- *Corresponding Author:
- Y. Zhai
Sports Science Institution,
Nanjing University,
Nanjing,
Jiangsu 210046,
P. R. China
E-mail: zyf@nju.edu.cn
| This article was originally published in a special issue,“New Advancements in Biomedical and PharmaceuticalSciences” |
| Indian J Pharm Sci 2022:84(2) Spl Issue “111-117” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In the present study, the high fat diet was used to establish a murine model of obesity and treadmill exercise was applied to improve obesity. After exercise intervention, the novel object recognition test and threechambered social test were performed to evaluate cognitive function. Enzyme-linked immunosorbent assay was employed to measure serum inflammatory factor levels and Western blot assay was performed to detect hippocampal protein levels in pro-inflammatory signaling pathway. We found that high fat diet mice spent less time on exploring novel objects and communication with unfamiliar mice in the novel object recognition and three-chambered social test, respectively. The cognitive decline in obese mice was significantly alleviated after treadmill exercise. Furthermore, serum levels of inflammatory cytokines, including interleukin-1 beta, tumor necrosis factor-alpha and interleukin-6 as well as hippocampal tolllike receptor 4/myeloid differentiation primary response 88/nuclear factor kappa B signaling pathway which can lead to the production of pro-inflammatory mediators were remarkably activated in obese mice. According to the random control experimental scheme and its big data analysis we found treadmill exercise inhibited the inflammatory response in the hippocampus. These results suggest that exercise may improve the high fat diet induced cognitive impairment by inhibiting hippocampal inflammation via the suppression of toll-like receptor 4/myeloid differentiation primary response 88/nuclear factor kappa B signaling pathway. Our work elucidates the mechanism underlying the improvement of exercise in obesity induced cognitive dysfunction and provides theoretical basis for the clinical application of exercise strategies for the treatment of obese patients with cognitive dysfunction.
Keywords
Statistical analysis, treadmill exercise, high-fat diet, hippocampus, cognitive dysfunction
Big data statistics show that cognitive dysfunction causes learning and memory impairment and may lead to aphasia, apraxia, ignorance as well as disturbance in executive function, which severely affect the normal life quality of patients [1]. It is noteworthy that obesity is often comorbid with cognitive dysfunction. As compared with normal-weight individuals, obese patients have a higher risk of neuropsychiatric disorders including cognitive [2,3]. Hippocampal neuroinflammation is a significant contributing factor for the occurrence and development of cognitive dysfunction [4]. Interestingly, previous research findings have shown that by the activation of microglia, obesity causes neuroinflammation and loss of hippocampal functional synapses [5]. However, whether obesity affects cognitive behaviors via neuroinflammatory pathway is still unclear.
Exercise forms an important part of life style intervention to control obesity without side effects. It is highly beneficial for human health such as bones and muscles growth, blood circulation, sleep quality and immunity [6-10]. Additionally, the latest research and statistical analysis found that exercise suppressed intestinal barrier damage and neuroinflammatory response and improved depression-like behavior in mice [11,12]. Recent studies reported that physical exercise increased the levels of growth factors in the brain and increased synaptic plasticity to promote cognitive formation [13]. Furthermore, in clinical practice, exercise alleviates cognitive decline and dementia progression and dementia progression and reverses the symptoms of mild cognitive impairment in the elderly [14]. However, the role of exercise in obesity-induced cognitive impairment and the underlying mechanism is not fully understood.
Our study is the first to evaluate the contribution of neuroinflammation in obese-induced cognitive dysfunction and explore the effect of exercise on cognition in obese mice and the underlying mechanism. In the present study, High Fat Diet (HFD) was used to establish an animal model of obesity and treadmill exercise was used as a therapeutic approach to treat obesity. Cognitive function of mice was evaluated by Novel Object Recognition (NOR) test and Three- Chambered Social (TCS) test followed by detection of serum levels of inflammatory cytokines, including Interleukin-1 Beta (IL-1β), Tumor Necrosis Factor- Alpha (TNF-α) and Interleukin-6 (IL-6). Since Toll- Like Receptor 4 (TLR4)/Myeloid Differentiation primary response 88 (MyD88)/Nuclear Factor kappa B (NF-κB) signaling pathway leads to the production of pro-inflammatory mediators [15], the protein levels of TLR4, MyD88, p-p65NF-κB, phosphorylated-IκB kinase alpha (p-IKKα) and p-IKKβ in the hippocampus were also measured in the present study.
Our results showed that obese produced significant hippocampal neuroinflammation via the activation of TLR4/MyD88/NF-κB signaling pathway. Moderateintensity aerobic exercise by treadmill suppressed the pro-inflammatory signaling pathway and significantly improved the cognitive impairment caused by obesity. These findings provide theoretical support for the further application of exercise training in the improvement of obese induced cognitive decline.
Materials and Methods
Animals:
4 w old male C57BL/6Cnc mice (n=60) were purchased from Zhejiang Vital River Laboratory Animal Technology Co. (Zhejiang, China) and housed with a 12 h light/dark cycle (8:00 am-8:00 pm), under controlled temperature (23°±2° 50 %-60 % relative humidity. The mice were divided into three groups (n=20 per group): Control group (control mice); HFD group (HFD mice) and HFD with treadmill exercise group (HFD-M mice). All procedures on animals followed guidelines established by the Animal Ethical and Welfare Committee of Nanjing University and the China Council on Animal Care at Nanjing University.
HFD model:
The mice were fed with normal diet (Table 1) or HFD (Table 2) chow and were provided with sterilized water or 20 % fructose water [16]. The obesity model was considered successful when the body weight was more than 20 % of the average body weight of normal mice.
|
|
Components | Water | ||
|---|---|---|---|---|
| Crude Fat (g) | Crude Protein (g) | Crude Fiber (g) | ||
| Normal diet | 40 | 180 | 50 | Sterile water |
Table 1: Components of Normal Diet
|
|
Components |
Water |
||
|---|---|---|---|---|
| Fat (%) | Protein (%) | Carbohydrates (%) | ||
| HFD | 60 | 20 | 20 | 20 % fructose water |
Table 2: Components of HFD
Exercise protocol:
This experiment included 60 male C57BL/6Cnc mice aged 4 w, 40 of which were fed with a HFD for 6 w to establish an obesity model. After achieving HFD induced obesity, HFD-M mice were selected to acclimate to a treadmill undergo a 6 w training protocol. The HFD-M mice performed treadmill exercise at a speed of 12 m/ min for 6 w, 5 d a week, once a day. For each exercise period, the mice were allowed 5 min of warm-up at 8 m/min and then continued to exercise at a speed of 12 m/min for 30 min/d at 2:00-4:00 pm [17] (Table 3).
| Exercise period (w) | Warm-up (m/min) |
Exercise speed (m/min) |
Slope grade (%) |
Time (min) |
|---|---|---|---|---|
| 0-1 | 5 | 0 | 30 | |
| 2-7 | 8 | 12 | 0 | 30 |
Table 3: Treadmill Exercise Started 6 W After the Intake of HFD
TCS test:
Social behavior or social memory was tested by the TCS test. The TCS test was conducted in a white plastic open box that was a 57 cm×45 cm×20 cm in size. There was a switchable channel at the bottom center of the two transparent partitions and metal cages at the corners of the left and right boxes. Before the initiation of the formal test, the test mice were placed in the middle box, in order to adapt for 5 min. During the first stage of the experiment, a stranger mouse of the same sex was placed in the metal cage at the corner of the left box. The test mice were placed in the middle box and then allowed to move freely in the three boxes for 10 min before being removed. In the second phase of the experiment, the second stranger mice of the same sex was placed in the metal cage at the corner of the right box and the test mice were placed in the middle box and then allowed to move freely in the three boxes for 10 min. During the experiment, a video camera was used to track the movement trajectory of the mice and the time and number of contacts between the test mouse and the metal cage were recorded [18].
NOR test:
Cognitive abilities of mice were tested by the NOR test, which was conducted using a 30×30×20 cm box. First, the mice adapted to the environment in the experimental box for 5 min. Then, two identical items were placed into a symmetrical position in the box and the mice were allowed to freely explore the box. A video camera was used to track the movement trajectory of the mice in the box for 10 min. After 1 h, one item was replaced with another item of similar size but with completely different colors and shapes. The time and the number of times that the mice explored the two different objects within 10 min were recorded and a discrimination index was used to evaluate the animal’s learning ability [19].
Enzyme-Linked Immunosorbent Assay (ELISA):
ELISA was used to detect serum levels of IL-1β, TNF-α and IL-6 inflammatory factors (n=10) according to the manufacturer’s protocol. ELISA kits for detection of TNF-α (MTA00B), IL-6 (M6000B) and IL-1β (MLB00C) were purchased from R&D Systems (Minneapolis, MN, USA).
Western blot assay:
The total protein in mice hippocampal tissue was extracted with tissue protein lysis buffer (P0013B, Shanghai Beyotime Biotechnology Co., Ltd., Shanghai, China) and a PierceTM Bicinchoninic Acid (BCA) assay protein analysis kit (23225, Thermo Fisher Scientific, Waltham, MA, USA) was used to evaluate protein extract concentration. The protein extract was directly mixed with the loading buffer and boiled. The samples were separated by Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis (SDS-PAGE) and analyzed using the indicated primary antibody, which was followed by Horseradish Peroxidase (HRP)-coupled secondary antibody (anti-mouse IgG, 6805020 and anti-rabbit IgG, 671280; 1:5000 dilution; Biosharp, Hirono, Japan). Chemiluminescent HRP substrates (Miribo, Trikala, CA, USA) and Tanon-5200 chemiluminescence imager (Tanon Technology Co., Ltd., China Shanghai) were used for visualization. The antibodies used in this study were as follows: TLR4 (1:1000 dilution, 14358; CST, Danvers, MA, USA); MyD88 (1:1000 dilution, 4283; CST); p-p65NF-κB (1:1000 dilution, 3039 ; CST); p65 NF-κB (1:1000 dilution, 8242; CST); p-IKKα (1:1000 dilution, ab38515; Abcam, Cambridge, Massachusetts); IKKα (1:1000 dilution, 2682; CST); p-IKKβ (1:1000 dilution, ab59195; MA, USA); IKKβ (1:1000 dilution, 2684; CST); GAPDH (1:1000 dilution, 5174, CST).
Statistical analysis:
All data are expressed as the mean±standard deviation and statistical analysis was performed using GraphPad Prism 7.0 software. The Analysis of Variance (ANOVA) test and the Bonferroni post-test were conducted. Significance was established at the p<0.05.
Results and Discussion
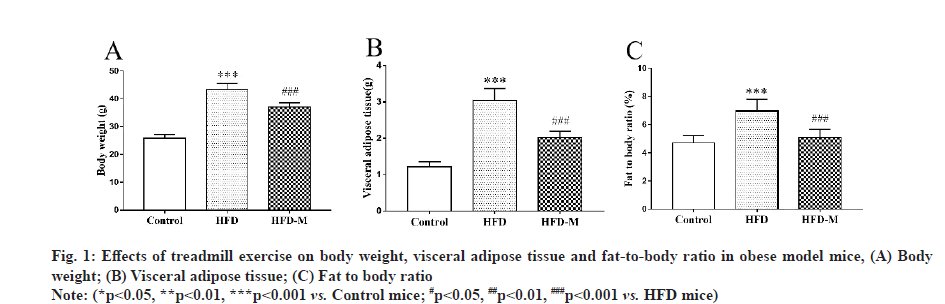
The body weight, visceral adipose tissue and fat-tobody ratio of all animals were determined before the test and no significant difference was found among each group. After 13 w, the weight of the HFD mice was significantly higher than that of the control mice and the weight of the HFD-M mice was lower than that of the HFD mice (p<0.001). In addition, we measured the visceral adipose tissue of the mice and calculate the fat-to-body ratio and we obtained the same results for both indicators. These results indicated that a HFD induced obesity, while treadmill exercise effectively improved the symptoms of obesity (fig. 1).
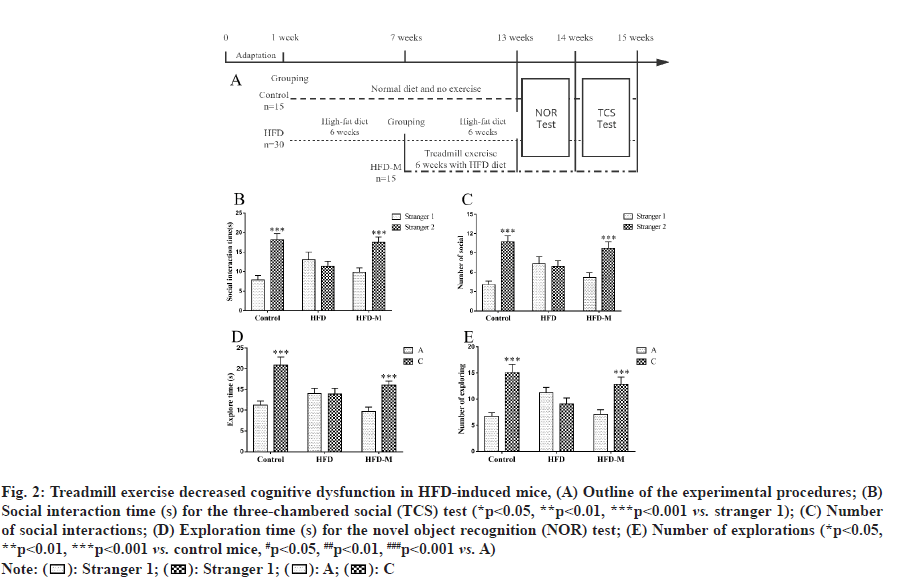
To examine the effect of treadmill exercise on cognitive impairment in HFD mice, the TCS and NOR tests were performed to evaluate learning and memory abilities. In the second phase of the TCS test, there was no difference in the exploration duration by HFD mice between the familiar mice (stranger 1) and stranger mice (stranger 2) (p>0.05), indicating that the HFD induced obese mice exhibited cognitive impairment and did not show any interest in the newly introduced stranger. However, the exploration time of the mice in the control and HFD-M groups were significantly higher than that in the first stage (p<0.001). The exploration times of HFD-M mice in examining stranger 2 were also significantly increased (p<0.001). For further confirmation, we subsequently carried out the NOR test.
In the second phase of the NOR test, there was no statistical difference in both time and frequency for HFD mice between the exploration of familiar and novel objects (p>0.05). However, consistent with the TCS test results, the NOR test results showed that the time taken by HFD mice to explore a novel object were significantly lower than those of control mice. And treadmill exercise obviously extended the time for obese mice to explore a novel object. All these results showed that treadmill exercise promoted short-term learning and memory abilities in obese mice (fig. 2).
Fig. 2: Treadmill exercise decreased cognitive dysfunction in HFD-induced mice, (A) Outline of the experimental procedures; (B) Social interaction time (s) for the three-chambered social (TCS) test (*p<0.05, **p<0.01, ***p<0.001 vs. stranger 1); (C) Number of social interactions; (D) Exploration time (s) for the novel object recognition (NOR) test; (E) Number of explorations (*p<0.05, **p<0.01, ***p<0.001 vs. control mice, #p<0.05, ##p<0.01, ###p<0.001 vs. A)
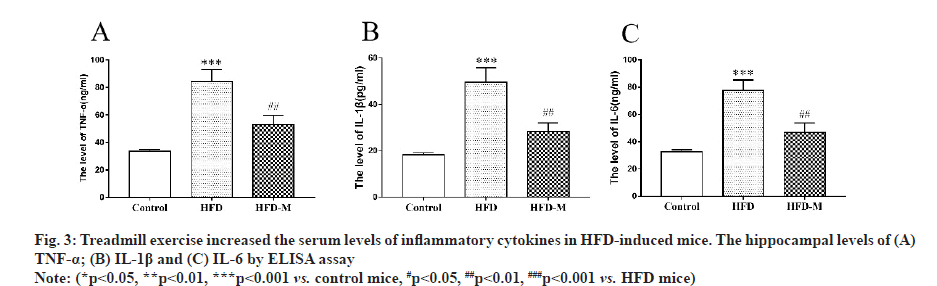
Next, we attempted to explore the possible mechanisms of exercise in the improvement of cognitive dysfunction in HFD mice. We measured serum levels of inflammatory cytokines and the results indicated that the pro-inflammatory cytokines, including IL-1β, TNF-α and IL-6 were significantly increased in HFD mice (***p<0.001) and were significantly decreased after moderate exercise (##p<0.01) (fig. 3).
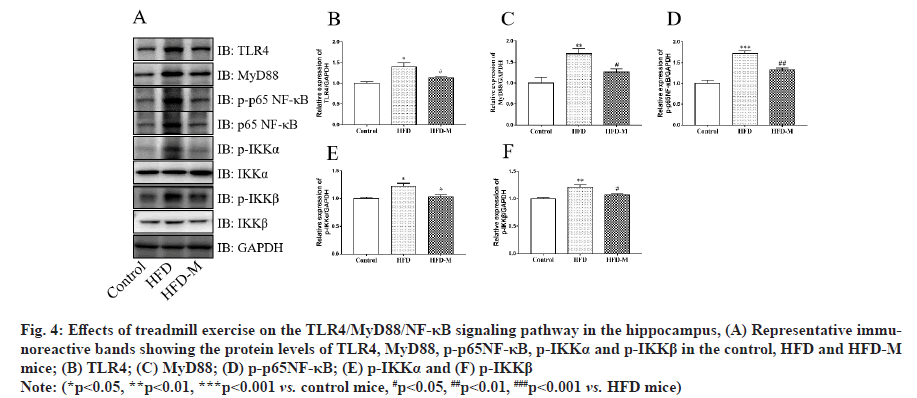
The TLR/MyD88/NF-κB signaling pathway is an important pathway in the inflammatory response. It plays an important role in the occurrence and regulation of autoimmune diseases, inflammatory diseases and infectious diseases. Notably, it has been reported that obesity activates TLR4/MyD88/NF-κB signaling pathway in adipose tissue [20]. However, its role in hippocampal neuroinflammation has been poorly studied. The protein levels of the TLR4/MyD88/NF-κB signaling pathway in the hippocampus were measured by Western blot assay. As shown in fig. 4, the results indicated that the protein levels of TLR4 (**p<0.01), MyD88 (*p<0.05), p-p65NF-κB (*p<0.05), p-IKKα (**p<0.01) and p-IKKβ (*p<0.05) were up-regulated in the HFD group compared with the control group. However, treadmill exercise down-regulated the levels of TLR4, MyD88, p-p65NF-κB, p-IKKα and p-IKKβ (#p<0.05) in HFD-M mice. Our results suggested that treadmill exercise may alleviate HFD induced cognitive impairment by inhibiting hippocampal inflammation through the suppression of TLR4/MyD88/NF-κB pathway (fig. 4).
Fig. 4: Effects of treadmill exercise on the TLR4/MyD88/NF-κB signaling pathway in the hippocampus, (A) Representative immunoreactive bands showing the protein levels of TLR4, MyD88, p-p65NF-κB, p-IKKα and p-IKKβ in the control, HFD and HFD-M mice; (B) TLR4; (C) MyD88; (D) p-p65NF-κB; (E) p-IKKα and (F) p-IKKβ
Note: (*p<0.05, **p<0.01, ***p<0.001 vs. control mice, #p<0.05, ##p<0.01, ###p<0.001 vs. HFD mice)
The global incidence of severe obesity in recent decades (Body Mass Index (BMI) ≥35 kg/m2) has been steadily increasing around the world [21,22]. Obesity is associated with the occurrence and development of a variety of diseases, including cognitive dysfunction [23-27]. In the present study, we reveal that the hippocampal neuroinflammation underlies the obese-induced cognitive decline and exercise rescues the cognitive impairment by suppressing of the pro-inflammatory TLR4/MyD88/NF-κB signaling pathway in the hippocampus.
Diet high in fat and sugar is a major cause of obesity, which is significantly correlated with inflammation [28,29]. In the present study, we have successfully developed a murine model of obesity through HFD. Animals of HFD model showed significant cognitive dysfunction, which manifested as significantly reduced communication time with strangers and reduced ability to recognize novel objects in NOR and TCS tests, respectively. Running at a speed of 12 m/min on a treadmill for rats is considered to be moderate intensity exercise, which can be interpreted as regular aerobic exercise [30]. Notably, we found that moderately intense exercise effectively reduced the weight gain and body fat mass of HFD mice. Furthermore, mice trained with moderate aerobic exercise showed significant improvement in cognitive dysfunction induced by obesity.
Currently, it has been found that inflammation is closely related to neurological diseases [31-33]. Notably, exercise can reduce inflammation and counteract the symptoms of nervous system diseases and these effects are closely related to exercise intensity [31]. Therefore, we examined the effects of obesity and moderate aerobic exercise on hippocampal inflammation. Serum levels of IL-1β, TNF-α and IL-6 are important indicators of hippocampal inflammation [28,34]. We found that serum levels of inflammatory factors significantly increased in HFD group and moderate intensity exercise inhibited the increase of inflammatory factors induced by obesity. In addition, TLR/MyD88/NF-κB signaling pathway plays an important role in the occurrence and regulation of inflammatory response. Our results indicated that obesity activates the TLR/MyD88/NF- κB signaling pathway and moderate intensity exercise significantly inhibits this pathway activation. These results are in support of ELISA data. Our findings suggest that moderate intensity exercise could rescue the cognitive dysfunction induced by obesity. The effect of moderate intensity exercise was mediated by reducing the inflammatory response in the hippocampus via inhibiting of the activation of TLR/MyD88/NF-κB signaling pathway. However, the effects of different exercise intensity levels on hippocampal inflammation and cognitive dysfunction induced by obesity should be further studied.
In summary, the current study revealed that HFDinduced obesity led to the upregulation of inflammatory cytokines and produced cognitive dysfunction in mice. Notably, we observed that moderate exercise can suppressed inflammatory responses and prevented cognitive damage caused by obesity. The activation and suppression of hippocampal TLR4/MyD88/NF- κB signaling pathway underlies the obesity-induced cognitive dysfunction and the exercise-induced improvement of cognitive decline, respectively. These results may provide theoretical basis for the clinical application of exercise strategies for the treatment of obese patients with cognitive dysfunction.
Obesity is one of major health problems; the number of severely and morbidly obese people worldwide is increasing every year. Exercise is one of the most effective methods to control obesity without side effects.
Especially, treadmill exercise is associated with a lower BMI and lower body fat mass. In our study, we found that regular treadmill exercise rescue the cognitive dysfunction in HFD-fed obese mice. Furthermore, moderate aerobic exercise suppressed the hippocampus inflammatory response of the obesity-induced activation of TLR/MyD88/NF-κB signaling pathway. Our work elucidates the mechanism by which exercise improves obesity-induced cognitive dysfunction and provides a novel exercise strategy for the clinical treatment of obese patients with cognitive dysfunction.
Acknowledgement:
This work was supported by the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX19_0026), the Social Science fund of Jiangsu Province (No. 20TYB006).
Conflict of interests:
The authors declared no conflict of interest.
References
- Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol 2011;7(3):137-52.
[Crossref] [Google Scholar] [PubMed]
- Boeka AG, Lokken KL. Neuropsychological performance of a clinical sample of extremely obese individuals. Arch Clin Neuropsychol 2008;23(4):467-74.
[Crossref] [Google Scholar] [PubMed]
- Fergenbaum JH, Bruce S, Lou W, Hanley AJ, Greenwood C, Young TK. Obesity and lowered cognitive performance in a Canadian First Nations population. Obesity 2009;17(10):1957-63.
[Crossref] [Google Scholar] [PubMed]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011;333(6048):1456-8.
[Crossref] [Google Scholar] [PubMed]
- Cope EC, LaMarca EA, Monari PK, Olson LB, Martinez S, Zych AD, et al. Microglia play an active role in obesity-associated cognitive decline. J Neurosci 2018;38(41):8889-904.
[Crossref] [Google Scholar] [PubMed]
- Becker CU, Sartório CL, Campos-Carraro C, Siqueira R, Colombo R, Zimmer A, et al. Exercise training decreases oxidative stress in skeletal muscle of rats with pulmonary arterial hypertension. Arch Physiol Biochem 2020:1-9.
[Crossref] [Google Scholar] [PubMed]
- Abbate M, Gallardo-Alfaro L, del Mar Bibiloni M, Tur JA. Efficacy of dietary intervention or in combination with exercise on primary prevention of cardiovascular disease: A systematic review. Nutr Metab Cardiovasc Dis 2020;30(7):1080-93.
[Crossref] [Google Scholar] [PubMed]
- Barrett B, Harden CM, Brown RL, Coe CL, Irwin MR. Mindfulness meditation and exercise both improve sleep quality: Secondary analysis of a randomized controlled trial of community dwelling adults. Sleep Health 2020;6(6):804-13.
[Crossref] [Google Scholar] [PubMed]
- Farrow M, Nightingale TE, Maher J, McKay CD, Thompson D, Bilzon JL. Effect of exercise on cardiometabolic risk factors in adults with chronic spinal cord injury: A systematic review. Arch Phys Med Rehabil 2020;101(12):2177-205.
[Crossref] [Google Scholar] [PubMed]
- Kido K, Sase K, Yokokawa T, Fujita S. Enhanced skeletal muscle insulin sensitivity after acute resistance-type exercise is upregulated by rapamycin-sensitive mTOR complex 1 inhibition. Sci Rep 2020;10(1):1-2.
- Gerecke KM, Kolobova A, Allen S, Fawer JL. Exercise protects against chronic restraint stress-induced oxidative stress in the cortex and hippocampus. Brain Res 2013;1509:66-78.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Xu F, Liu S, Liu G, Yang X, Gao W, et al. Significance of gastrointestinal tract in the therapeutic mechanisms of exercise in depression: Synchronism between brain and intestine through GBA. Prog Neuropsychopharmacol Biol Psychiatry 2020;103:109971.
[Crossref] [Google Scholar] [PubMed]
- Chen K, Zheng Y, Wei JA, Ouyang H, Huang X, Zhang F, et al. Exercise training improves motor skill learning via selective activation of mTOR. Sci Adv 2019;5(7):eaaw1888.
[Crossref] [Google Scholar] [PubMed]
- Blumenthal JA, Smith PJ, Mabe S, Hinderliter A, Lin PH, Liao L, et al. Lifestyle and neurocognition in older adults with cognitive impairments: A randomized trial. Neurology 2019;92(3):e212-23.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Lu B, Fu J, Zhu X, Song E, Song Y. Amorphous silica nanoparticles induce inflammation via activation of NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-kb signaling pathway in HUVEC cells. J Hazard Mater 2021;404:124050.
- Whitt J, Woo V, Lee P, Moncivaiz J, Haberman Y, Denson L, et al. Disruption of epithelial HDAC3 in intestine prevents diet-induced obesity in mice. Gastroenterology 2018;155(2):501-13.
[Crossref] [Google Scholar] [PubMed]
- Han TK, Leem YH, Kim HS. Treadmill exercise restores high fat diet-induced disturbance of hippocampal neurogenesis through β2-adrenergic receptor-dependent induction of thioredoxin-1 and brain-derived neurotrophic factor. Brain Res 2019;1707:154-63.
[Crossref] [Google Scholar] [PubMed]
- Do Gyeong Kim EL, Kim S, Kim Y, Adil KJ, Jeon SJ, Cho KS, et al. Social interaction test in home cage as a novel and ethological measure of social behavior in mice. Exp Neurobiol 2019;28(2):247-60.
- Antunes M, Biala G. The novel object recognition memory: Neurobiology, test procedure and its modifications. Cogn process 2012;13(2):93-110.
[Crossref] [Google Scholar] [PubMed]
- Kim SJ, Choi Y, Choi YH, Park T. Obesity activates toll-like receptor-mediated proinflammatory signaling cascades in the adipose tissue of mice. J Nutr Biochem 2012;23(2):113-22.
[Crossref] [Google Scholar] [PubMed]
- Basterra-Gortari FJ, Beunza JJ, Bes-Rastrollo M, Toledo E, García-López M, Martínez-González MA. Increasing trend in the prevalence of morbid obesity in Spain: From 1.8 to 6.1 per thousand in 14 years. Rev Esp Cardiol 2011;64(5):424-6.
[Crossref] [Google Scholar] [PubMed]
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387(10026):1377-96.
[Crossref] [Google Scholar] [PubMed]
- Vecchié A, Dallegri F, Carbone F, Bonaventura A, Liberale L, Portincasa P, et al. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med 2018;48:6-17.
[Crossref] [Google Scholar] [PubMed]
- Mili? S, Luli? D, Štimac D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J Gastroenterol 2014;20(28):9330.
[Google Scholar] [PubMed]
- Jantaratnotai N, Mosikanon K, Lee Y, McIntyre RS. The interface of depression and obesity. Obes Res Clin Pract 2017;11(1):1.
[Crossref] [Google Scholar] [PubMed]
- Milaneschi Y, Simmons WK, van Rossum EF, Penninx BW. Depression and obesity: Evidence of shared biological mechanisms. Mol Psychiatry 2019;24(1):18-33.
[Crossref] [Google Scholar] [PubMed]
- Carpaij OA, van den Berge M. The asthma–obesity relationship: Underlying mechanisms and treatment implications. Curr Opin Pulm Med 2018;24(1):42-9.
[Crossref] [Google Scholar] [PubMed]
- Hou Y, Gu D, Peng J, Jiang K, Li Z, Shi J, et al. Ginsenoside Rg1 regulates liver lipid factor metabolism in NAFLD model rats. ACS omega 2020;5(19):10878-90.
- Cox AJ, West NP, Cripps AW. Obesity, inflammation and the gut microbiota. Lancet Diabetes Endocrinol 2015;3(3):207-15.
[Crossref] [Google Scholar] [PubMed]
- Han TK, Leem YH, Kim HS. Treadmill exercise restores high fat diet-induced disturbance of hippocampal neurogenesis through β2-adrenergic receptor-dependent induction of thioredoxin-1 and brain-derived neurotrophic factor. Brain Res 2019;1707:154-63.
[Crossref] [Google Scholar] [PubMed]
- Paolucci EM, Loukov D, Bowdish DM, Heisz JJ. Exercise reduces depression and inflammation but intensity matters. Biol Psychol 2018;133:79-84.
[Crossref] [Google Scholar] [PubMed]
- Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology 2017;153(2):448-59.
- Kiecolt-Glaser JK, Fagundes CP, Andridge R, Peng J, Malarkey WB, Habash D, et al. Depression, daily stressors and inflammatory responses to high-fat meals: When stress overrides healthier food choices. Mol Psychiatry 2017;22(3):476-82.
[Crossref] [Google Scholar] [PubMed]
- Zhang C, Jia Y, Liu B, Wang G, Zhang Y. TLR4 knockout upregulates the expression of Mfn2 and PGC-1α in a high-fat diet and ischemia-reperfusion mice model of liver injury. Life Sci 2020;254:117762.