fungal strain, C. albicans shows higher sensitivity near to standard. Chloramphenicol and amphotericin B have shown ZI ranged from 18±0.12 to 20.4±1.02 mm at a concentration of 30 μg/bore (Table 1). The ethanol extract of leaves of W. biflora was tested at different concentrations for their antimicrobial activity. The extent of their inhibitory by comparing with their MIC values was also determined. The results indicated that C. albicans (MIC‑39 μg/ml) is more sensitive to ethanol extract of W. biflora. Whereas MIC value for chloramphenicol and amphotericin B were ranged from 4.8 to 19.50 μg/ml. The antimicrobial activity could be due to the presence of terpenes and flavonoids in ethanol extract of W. biflora.
| Micro-organism | Zone of inhibition in mm | MIC (µg/ml) | ||
|---|---|---|---|---|
| Test drug (1.5 mg/bore) |
Standard (30 µg/bore) |
Test drug |
Standard drug |
|
| Bacillus subtilis | 11.3 ± 0.6 | 18.7 ± 3.11 | 312.5 | 6.25 |
| Staphyloccocus aureus |
15.2 ± 1.8 | 20.8 ± 2.30 | 312.5 | 3.13 |
| Escherichia coli | 14.6 ± 2.5 | 20.6 ± 2.22 | 625 | 12.5 |
| Klebsiella pneumoniae |
20.4 ± 0.9 | 20.2 ± 2.32 | 78.3 | 25 |
| Candida albicans | 21.6 ± 2.15 | 20.4 ± 1.02 | 78.1 | 6.25 |
| Aspergillus niger | 12.8 ± 1.7 | 19.8 ± 1.19 | 156.2 | 3.13 |
All values are expressed as mean±standard error mean, n=3, (chloramphenicol and amphotericin B are the standards for bacteria and fungus, respectively). MIC=Minimum inhibitory concentration
Table 1: Zone Inhibition and Minimum Inhibitory Concentration Effects of Ethanol Extract of W. Biflora on Experimented Microbs
Wound healing is a combination of inflammation, cell proliferation and collagen lattice formation. When wound occurs, it is accompanied, within a short time by pain, reddening oedemas, which are the classical symptoms of inflammation. These symptoms are caused by the release of eicosanoids, leukotriene and reactive oxygen species (ROS) [22]. Since, ROS is produced in high amounts at the site of wound as a defence mechanism against invading bacteria [23,24]. Several phytoconstituents viz. terpenoids, tannins, alkaloids and flavonoids are identified to initiate wound healing process because of their antioxidant properties and antimicrobial activities. In present in vitro antioxidant study W. biflora extract demonstrated dose dependent DPPH and nitric oxide radical scavenging activity (Table 2).
| Sample | IC50 value (µg) | |
|---|---|---|
| DPPH radical scavenging activity |
Nitric oxide scavenging activity |
|
| Ethanol extract of W. Biflora |
377.03 ± 0.15 | 638.92 ± 0.43 |
| Standard (Rutin) | 92.66 ± 0.50 | 82.45 ± 0.78 |
All values are expressed as a mean±standard error mean (n=3), IC50=Inhibitory concentration 50, DPPH= 2,2-diphenyl-1-picrylhydrazyl
Table 2: In Vitro Antioxidant Activity of Ethanol Extract of W. Biflora
In in vivo wound model, the test group animals, treated with W. biflora extract ointment, has shown high sensitivity in repairs the wound tissues compared with control groups of animals. A very rapid closure of the wound in treated groups observed between 4 and 8 days of postsurgery (P<0.05). After day 8 of postsurgery, wound closure was gradual until the total closure of wound. However, in the standard group, treated with nitrofurazone ointment has shown gradual closure of the wound. On the day 16 of postsurgery, mean wound area of ethanol extract of W. Biflora (10% w/w) treated group was 97.90%, whereas in control it was 58.50%, indicating that the extract shows better wound healing property comparable to that of standard drug. Total wound closure was observed on 18th day of postsurgery in all the treated groups and in case of control group on 20th day (Table 3).
| Post wounding days |
Wound area (mm2) and % of wound contraction | |||
|---|---|---|---|---|
| Simple ointment base |
Nitrofurazone ointment |
W. biflora extract 5% ointment |
W. biflora extract 10% ointment |
|
| 0 | 526 ± 3.1 | 512 ± 2.7 | 531 ± 2.9 | 522 ± 4.2 |
| 2 | 438 ± 2.2 (16.7) | 414 ± 14.1 (19.1) | 426 ± 1.7 (19.8) | 407 ± 2.2 (22.0) |
| 4 | 392 ± 3.4 (25.5) | 306 ± 2.6 (40.2) | 352 ± 1.6 (33.7) | 331 ± 5.1 (36.6) |
| 6 | 314 ± 4.2 (35.2) | 233 ± 2.8 (54.5) | 293 ± 3.3 (44.8) | 246 ± 2.8 (52.9) |
| 8 | 306 ± 3.9 (41.8) | 189 ± 1.6 (63.1) | 226 ± 4.3 (57.1) | 176 ± 5.2 (66.3) |
| 10 | 289 ± 0.8 (45.1) | 108 ± 2.2 (78.9) | 165 ± 1.7 (68.9) | 117 ± 3.6 (77.6) |
| 12 | 268 ± 2.7 (49.0) | 64 ± 1.8(87.5) | 128 ± 3.4 (75.9) | 73 ± 1.8(86.0) |
| 14 | 242 ± 1.6 (54.0) | 30 ± 2.2(94.1) | 82 ± 1.4(84.5) | 34 ± 2.1(93.5) |
| 16 | 218 ± 0.8 (58.5) | 08 ± 0.2* (98.4) | 36 ± 1.1(93.2) | 11 ± 0.1* (97.9) |
| 18 | 196 ± 2.4 (62.7) | 00 ± 00(100) | 12 ± 0.8* (97.7) | 00 ± 00(100) |
| 20 | 187 ± 3.6 (64.4) | - | 00 ± 00(100) | - |
All data were expressed as mean±standard error mean (SEM). Differences were considered significant at *P<0.05 when compared test groups versus control (simple ointment) group (n=6). P values were calculated by Student t test
Table 3: Effect of Ethanol Extract of W. Biflora Excision Wound Model In Rats
The amount mucopolysaccharide, collagen and DNA are plays a key role for wound healing activity. They permit the sharp twisting of the collagen helix. They help in providing stability to the triple‑helical structure of collagen by forming hydrogen bonds. Hydroxyproline is found in few points other than collagen. The only other mammalian protein which includes hydroxyproline is elastin. For this reason, hydroxyproline content has been used as an indicator to determine collagen content. Collagen formation, hydroxyproline and DNA content will affect the breaking strength of the skin [25]. The tensile strength of the ethanol extract of W. Biflora (10% w/w) treated groups and the nitrofurazone ointment treated group was near to each other. The 5% w/w extract treated group showed lesser but significant increase in tensile strength compared to the control group (P<0.05) on the 10th day of postwounding surgery.
Mucopolysaccharide and hydroxyproline content was increased significantly in the groups treated with 5% and 10% w/w extract ointment (65.33±3.11 and 72.22±2.11 respectively) than the control group (51.2±4.21), which implies more collagen deposition in treated group than control group (Table 4).
| Parameters | Days | Simple ointment base |
Nitrofurazone ointment |
W. biflora extract 5% ointment |
W. biflora extract 10% ointment |
|---|---|---|---|---|---|
| Mucopolysaccharide content | 4 | 0.97 ± 0.11 | 1.51 ± 0.02 | 1.21 ± 0.02 | 1.43 ± 0.01 |
| 8 | 0.73 ± 0.06 | 0.97 ± 0.03 | 0.61 ± 0.02 | 0.68 ± 0.02 | |
| 12 | 0.50 ± 0.15 | 0.81 ± 0.05 | 0.53 ± 0.01 | 0.67 ± 0.01 | |
| Hydroxyproline content | 4 | 2.1 ± 0.24 | 3.57 ± 0.01 | 2.23 ± 0.18 | 3.0 ± 0.05 |
| 8 | 3.4 ± 0.10 | 6.49 ± 0.07 | 5.53 ± 0.03 | 5.9 ± 0.06 | |
| 12 | 3.9 ± 0.30 | 8.00 ± 0.04 | 4.53 ± 0.02 | 5.1 ± 0.06 | |
| DNA content | 4 | 5.6 ± 0.13 | 9.83 ± 0.12 | 8.25 ± 0.02 | 7.18 ± 0.04 |
| 8 | 4.6 ± 0.17 | 6.76 ± 0.07 | 5.06 ± 0.04 | 6.16 ± 0.02 | |
| 12 | 2.02 ± 0.05 | 3.33 ± 0.10 | 2.53 ± 0.07 | 3.26 ± 0.10 | |
| Tensile strength (g) | - | 262.16 ± 10.12 | 389.06 ± 4.45* | 322.16 ± 7.14 | 366 ± 2.41* |
All data were expressed as mean±standard error mean. Differences were considered significant at *P<0.05 when compared test groups versus control (simple ointment) group (n=6). P values were calculated by Student ‘t’ test
Table 4: Effect of Ethanol Extract of W. Biflora on Incision Wound Model in Rats
Treatment of rat wounds with 5% and 10% w/w ointments have led to reduction in scar formation and promote fibroblast proliferation, angiogenesis, keratinasation and epithelisation as compared to vehicle treated group or control group. Photograph of skins are presented in fig. 1.
The results discussed above indicate that the ethanol extract of W. biflora leaves established the scientific basis of traditional claim for the use of W. biflora leaves as a wound healing agent. The wound healing activity may facilitated by its antimicrobial property against experimented microbial strains viz. B. subtiles, S. aureus, E. coli, K. pneumoniae, C. albicuns, A. Niger and also the presence of phytochemicals, which inhibits ROS activity. Therefore, the external application of the extract on the wound prevented the microbes to invade the surface wounds and subsequently accelerates the wound healing process. Thus W. biflora could be a potent wound healing agent for use in future.
References
- Kiran K, Asad M. Wound healing activity of Sesamum indicum L seed and oil in rats. Indian J Exp Biol 2008;46:777-82.
- Patil MB, Jalalpure SS, Ali A. Preliminary phytochemical investigation on wound healing activity of the leaves of Argemone Mexicana. Indian Drugs 2001;38:288.
- Tara Shanbhag V, Chandrakala S, Sachidananda A, Kurady BL, Smita S, Ganesh S. Wound healing activity of alcoholic extract of Kaempferia galanga in Wistar rats. Indian J Physiol Pharmacol 2006;50:384-90.
- Anonymous: The useful plants of India. Government of India, New Delhi: Council of Scientific and Industrial Research (CSIR); 1986.
- Yoganarasimhan SN. Medical Plants of India. Vol. 2. Bangalore: Cybermedia; 2000.
- Yoganandam GP, Ilango K, Biswas D. Pharmacognostical and preliminary phytochemical studies on the leaves of Wedelia biflora (Linn) D.C. J Pharm Res 2009;2:1113-5.
- Miles DH, Chittawong V, Hedin PA, Kokpol U. Potential agrochemicals from leaves of Wedelia biflora. Phytochemistry 1993;32:1427-9.
- Meena AK, Rao MM, Meena RP, Renu PP. Pharmacological and phytochemical evidences for the plants of Wedelia Genus – A Review. Asian J Pharm Res 2011;1:7-12.
- Kokate CK. Practical Pharmacognosy. 4th ed. New Delhi: Vallabh Prakashan; 1994. p. 107-11.
- Ekpo M, Mbagwu H, Jackson C, Eno M. Antimicrobial and wound healing activities of Centrosema pubescens (Leguminosae). J Pharm Clin Sci 2011;1:1-6.
- Mathew J. Antimicrobial activity of selected aromatic plants. Indian Drugs 2005;42:28-32.
- Zyoda JR, Porter JR. A convenient microdilution method screening natural products against bacteria and fungi. Pharm Biol 2001;39:221-5.
- Mitra MP, Sasmal D, Narayan S. Wound healing activity of the methanolic extracts of the leaves of Salvia splendens Sallow Ex Roem and Schult. J Pharm Res 2008;7:39-43.
- Priya KS, Gnanamani A, Radhakrishnan N, Babu M. Healing potential of Datura alba on burn wounds in albino rats. J Ethnopharmacol 2002;83:193-9.
- Edwin S, Jarald EE, Deb L, Jain A, Kinger H, Dutt KR, et al. Wound healing and antioxidant activity of Achyranthes aspera. Pharm Biol 2008;46:824-8.
- Mustafa MR, Mohamed AA, Sidik K, Noor SM. Evaluation of wound healing potential of Ageratum conyzoides leaf extract in combination with honey in rats as animal model. Int J Mol Med Adv Sci 2005;1:406-10.
- Diegelmann RF, Evans MC. Wound healing: An overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283-9.
- Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc 2008;2008:4986.
- Jain A, Soni M, Deb L, Jain AR, Rout SP, Gupta VB, et al. Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb. Leaves. J Ethnopharmacol 2008;115:61-6.
- Manganthayaru K, Sravan K, Praveen A, Reddy K, Kumar RM, Swetha B, et al. In vitro antioxidant studies on aerial part of Origanum majoram (Linn) and Artemesia sieversiana (Ehrh). Pharmacogn Mag2007;3:90-4.
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: Molecular and cellular mechanisms. J Invest Dermatol 2007;127:514-25.
- Healy B, Freedman A. Infections. BMJ 2006;332:838-41.
- Houghton PJ, Hylands PJ, Mensah AY, Hensel A, Deters AM. In vitro tests and ethnopharmacological investigations: Wound healing as an example. J Ethnopharmacol 2005;100:100-7.
- Sen CK, Khanna S, Gordillo G, Bagchi D, Bagchi M, Roy S. Oxygen, oxidants, and antioxidants in wound healing: An emerging paradigm. Ann N Y Acad Sci 2002;957:239-49.
- Udupa SL, Udupa AL, Kulkarni DR. Studies on the antiinflammatory and wound healing properties of Moringa oleifera and Aegle marmelos. Fitoterapia 1994;65:119-23.