- *Corresponding Author:
- V. B. Tatipamula
Center for Molecular Biology, College of Medicine and Pharmacy, Duy Tan University, Danang 550000, Vietnam
E-mail: vinaybharadwajtatipamula@duytan.edu.vn
| Date of Received | 10 June 2021 |
| Date of Revision | 14 August 2022 |
| Date of Acceptance | 24 March 2023 |
| Indian J Pharm Sci 2023;85(2):525-531 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The chemical and biological profile of the ethanolic extract of Fabronia secunda examined for the first time. The gas chromatography-mass spectrometric analysis of ethanolic extract of Fabronia secunda identified 200 components, which mainly composed of lup-20(29)-ene-3,28-diol (4.01 %), guanosine (3.99 %), and 1,2-benzene-dicarboxylic acid (3.79 %). Also, the chromatographic analysis yielded three compounds, namely stigmasterol (1), β-sitosterol (2) and lupeol (3). The ethanolic extract of Fabronia secunda exhibited superior inhibition of superoxide and 2,2-diphenyl-1-picrylhydrazyl radicals with an half maximal inhibitory concentration value of 33.5 and 34.5 μg/ml respectively. Additionally, ethanolic extract of Fabronia secunda depicted prominent inhibitory profiles against alpha-glucosidase and pancreatic alpha-amylase with half maximal inhibitory concentration values of 48.5 and 58.0 μg/ml respectively. To conclude, this preliminary chemical analysis provides a piece of evidence to know the metabolism of mosses and the biological investigation proved the therapeutic importance of mosses like Fabronia secunda.
Keywords
Fabronia secunda, moss, ferric ions, 2,2-diphenyl-1-picrylhydrazyl, superoxide, α-glucosidase inhibitory assay, pancreatic α-amylase inhibitory assay, aldose reductase inhibitory assay
Mosses were simplest-level plants that belong to the second-largest taxonomic group among bryophytes. Amongst 25 000 bryophytes species, mosses include 14 000 species around the world[1]. Mosses survive in wet and humid places and mostly they live in rocks, soil, woods and walls of a building. Long-time ago, mosses less considered for the identification of bioactive substances due to their identification problems.
Nevertheless, in recent times, the research attention in mosses chemical profile is increasing, as several biologically active components identified from them due to their unique adaptations. However, from a large number of mosses, only very few species have been studied additionally, the study of the chemical composition of moss assists in knowing their metabolism[2].
Mosses are widely present in forest ecosystems and the Northern Hemisphere. Traditionally, tribes of North American utilized mosses for the management of convulsions, neurasthenia, pneumonia, scald, burns, tuberculosis and others. In the folklore of China and India, extracts of mosses are well-known for antimicrobial activity and the treatment of anxiety, snake-bites, heart problems, tuberculosis, cancer and diabetes[3,4]. The major chemical constituents of mosses are carbohydrates, fatty acids, lipids, flavonoids, benzoic acid derivatives, polyphenols, terpenoids, steroids and some nitrogen-containing aromatic compounds[5]. Mosses extracts possess to have antimicrobial, sedatives, cytotoxic, anti-human immunodeficiency virus, antioxidant, antifeedant and nematocidal activities, which is due to the terpenoids and aromatic compounds found in mosses[4]. Polytrichum moss species also present diuretic, antipyretic and anti-todal activities and can be used to promote hair growth[4]. Moss Taxithelium nepalense has antioxidant and antidiabetic activities[6].
Fabronia genus belonging to family Fabroniaceae with about 95 species around the globe in tropical and warm temperate regions. Particularly, moss Fabronia secunda (F. secunda) Mont. reported on the flora of India[7]. The literature survey did not reveal any chemical and biological profiles of the genus Fabronia. Hence, to know the chemical profile of F. secunda, we performed phytochemical and chromatographic analysis of its Ethanolic Extract of F. secunda (Et-Fs) and correlated to its biological profile. The aim of the present research analysis to analyze the chemical composition of Et-Fs through Gas Chromatography-Mass Spectrometry (GC-MS) and to monitor their antioxidant and antidiabetic activities.
Materials and Methods
Collection:
The specimens of moss F. secunda Mont. collected from Bhitharkanika Island, Rajnagar, Orissa, India, in April 2019. The specimens examined by Dr. Ankita Srivastava, and a voucher specimen (LWG- 29/VB-Orissa-2019) deposited at Lucknow Lichen herbarium, National Botanical Research Institute, Lucknow, India.
Chemicals used in present research work:
1,1-diphenyl-2-picrylhydrazyl (DPPH) and intestinal acetone powders from rat purchased from Sigma Aldrich (Mumbai, India). Amylase HR reagent obtained from Pro Lab Marketing Pvt. Ltd. (New Delhi, India).
Extraction and isolation of moss material:
The dried moss F. secunda Mont. (about 150 g) extracted thrice with ethanol and concentration at a reduced pressure to obtain Et-Fs as a dark greenish solid (10.2 g, 6.8 % w/w), preserved at 4° for further use. Et-Fs (1.0 g) exposed to column chromatography using hexane and ethyl acetate (step-gradient flow) to obtain three fractions. Further, each fraction purified using hexane in acetone (1:9) solvent system yielded three compounds, namely stigmasterol (15 mg) as a colorless crystalline solid, β-sitosterol (10 mg) as a colorless to white needles and lupeol (20 mg) as a white powder.
GC-MS analysis of Et-Fs:
The phytochemical investigation of Et-Fs performed on GC-MS equipment (GCMS-QP2010 Plus, Shimadzu, Europe). Experimental parameters of the GC-MS system set according to the earlier procedures of Tatipamula et al.[8].
Antioxidant activity of Et-Fs:
DPPH assay of Et-Fs: The Et-Fs subjected to DPPH assay in triplicate[9]. Known concentrations of the sample added 0.004 % DPPH in methanol. After that incubated at 37° for 30 min and recorded for absorbance at 517 nm against the blank using Ultraviolet (UV)-Visible spectrophotometry (Spectra MAX plus 384, USA).
Ferric ion (Fe3+) reducing power assay of Et-Fs: The Et-Fs were exposed to Ferric ion (Fe3+), reducing power assay in triplicate[10]. To 2.5 ml phosphate buffer (pH 6.6, 0.2 M), added 2.5 ml potassium ferricyanide (1 %) and added known concentrations of sample and incubated for 20 min. Later to each sample, added 0.1 % of 0.5 ml of ferric chloride and 10 % of 2.5 ml trichloroacetic acid and recorded for absorbance at 700 nm against the blank.
Superoxide radical scavenging assay of Et-Fs: The Et-Fs subjected to scavenging assay of superoxide in triplicate[11]. Known concentrations of the sample added 1 ml of a standardized solution containing 50 µM nitro blue tetrazolium+73 µM Nicotinamide adenine dinucleotide+15 µM of peroxymonosulfate in phosphate buffer (pH 7.4) and incubated for 30 min. After that, absorbance recorded at 562 nm against the blank.
Anti-diabetic activity of Et-Fs:
α-Glucosidase inhibitory assay of Et-Fs: The inhibitory assay of α-glucosidase[12] estimated in a triplet (n=3). 20 µl of known concentrations of the sample added 2.0 µl of α-glucosidase prepared from rat intestine acetone powder and incubated at 37° for 5 min. After incubation, 50 µl of p-nitrophenyl-α- D-glucopyranoside (substrate) added and incubated at 37° for 20 min. After that, the addition of 0.5 µl of 1 M Na2CO3 terminate the reaction and noted absorbance at 405 nm. Plotting concentrations against their percentage inhibition determined half maximal inhibitory concentration (IC50) values of Et-Fs.
Porcine pancreatic α-amylase of Et-Fs: The porcine pancreatic α-amylase inhibitory assay[13] determined in a triplet (n=3). 100 µl of amylase HR reagent and 40 µl of the samples at different concentrations added and incubated at 37° for 10 min. Then 60 µl of 0.1 mg/ml substrate i.e., blocked p-nitrophenyl maltoheptaoside (BPNPG7) in 0.1 M 4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid (HEPES) buffer of pH 6.9 was added and incubated for 10 min at 37° and noted absorbance at 405 nm.
Aldose-reductase activity of Et-Fs: Aldose reductase activity estimated by using the procedures of Talluri et al.[14]. Eye lenses (free from disease) were separated and washed with saline from the eyes of normal albino rats. 10 % of the homogenate prepared with 0.1 M of pH 7.4 buffer (phosphate) for 10 min centrifuged at 5000 rpm and the supernatant was separated and kept at 4°. To the 0.1 ml lens supernatant added 0.7 ml of 0.067 M phosphate buffer, 0.1 ml of 25×10-5 M NADPH, 0.1 ml of 5×10-4 M substrate (DL-glyceraldehyde) except reference and made up to 1 ml volume. After that, absorbance measured at 340 nm for every 30 sec interval of up to 3 min. Likewise, various concentrations of Et-Fs (50, 100, 150 and 200 µg/ml) prepared with phosphate buffer and noted for their absorbance at 340 nm. Phosphate buffer saline was used as a negative control.
Results and Discussion
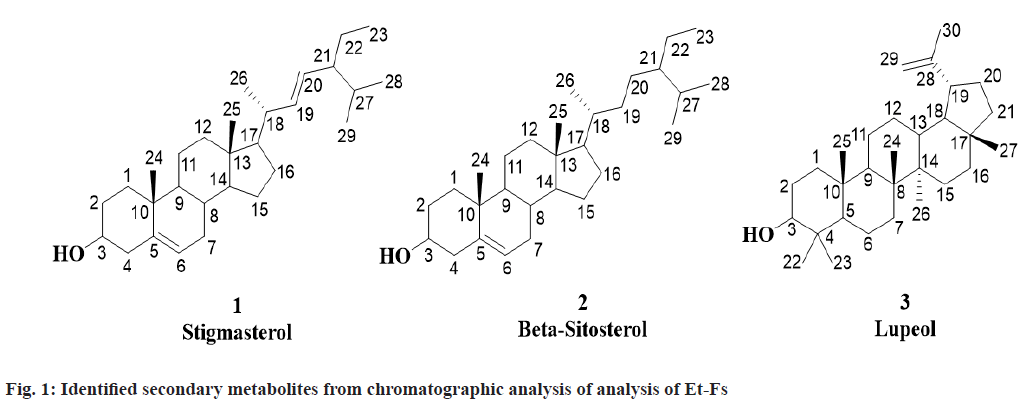
For the first time, three known compounds Stigmasterol, β-sitosterol and Lupeol identified from the Et-Fs utilizing chromatographic methods and analyses of spectral data namely 1H Nuclear Magnetic Resonance (NMR), Mass Spectrometry (MS), Infrared (IR) and 13C NMR and correlated with those reported in the literature (fig. 1).
Stigmasterol was obtained from first fraction with Rf value of 0.57 (hexane:ethyl acetate, 9:1). Molecular formula: C29H48O; m.p.: 140-141°; UV absorption in methanol at λmax 257. 1H NMR (CDCl3, 400 MHz): δ 0.94-0.96 (3H, t, CH3-23), 0.97 (3H, s, CH3-25), 0.99 (3H, s, CH3-28), 1.00 (3H, s, CH3-29), 1.05-1.06 (1H, dd, J= 4 Hz, CH-14), 1.07-1.10 (1H, m, CH- 12a), 1.13 (3H, s, CH3-24), 1.18-1.19 (3H, d, J= 4 Hz, CH3-26), 1.21-1.26 (1H, m, CH-1b), 1.24-1.26 (1H, m, CH-17), 1.27-1.31 (1H, m, CH-22a), 1.32- 1.38 (3H, m, CH-2b,11b,16a), 1.41 (1H, m, CH-3), 1.42-1.51 (3H, m, CH-1a,22b,27), 1.53-1.64 (4H, m, CH-11a,12b,15b,16b), 1.74-1.80 (1H, m, CH-7a), 1.84-1.90 (3H, m, CH-2a,15a,21), 1.94-1.97 (1H, m, CH-4a), 1.99-2.05 (1H, m, CH-7b), 2.06-2.10 (1H, m, CH-18), 2.15-2.22 (1H, m, CH-8), 2.28-2.32 (1H, m, CH-4b), 2.47-2.52 (1H, m, CH-9), 3.40-3.44 (1H, m, OH-3), 5.22-5.23 (2H, m, =CH-19,20), 5.46-5.49 (1H, t, J=4, 8 Hz, =CH-6); 13C NMR (CDCl3, 400 MHz): δ 11.58 (C-23), 13.94 (C-25), 19.83 (C-28/29), 19.90 (C-26), 20.81 (C-24), 22.38 (C-11), 23.15 (C- 22), 24.86 (C-15), 29.04 (C-16), 31.80 (C-27), 31.88 (C-7), 32.97 (C-2), 33.94 (C-8), 37.27 (C-1), 39.11 (C-12), 39.86 (C-10/18), 43.54 (C-4), 43.63 (C- 13), 50.51 (C-9), 51.89 (C-21), 55.88 (C-17), 58.13 (C-14), 72.16 (C-3), 121.66 (C-6), 130.38 (C-20), 137.38 (C-19), 140.58 (C-5). Elemental Analysis for C29H48O: found C-84.76, H-11.82 (%), calcd. C, 84.40, H, 11.72 (%); Electrospray Ionisation Mass Spectrometry (ESI-MS) (positive mode) m/z: 413.50 [M+H+], calcd. m/z for C29H48O: 412.37 [M].
β-sitosterol was obtained from second fraction with Rf value of 0.5 (hexane: ethyl acetate, 9:1). Molecular formula: C29H50O; m.p.: 136-137°; UV absorption in methanol at λmax 206. 1H NMR (CDCl3, 400 MHz): δ 0.97 (3H, s, CH3-25), 0.98-1.01 (3H, t, J=4, 8 Hz, CH3-23), 1.02-1.04 (9H, dd, J=2, 4 Hz, CH3-26,28,29), 1.05 (1H, d, J= 1.8 Hz, CH-14), 1.06- 1.07 (1H, d, J= 4 Hz, CH-12a), 1.08-1.12 (2H, m, CH-17,21), 1.13 (3H, s, CH3-24), 1.21-1.26 (2H, m, CH-1b, 22a), 1.27-1.31 (1H, m, CH-19b), 1.31-1.36 (7H, m, CH-2b,11b,16b,19a,20ab,22b), 1.38-1.39 (1H, m, CH-18), 1.40 (1H, s, CH-3), 1.46-1.64 (5H, m, CH-1a,11a,15b,16a,27), 1.74-1.80 (2H, m, CH- 7), 1.84-1.89 (2H, m, CH-2a,15a), 1.94-1.96 (1H, m, CH-4a), 1.99-2.05 (1H, m, CH-8), 2.28-2.31 (1H, dd, J=4 Hz, CH-4b), 2.43-2.47 (1H, m, CH-9), 3.40-3.44 (1H, s, OH-3), 5.46-5.49 (1H, t, J=4, 8 Hz, CH3-6); 13C NMR (CDCl3, 400 MHz): δ 12.11 (C-23), 13.94 (C-25), 18.77 (C-26), 19.87 (C-28/29), 20.81 (C-24), 22.38 (C-11), 24.86 (C-15), 24.95 (C-22), 28.15 (C- 16), 28.30 (C-20), 31.13 (C-27), 31.88 (C-7), 32.97 (C-2), 33.95 (C-8), 35.41 (C-19), 36.29 (C-18), 37.27 (C-1), 37.28 (C-10), 39.11 (C-12), 43.44 (C- 13), 43.54 (C-4), 45.01 (C-21), 50.51 (C-9), 56.86 (C-17), 58.13 (C-14), 72.16 (C-3), 121.66 (C-6), 140.58 (C-5). Elemental Analysis for C29H50O: found C-83.63, H-12.48 (%), calcd. C, 83.99, H, 12.15 (%); ESI-MS (positive mode) m/z: 415.30 [M+H+], calcd. m/z for C29H50O: 414.39 [M].
Lupeol was obtained from third fraction with Rf value of 0.64 (hexane:ethyl acetate, 4:1). Molecular formula: C30H50O; m.p.: 210-211°; UV absorption in methanol at λmax 350. 1H NMR (CDCl3, 400 MHz): δ 0.83-0.90 (1H, m, CH-12b), 0.98 (3H, s, CH3- 27), 1.00 (6H, s, CH3-24,26), 1.01 (9H, s, CH3- 22,23,25), 1.03-1.08 (2H, m, CH-13,18), 1.14-1.20 (3H, m, CH-9,11b,16a), 1.25-1.30 (1H, m, CH-6a), 1.35-1.41 (5H, m, CH-1b,7a,15a,16b,20b), 1.42- 1.49 (4H, m, CH-3,11a,12a,21b), 1.55-1.73 (7H, m, CH-1a,2a,6b,7b,15b,20a,21a), 1.78 (3H, s, CH3-30), 1.90-1.98 (2H, m, CH-2b,5), 2.07-2.11 (1H, m, CH- 19), 3.46-3.48 (1H, m, OH-3), 4.54 (1H, s, =CH- 29b), 4.79 (1H, s, =CH-29a); 13C NMR (CDCl3, 400 MHz): δ 16.08 (C-25), 17.47 (C-24/26), 18.87 (C- 6), 21.33 (C-30), 21.48 (C-11), 23.21 (C-27), 23.78 (C-22/23), 26.55 (C-12), 27.72 (C-2), 28.96 (C-15), 30.10 (C-20), 35.48 (C-7), 35.68 (C-16), 37.70 (C- 10), 37.97 (C-1/13), 39.08 (C-21), 39.59 (C-4), 41.12 (C-8), 41.98 (C-14), 42.94 (C-17), 48.17 (C-19), 49.26 (C-18), 51.06 (C-9), 54.89 (C-5), 78.56 (C-3), 109.99 (C-29), 151.14 (C-28). Elemental Analysis for C30H50O: found C-84.63, H-11.39 (%), calcd. C, 84.44, H, 11.81 (%); ESI-MS (positive mode) m/z: 427.90 [M+H+], calcd. m/z for C30H50O: 426.39 [M].
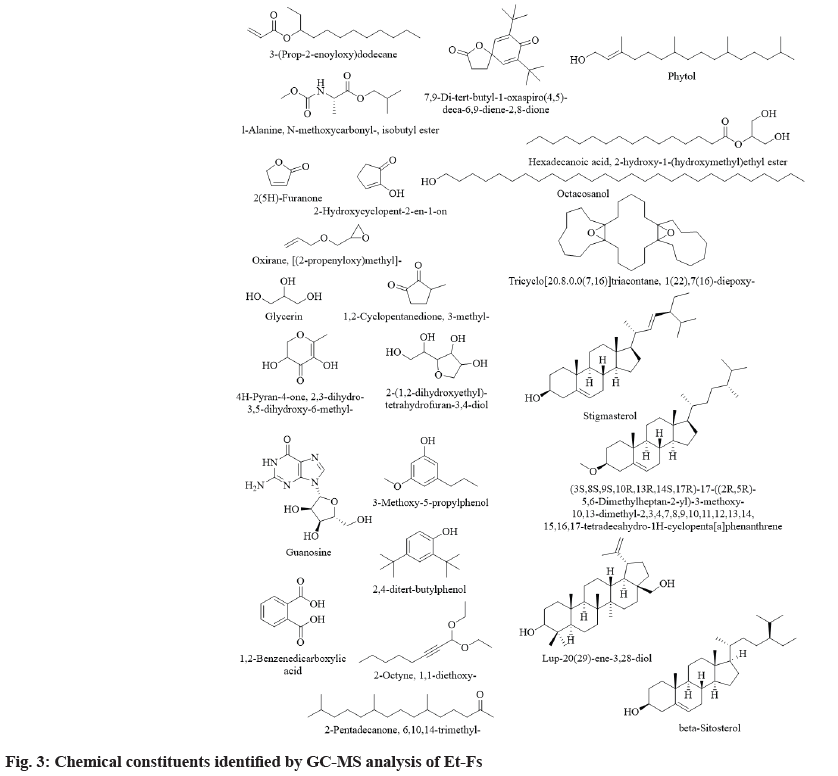
The results pertaining to GC-MS analysis of Et-Fs identifies 200 different components (in total) probably affecting the biological profile of Et-Fs (fig. 2). The studied moss F. secunda contains a significant number of steroids, aliphatic hydrocarbons. Among them, the following 24 substances have been identified in Et-Fs in major quantities for the first time: lup-20(29)-ene-3,28-diol (4.01 %), guanosine (3.99 %), 1,2-benzenedicarboxylic acid (3.79 %), 1(22),7(16)-diepoxy-tricyclo[20.8.0.0(7,16)] triacontane (3.69 %), 2-hydroxycyclopent-2-en- 1-on (3.38 %), [(2-propenyloxy)methyl]-oxirane (2.11 %), glycerin (2.04 %), stigmasterol (1.99 %), 2,4-ditert-butylphenol (1.90 %), phytol (1.88 %), N-methoxycarbonyl-l-alanine-isobutyl ester (1.79 %), 2(5H)-furanone (1.62 %), 3-(prop-2-enoyloxy) dodecane (1.58 %), octacosanol (1.48 %), 7,9-ditert- butyl-1-oxaspiro(4,5)-;deca-6,9-diene-2,8-dione (1.43 %), 2-hydroxy-1-(hydroxymethyl)ethylesterhexadecanoic acid (1.41 %), 2-(1,2-dihydroxyethyl)- tetrahydrofuran-3,4-diol (1.37 %), 3-methoxy- 5-propylphenol (1.36 %), 6,10,14-trimethyl-2- pentadecanone (1.18 %), beta-sitosterol (1.10 %), 1,1-diethoxy-2-octyne (1.10 %), 3-methyl- 1,2-cyclopentanedione (1.05 %), 2,3-dihydro-3,5- dihydroxy-6-methyl-4H-pyran-4-one (1.04 %) and (3S,8S,9S,10R,13R,14S,17R)-17-((2R,5R)-5,6- dimethylheptan-2-yl)-3-methoxy-10,13-dimethyl- 2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro- 1H-cyclopenta[a]phenanthrene (1.01 %) (Table 1 and fig. 3). Also the GC-MS analysis depicted the presence of a higher amount of phenolic compounds, which proves the higher content of phenolics in Et- Fs. Among the phytochemicals from natural sources, phenolics usually acknowledged as substances with high antioxidant abilities. Along with it, they also reported preventing ailments such as Alzheimer’s, diabetes, eye disorders, cancer and heart problems. Additionally, the most vital feature of phenolic comprises their aptitude to defend against oxidative diseases like diabetes by reducing the LDL oxidation[15,16].
| S. No | Retention Time | Area % | Compound Name |
|---|---|---|---|
| 1 | 4.021 | 1.58 | 3-(Prop-2-enoyloxy)dodecane |
| 2 | 4.192 | 1.79 | N-methoxycarbonyl-l-alanine-isobutyl ester |
| 3 | 6.19 | 1.62 | 2(5H)-Furanone |
| 4 | 6.435 | 3.38 | 2-Hydroxycyclopent-2-en-1-on |
| 5 | 7.778 | 2.11 | [(2-propenyloxy)methyl]-Oxirane |
| 6 | 8.192 | 2.04 | Glycerin |
| 7 | 8.265 | 1.05 | 1,2-Cyclopentanedione, 3-methyl- |
| 8 | 10.393 | 1.04 | 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one |
| 9 | 12.091 | 1.37 | 2-(1,2-dihydroxyethyl)-tetrahydrofuran-3,4-diol |
| 10 | 15.14 | 3.99 | Guanosine |
| 11 | 15.323 | 1.36 | 3-Methoxy-5-propylphenol |
| 12 | 15.63 | 1.9 | 2,4-ditert-butylphenol |
| 13 | 16.1 | 1.1 | 1,1-diethoxy-2-Octyne |
| 14 | 16.683 | 3.79 | 1,2-Benzenedicarboxylic acid |
| 15 | 19.601 | 1.18 | 6,10,14-trimethyl-2-pentadecanone |
| 16 | 20.425 | 1.43 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)-deca-6,9-diene-2,8-dione |
| 17 | 22.409 | 1.88 | Phytol |
| 18 | 26.086 | 1.41 | 2-hydroxy-1-(hydroxymethyl)ethylester-hexadecanoic acid |
| 19 | 30.796 | 3.69 | 1(22),7(16)-diepoxy-tricyclo[20.8.0.0(7,16)]triacontane |
| 20 | 31.763 | 1.48 | Octacosanol |
| 21 | 34.273 | 1.01 | (3S,8S,9S,10R,13R,14S,17R)-17-((2R,5R)-5,6-Dimethylheptan-2-yl)-3-methoxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene |
| 22 | 34.735 | 1.99 | Stigmasterol |
| 23 | 35.105 | 4.01 | Lup-20(29)-ene-3,28-diol |
| 24 | 35.922 | 1.1 | Beta-Sitosterol |
Table 1: Major Chemical Constituents Identified by GC-MS Analysis of Et-Fs
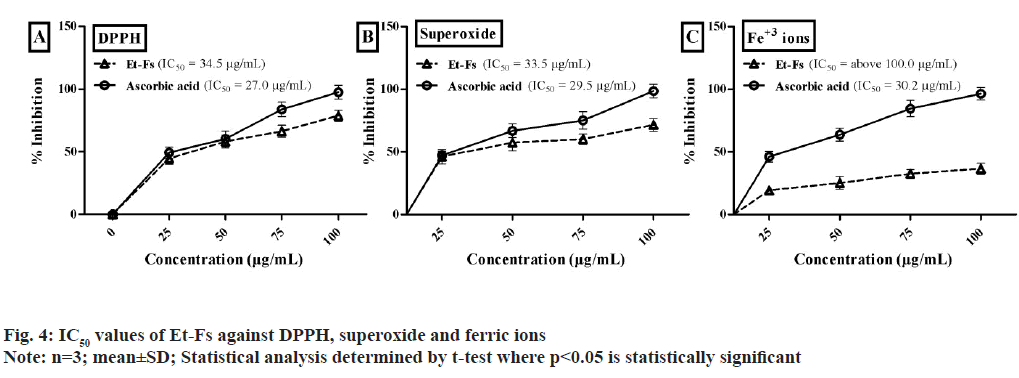
Based on the points mentioned above, Et-Fs exposed to an initial test against DPPH, superoxide, and Ferric ion assays illustrated in fig. 4. The IC50 values of Et-Fs on DPPH found to be 34.5 μg/ml, whereas standard (ascorbic acid) value was 27.0 μg/ml. The concentration of Et-Fs needed for 50 % inhibition of superoxide radical found to be 33.5 μg/ml, while ascorbic acid was 29.5 μg/ml. The IC50 values of Et- Fs found to be above 100.0 μg/ml whereas ascorbic acid value was 30.2 μg/ml (fig. 4).
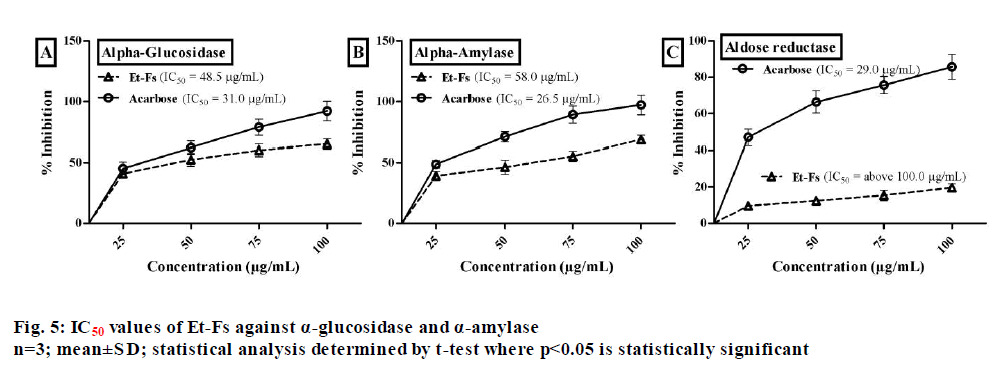
Natural sources with a high amount of phenolics which displaying effective antioxidant profile have recommended being useful in the treatment of diabetes. Generally, the main biological target in diabetes of phenolics is α-glycosidase and DPP-4, by acting as radical scavengers[17]. Therefore, as the moss F. secunda revealed good phenolics content with prominent antioxidant activity, we extended our study towards the management of diabetes. The inhibitory assay of α-glucosidase assessed by employing p-nitrophenyl-α-?-glucopyranoside and acarbose as a substrate and standard respectively. From the assay, it estimated that 50 % concentration needed for Et-Fs to inhibit α-glucosidase enzyme found to be 48.5 µg/ml, while standard (acarbose) was 31.0 µg/ml (fig. 5).
The porcine pancreatic α-amylase inhibitory assay performed by using BPNPG7 as a substrate. The concentration of Et-Fs needed for 50 % inhibition of porcine pancreatic α-amylase found to be 58.0 µg/ ml, while acarbose was 26.5 µg/ml (fig. 5). The effect of Et-Fs evaluated with aldose reductase enzyme assay by using the DL-glyceraldehyde substrate. The Et-Fs showed a very mild inhibitory profile against aldose reductase with IC50 values of above 100.0 µg/ml whereas acarbose value was 29.0 µg/ml (fig. 5). From the outcomes of in vitro assays, it has confirmed that the Et-Fs showed antidiabetic profile by inhibition of, particularly digestive enzymes.
To draw to a close, mosses poorly explored as much as their chemical and biological profiles. This present research study is the primary information on the chemical composition, antioxidant, α-glucosidase, α-amylase and aldose reductase inhibitory properties of F. secunda.
The examination of the extract reveals the existence of 200 components and all reported for the first time in F. secunda. The main phytochemicals identified from GC-MS analysis of Et-Fs are steroids, polyhydroxy compounds, and aliphatic fatty acids. Also, the chromatographic examination yielded three metabolites stigmasterol, β-sitosterol and lupeol. Additionally, the preliminary evaluation of its antioxidant and antidiabetic activities suggests that Et-Fs acting by suppression of free radicals (DPPH and superoxide), and particularly inhibiting the digestive enzymes. The outcomes of the chemical examination of the moss and its biological profile information display a variety of constituents, which could be isolated and examined to confirm the outcomes and know the mechanism of exactly how F. secunda reduced free radicals in diabetes.
Conflict of interest:
Authors of this manuscript declared that there is no conflict of interest.
References
- Peters K, Gorzolka K, Bruelheide H, Neumann S. Seasonal variation of secondary metabolites in nine different bryophytes. Ecol Evol 2018;8(17):9105-17.
[Crossref] [Google Scholar] [PubMed]
- Cheng X, Xiao Y, Wang X, Wang P, Li H, Yan H, et al. Anti-tumor and pro-apoptotic activity of ethanolic extract and its various fractions from Polytrichum commune L. ex Hedw in L1210 cells. J Ethnopharmacol 2012;143(1):49-56.
[Crossref] [Google Scholar] [PubMed]
- Singh M, Govindarajan R, Nath V, Rawat AK, Mehrotra S. Antimicrobial, wound healing and antioxidant activity of Plagiochasma appendiculatum Lehm. et Lind. J Ethnopharmacol 2006;107(1):67-72.
[Crossref] [Google Scholar] [PubMed]
- Klavina L, Springe G, Nikolajeva V, Martsinkevich I, Nakurte I, Dzabijeva D, et al. Chemical composition analysis, antimicrobial activity and cytotoxicity screening of moss extracts (moss phytochemistry). Molecules 2015;20(9):17221-43.
[Crossref] [Google Scholar] [PubMed]
- Pejin B, Bogdanovi?-Pristov J. ABTS cation scavenging activity and total phenolic content of three moss species. Hem Ind 2012;66:723-6.
- Tatipamula VB, Killari KN, Ketha A, Sastry VG. Taxithelium napalense acts against free radicals and diabetes mellitus. Bangladesh J Pharmacol 2017;12(2):197-203.
- Nath V, Asthana AK, Sahu V. Fabronia secunda Mont. A New addition to western Himalayas. Indian J Forestry 2007;30(3):353-4.
- Tatipamula VB, Killari KN, Gopaiah KV, Ketha A. GC-MS analysis of ethanol extract of Taxithelium napalense (Schwaerg) Broth along with its alpha-glucosidase inhibitory activity. Indian J Pharm Sci 2019;81(3):569-74.
- Bharadwaj V, Alekya K. Qualitative analysis and free radicals scavenging ability of marine algae, Chara baltica. J Integr Sci 2018:1-5.
- Haritha P, Patnaik SK, Tatipamula VB. Chemical and pharmacological evaluation of manglicolous lichen Graphis ajarekarii Patw. and CR Kulk. Vietnam J Sci Technol 2019;57:300-8.
- Tatipamula VB, Vedula GS, Sastry AV. Chemical and pharmacological evaluation of manglicolous lichen Roccella montagnei Bel em. DD Awasthi. Futur J Pharm Sci 2019;5:1-9.
- Tatipamula VB, Kolli MK, Lagu SB, Paidi KR, Reddy P R, Yejella RP. Novel indolizine derivatives lowers blood glucose levels in streptozotocin-induced diabetic rats: A histopathological approach. Pharmacol Rep 2019;71:233-42.
[Crossref] [Google Scholar] [PubMed]
- Tatipamula VB, Haritha P, Rao GS, Ketha A, Yejella PR. Isolation and Characterization of metabolites from Clathria procera Ridley extract and evaluation of its antidiabetic effects in Streptozotocin-induced diabetic rats. J Exp Appl Anim Sci. 2019;3(1):35-56.
- Talluri MR, Ketha A, Battu GR, Tadi RS, Tatipamula VB. Protective effect of Aurelia aurita against free radicals and streptozotocin-induced diabetes. Bangladesh J Pharmacol 2018;13:287-95.
- Huyut Z, Beydemir ?, Gülçin ?. Antioxidant and antiradical properties of selected flavonoids and phenolic compounds. Biochem Res Int 2017;2017:7616791.
[Crossref] [Google Scholar] [PubMed]
- Okpuzor J, Ogbunugafor H, Kareem GK, Igwo-Ezikpe MN. In vitro investigation of antioxidant phenolic compounds in extracts of Senna alata. Res J Phytochem 2009;3(4):68-76.
- Sarian MN, Ahmed QU, So’ad M, Zaiton S, Alhassan AM, Murugesu S, et al. Antioxidant and antidiabetic effects of flavonoids: A structure-activity relationship based study. Biomed Res 2017;1-14.
[Crossref] [Google Scholar] [PubMed]