- *Corresponding Author:
- S. Yuan
Orthopedics department,
Zhuji People’s Hospital,
Zhejiang 312500,
China
E-mail: guqingjushi@126.com
| This article was originally published in a special issue, “New Advancements in Biomedical and Pharmaceutical Sciences” |
| J Pharm Sci 2022:84(2) Spl issue “207-211” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Although ginsenoside Rg3 has been shown to exert anticancer effects on a variety of malignancies, the effects and molecular mechanisms of ginsenoside Rg3 on oesophageal cancer remain unclear. Therefore, these factors must be explored. Here, we analyzed the effect of ginsenoside Rg3 on oesophageal cancer cell proliferation and migration, as well as its effect on apoptosis. Furthermore, we detected downstream pathways that ginsenoside Rg3 could act on. The results showed that ginsenoside Rg3 significantly inhibited cell proliferation and migration and promoted apoptosis. We found that ginsenoside Rg3 regulates the phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin pathway in oesophageal cancer development. In general, ginsenoside Rg3 inhibits the proliferation and migration of oesophageal cancer cells via induction of apoptosis and down regulation of the phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin pathway. This study aimed to suggest new treatment options for oesophageal cancer.
Keywords
Oesophageal cancer, ginsenoside Rg3, proliferation, migration, apoptosis
Oesophageal Cancer (OC) remains an important cause of cancer-related deaths and is one of the most aggressive malignancies worldwide [1]. Oesophageal Squamous Cell Carcinoma (OSCC) is highly prevalent in East Asia, eastern and southern Africa and southern America [2]. OC is the sixth leading cause of cancerrelated death worldwide, with a 5 y overall survival rate of only 20 %-40 % [3]. Chemotherapy is one of the most important treatments for patients with advanced OC [4]. Although chemotherapeutic drugs can interfere with the nucleic acid metabolism of tumor cells and inhibit their proliferation, invasion and migration, they are also toxic to normal cells, causing serious adverse reactions and even endangering the lives of patients, severely limiting their clinical application [5,6].
Compared with chemical and biological drugs, Traditional Chinese Medicine (TCM) has the unique advantages of being comprised of multiple components, having multiple targets and causing fewer adverse reactions when treating diseases. Ginsenoside Rg3, a steroidal saponin derivative, is abundant in hotprocessed ginseng extracts [7,8]. Ginsenoside Rg3 has powerful anticancer properties and is known to regulate a variety of cellular events, such as cell proliferation, immune response, autophagy, metastasis and angiogenesis [9]. Ginsenoside Rg3 inhibits angiogenesis, induces tumor cell apoptosis, selectively inhibits tumor cell metastasis, and enhances immune function [10]. Previous studies have shown that ginsenoside Rg3 can inhibit the proliferation, invasion and migration of human tumor cells such as lung cancer [11], gastric cancer [12], prostate cancer [13] and colon cancer [14].
There is growing evidence of the role of ginsenoside Rg3 in cancer prevention. However, the anti-OC effect of ginsenoside Rg3 and its underlying mechanism remain unclear. Therefore, the purpose of this study was to investigate the antitumor effect of ginsenoside Rg3 on OC cell lines and to explore the molecular mechanism underlying its antitumor effect. In the present study, we identified and evaluated the potential role of ginsenoside Rg3 and revealed the potential molecular mechanism of its role in providing information for the diagnosis and treatment of OC.
Materials and Methods
Cell lines and culture:
The OSCC cell lines Esophageal Carcinoma (ECA109) and Terminal Equipment-1 (TE-1) were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences and cultured in Roswell Park Memorial Institute (RPMI) 1640 with 10 % Fetal Bovine Serum (FBS) (GIBCO, Carlsbad, CA, USA) and 1 % penicillin/streptomycin at 37° in a 5 % Carbon Dioxide (CO2) humidified incubator.
Flow cytometry:
The collected cells were used for apoptosis detection. The cells were harvested and stained with Propidium Iodide (PI) in accordance with the instructions of the Fluorescein Isothiocyanate (FITC) Annexin V Apoptosis Assay Kit (BD Biosciences, USA). The cells were fixed in 75 % cold ethanol for 24 h for periodic detection.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay:
The viability of ECA109 and TE-1 cells was determined using the MTT assay [15]. First, cells were treated with 6.25, 12.5, 25, 50 or 100 μg/ml of ginsenoside Rg3. A total of 2×103 cells/well were then inoculated in a 96-well culture plate containing the indicated medium (RPMI1640 plus 10 % FBS). After 4 h of culture, the MTT cell viability assay kit (thermo fisher scientific, USA) was used according to the manufacturer’s protocol. The Optical Density (OD) value was determined at 0 h, 12 h, 24 h and 48 h using a micro plate reader to evaluate the absorbance at 570 nm.
Wound assay:
Cell migration was detected using a wound assay. The cells were inoculated on 6-well plates (1×106 cells) and the single cell layer was scratched with a 200 μl pipette tip. The cells were washed with Phosphate Buffered Saline (PBS) three times to remove cell fragments. RPMI1640 without FBS was added to each well and incubated at 37° and 5 % CO2 for 48 h. Wound images were observed using a microscope at the same scratch location at 0 h, 24 h and 48 h.
Hoechst 33342 nuclear staining analysis:
Hoechst 33342 staining was used to assess changes in nuclear morphology. Briefly, the treated cells were stained with Hoechst 33342 dye (5 μg/ml) for 20 min in the dark at room temperature. The Hoechst stained nuclei were observed using NiKon ECLIPSE Ci after washing with PBS twice.
Western blot:
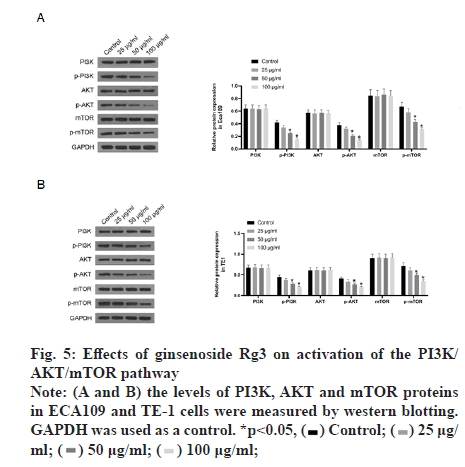
Briefly, the protein was extracted from cells using Radioimmuneprecipitation Assay (RIPA) lysis buffer (Thermo Fisher Scientific) and quantified using a Bicinchoninic Acid (BCA) kit (Beyotime Biotechnology). The proteins were separated on a 10 % Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking with Tris- Buffered Saline and Tween (TBST) containing 5 % skimmed milk, the blots were incubated overnight at 4° with a primary antibody (p-Akt (1:1000; Proteintech), p-PI3K (1:1000; Cell Signalling Technology), p-mTOR (1:1000; Proteintech) and GAPDH (1:1000; Beyotime Biotechnology) and then incubated with an Horseradish Peroxidase (HRP)-conjugated secondary antibody (1:10 000, Solarbio, Beijing, China). A chemiluminescence detection system (Millipore, Billerica, MA, USA) was used to visualise the results and the bands were quantified using quantity one software (Bio-Rad, Hercules, CA, USA).
Statistical analysis:
Data are expressed as mean±Standard Deviation (SD). Student’s t-test was used for between-group comparisons. Differences between groups were analyzed using one-way analysis of variance. Differences were considered statistically significant at p<0.05. All statistical analyses were performed using Graph Pad Prism 5 (Graph Pad Software, La Jolla, CA, USA).
Results and Discussion
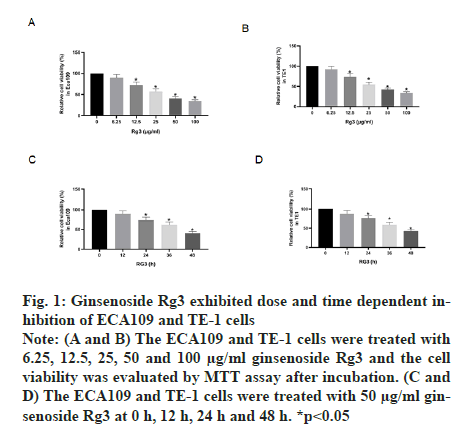
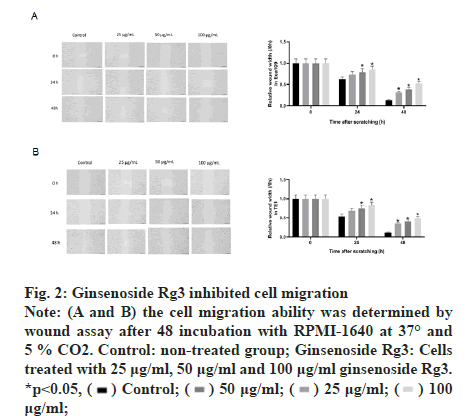
The MTT assay was performed after treatment with different doses of ginsenoside Rg3. The results showed that ECA109 and TE-1 cell viability decreased with an increase in Rg3 dose. Thus, we chose 50 μg/ml ginsenoside Rg3 for subsequent experiments (fig. 1A and fig. 1B). In addition, ECA109 and TE-1 cell viability decreased gradually over time (0 h, 12 h, 24 h, 36 h and 48 h) (fig. 1C and fig. 1D). The ability of tumour cells to migrate is the key to tumour progression. From the wound assay, the cell migration rate in the ginsenoside Rg3 treatment group was lower than that in the Normal Control (NC) group, indicating that ginsenoside Rg3 reduced the migration of ECA109 and TE-1 cells (fig. 2).
Figure 1: Ginsenoside Rg3 exhibited dose and time dependent inhibition of ECA109 and TE-1 cells Note: (A and B) The ECA109 and TE-1 cells were treated with 6.25, 12.5, 25, 50 and 100 μg/ml ginsenoside Rg3 and the cell viability was evaluated by MTT assay after incubation. (C and D) The ECA109 and TE-1 cells were treated with 50 μg/ml ginsenoside Rg3 at 0 h, 12 h, 24 h and 48 h. *p<0.05
Figure 2: Ginsenoside Rg3 inhibited cell migration Note: (A and B) the cell migration ability was determined by wound assay after 48 incubation with RPMI-1640 at 37° and 5 % CO2. Control: non-treated group; Ginsenoside Rg3: Cells treated with 25 μg/ml, 50 μg/ml and 100 μg/ml ginsenoside Rg3. *p<0.05,  μg/ml;
μg/ml;
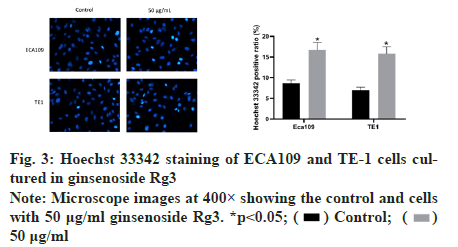
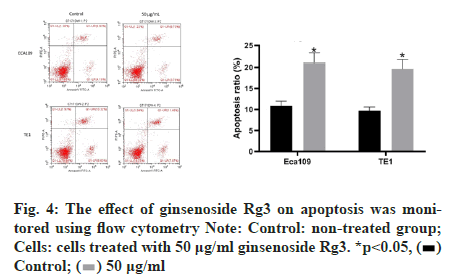
Subsequently, we examined the effect of ginsenoside Rg3 on the apoptosis of ECA109 and TE-1 cells. Hoechst 33342 staining showed weak fluorescence in the nuclei of normal cells. In contrast, the chromatin of cells treated with 50 μg/ml ginsenoside Rg3 was significantly condensed, with some chromatin accumulating on the nuclear membrane in a typical half-moon shape (fig. 3). In addition, flow cytometry showed that apoptosis of the cancer cells treated with ginsenoside Rg3 was significantly higher than that in the control group (fig. 4). These results indicate that ginsenoside Rg3 promoted apoptosis. The Phosphatidylinositol- 3-Kinase/protein kinase B/mammalian Target of Rapamycin (PI3K/Akt/mTOR) signalling pathway is involved in the proliferation, invasion and migration of cancer cells and its abnormal activation can lead to high proliferation, invasion and migration of cancer cells. Western blot analysis showed that the levels of PI3KAkt and mTOR expression in ECA109 and TE-1 cells were significantly lower following ginsenoside Rg3 treatment than those in the control group (fig. 5). The results indicated that ginsenoside Rg3 could effectively inhibit the expression of PI3K-Akt and mTOR.
Figure 3: Hoechst 33342 staining of ECA109 and TE-1 cells cultured in ginsenoside Rg3 Note: Microscope images at 400× showing the control and cells with 50 μg/ml ginsenoside Rg3. *p<0.05;  50 μg/ml
50 μg/ml
Figure 4: The effect of ginsenoside Rg3 on apoptosis was monitored using flow cytometry Note: Control: non-treated group; Cells: cells treated with 50 μg/ml ginsenoside Rg3. *p<0.05, 
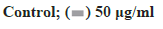
Ginsenoside Rg3 is one of the main active ingredients extracted from ginseng. Studies have shown that ginsenoside Rg3 has an inhibitory effect on a variety of cancers [16]. Ginsenoside Rg3 can improve the sensitivity of chemotherapy, reduce adverse reactions and enhance the immunity of tumour-bearing models. In addition, ginsenoside Rg3 is a beneficial adjuvant for traditional cancer treatment. The results of this study indicated that ginsenoside Rg3 could affect the proliferation, migration and survival of OC cells. The results of proliferation and wound assays showed that 50 μg/ml ginsenoside Rg3 promoted the apoptosis of OC cells and significantly inhibited the migration and invasion of tumour cells. Therefore, ginsenoside Rg3 is a potential antimetastatic agent for OC.
In this study, we found that ginsenoside Rg3 inhibited EC cell proliferation. Tumour growth, development and metastasis are closely related to cell proliferation. Studies have shown that mTOR is the centre of the autophagy-regulated cascade and it is directly related to the PI3K/Akt pathway [19]. Moreover, previous studies have shown that ginsenoside Rg3 can inhibit the proliferation of human osteosarcoma by regulating PI3K/Akt/mTOR [20]. However, there is no report on whether ginsenoside Rg3 can attenuate OC via the PI3K/Akt/mTOR signalling pathway.
In the current study, ginsenoside Rg3 reduced the phosphorylation of mTOR, suggesting that ginsenoside Rg3 inhibited the PI3K/Akt pathway in EC cells. In addition, ginsenoside Rg3 may inhibit the proliferation, migration and apoptosis of OC cells by inhibiting the PI3K/Akt/mTOR pathway. Collectively, these results suggest that ginsenoside Rg3 can trigger apoptosis and inhibit proliferation and slow migration in EC cells by regulating the PI3K/Akt/mTOR pathway.
However, due to the limitation of time and resources, there are some deficiencies in our experimental design. The ginsenoside Rg3 has multiple targets in cancer and many of these targets may have the same or opposite effect on cancer. In our study, Rg3-targeted non-coding Ribonucleic Acids (ncRNAs) were not analysed to explore how it regulates OC cell capacity and apoptosis. In addition, in vivo experiments of Rg3 for OC were lacking. Therefore, these will be further explored in future studies.
In conclusion, this study for the first time found that ginsenoside Rg3 had a significant inhibitory effect on OC cell proliferation. 50 μg/ml ginsenoside Rg3 could inhibit OC cell metastasis and promote cell apoptosis. In addition, this study also demonstrated that ginsenoside Rg3 mitigated the carcinogenic behaviour of OC cells by inhibiting the PI3K/Akt signalling pathway. This study laid a theoretical foundation for the further promotion and application of ginsenoside Rg3 and provided a new idea for the treatment of OC.
Acknowledgements
This work was financially supported by the Clinical Research Fund project of Zhejiang Medical Association, China (Grant No. 2021ZYC-A193).
Conflict of Interests
The authors declare that they have no conflicts of interest or competing interests.
References
- Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin 2012;62(2):118-28.
[Crossref] [Google Scholar] [Pub Med]
- Pickens A, Orringer MB. Pickens A, Orringer MB. Geographical distribution and racial disparity in esophageal cancer. Ann Thoracic Surg 2003;76(4):S1367-9.
[Crossref] [Google Scholar] [Pub Med]
- Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391(10125):1023-75.
[Crossref] [Google Scholar] [Pub Med]
- Huang FL, Yu SJ. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg 2018;41(3):210-5.
[Crossref] [Google Scholar] [Pub Med]
- Li J, Hou N, Faried A, Tsutsumi S, Kuwano H. Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur J Cancer 2010;46(10):1900-9.
[Crossref] [Google Scholar] [Pub Med]
- Sanoff HK, Carpenter WR, Freburger J, Li L, Chen K, Zullig LL, et al. Comparison of adverse events during 5‐fluorouracil vs. 5‐fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: A population‐based analysis. Cancer 2012;118(17):4309-20.
[Crossref] [Google Scholar] [Pub Med]
- Liu T, Duo L, Duan P. Ginsenoside Rg3 sensitizes colorectal cancer to radiotherapy through downregulation of proliferative and angiogenic biomarkers. Evid Based Complement Alternat Med 2018;2018.
[Crossref] [Google scholar] [Pub Med]
- Bai CX, Sunami A, Namiki T, Sawanobori T, Furukawa T. Electrophysiological effects of ginseng and ginsenoside Re in guinea pig ventricular myocytes. Eur J Pharmacol 2003;476(1-2):35-44.
[Crossref] [Google Scholar] [Pub Med]
- Sun HY, Lee JH, Han YS, Yoon YM, Yun CW, Kim JH, et al. Pivotal roles of ginsenoside Rg3 in tumor apoptosis through regulation of reactive oxygen species. Anticancer Res 2016;36(9):4647-54.
[Crossref] [Google Scholar] [Pub Med]
- Tang YC, Zhang Y, Zhou J, Zhi Q, Wu MY, Gong FR, et al. Ginsenoside Rg3 targets cancer stem cells and tumor angiogenesis to inhibit colorectal cancer progression in vivo. Int J Oncol 2018;52(1):127-38.
[Crossref] [Google Scholar] [Pub Med]
- Wang J, Tian L, Khan MN, Zhang L, Chen Q, Zhao Y, et al. Ginsenoside Rg3 sensitizes hypoxic lung cancer cells to cisplatin via blocking of NF-κB mediated epithelial-mesenchymal transition and stemness. Cancer Lett 2018;415:73-85.
[Crossref] [Google Scholar] [Pub Med]
- Kim BJ, Nah SY, Jeon JH, So I, Kim SJ. Transient receptor potential melastatin 7 channels are involved in ginsenoside Rg3‐induced apoptosis in gastric cancer cells. Basic Clin Pharmacol Toxicol 2011;109(4):233-9.
[Crossref] [Google Scholar] [Pub Med]
- Kim SM, Lee SY, Cho JS, Son SM, Choi SS, Yun YP, et al. Combination of ginsenoside Rg3 with docetaxel enhances the susceptibility of prostate cancer cells via inhibition of NF-κB. Eur J Pharmacol 2010;631(1-3):1-9.
[Crossref] [Google Scholar] [Pub Med]
- Hong S, Cai W, Huang Z, Wang Y, Mi X, Huang Y, et al. Ginsenoside Rg3 enhances the anticancer effect of 5‑FU in colon cancer cells via the PI3K/Akt pathway. Oncol Rep 2020;44(4):1333-42.
[Crossref] [Google Scholar] [Pub Med]
- Kumar P, Nagarajan A, Uchil PD. Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb Protoc 2018;2018(6):095497.
[Crossref] [Google Scholar] [Pub Med]
- Pan L, Zhang T, Sun H, Liu G. Ginsenoside Rg3 (Shenyi capsule) combined with chemotherapy for digestive system cancer in China: A meta-analysis and systematic review. Evid Based Complement Alternat Med 2019;2019:2417418.
[Crossref] [Google Scholar] [Pub Med]
- Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res 2014;115(1):148-64.
[Crossref] [Google Scholar] [Pub Med]
- Shi Y, Fang N, Li Y, Guo Z, Jiang W, He Y, et al. Circular RNA LPAR3 sponges microRNA‐198 to facilitate esophageal cancer migration, invasion, and metastasis. Cancer Sci 2020;111(8):2824-36.
[Crossref] [Google Scholar] [Pub Med]
- Yu X, Li R, Shi W, Jiang T, Wang Y, Li C, et al. Silencing of MicroRNA-21 confers the sensitivity to tamoxifen and fulvestrant by enhancing autophagic cell death through inhibition of the PI3K-AKT-mTOR pathway in breast cancer cells. Biomed Pharmacother 2016;77:37-44.
[Crossref] [Google Scholar] [Pub Med]
- Li Y, Lu J, Bai F, Xiao Y, Guo Y, Dong Z. Ginsenoside Rg3 suppresses proliferation and induces apoptosis in human osteosarcoma. Biomed Res Int 2018;2018.
[Crossref] [Google Scholar] [Pub Med]