- *Corresponding Author:
- Dongxu Kang
Department of Oncology
Affiliated Hospital of Yanbian University
Yanji, Jilin Province 133000, P. R. China
E-mail: zhangbaolian0323@163.com
| This article was originally published in a special issue, “New Advancements in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2021:84(1) Spl Issue “84-91” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Hepatocellular carcinoma is one of the most common malignances worldwide. Sorafenib, a multikinase inhibitor, is the standard therapy for patients with advanced-stage hepatocellular carcinoma. Therefore, it is essential to develop more effective therapeutic strategies to overcome resistance and improve the efficacy of sorafenib in treating hepatocellular carcinoma patients. In this study, we demonstrate that sorafenib chemotherapy combined with gene therapy, which expressed short hairpin ribonucleic acid against heat shock protein 27, effectively overcomes the sorafenib resistance of hepatocellular carcinoma cells and improves the anti-tumor effect of sorafenib. This combinational therapy will be a new hope for hepatocellular carcinoma patients who have sorafenib resistance, can be a better therapeutic strategy to control the malignant liver cancer. Our study provides a new therapeutic strategy for hepatocellular carcinoma that not only overcomes the resistance of hepatocellular carcinoma to sorafenib, but also utilizes cutting edge of gene therapies.
Keywords
Hepatocellular carcinoma, sorafenib, resistance, heat shock protein 27
Hepatocellular carcinoma (HCC) is one of the most common malignancies across the globe, especially in Asia and Southern Africa[1]. Via a diagnosis followed by systemic examination, HCC can be classified into early, mid and late (advanced) stages. Surgical resection, liver transplantation[2] or local ablation, have been generally used to treat early- and mid-stage HCC, where the 5 y survival rate could be as high as to 60 %~70 %. However, owing to the lack of effective treatment options and underlying liver disease, patients who are diagnosed at an advanced stage or with progression experience a much more dismal prognosis after locoregional therapy[3]. Until sorafenib was used as a first-line therapy, there was no systemic therapy to improve survival in patients with late-stage HCC[4-6].
Sorafenib, which has anti-proliferative and anti-angiogenic effects, is an oral multikinase inhibitor. It can inhibit several cellular signaling pathways, including those of Raf/Mitogen-Activated Protein Kinase (MAPK), Extracellular Signal-Regulated Kinase (ERK), as well as the receptors of Vascular Endothelial Growth Factor (VEGF) and Platelet-Derived Growth Factor (PDGF). As the standard care for patients with advanced HCC, sorafenib has been proven to have the capacity to inhibit HCC cell proliferation, angiogenesis and so on[7-9]. However, the promising treatment of HCC with sorafenib has limited benefits to survival and very low rates of tumor response, where some patients with HCC even exhibit no initial response to sorafenib[3,10], indicating the existence of both primary and acquired resistances of HCC cells to sorafenib[11]. The primary resistance of HCC to sorafenib has been shown to be attributed to genetic heterogeneity. While sorafenib inhibits several cellular signaling pathways as a multikinase inhibitor, it simultaneously or sequentially activates the addiction switches and compensatory pathways. As for the acquired resistance of HCC cells to sorafenib, although several mechanisms have been proposed, the exact mechanisms underlying sorafenib resistance in HCC cells remain unclear[12,13].
Heat shock protein 27 (HSP27), an important chaperone molecule, belongs to the HSP family, which is responsible for modulating more than 200 client proteins[14]. The most common functions of HSP27 are chaperone activity, thermotolerance, inhibition of apoptosis, regulation of cell development and cell differentiation. It has also been reported that HSP27 functions as a pro-metastatic protein in different types of cancer and is closely linked to the aggressiveness of tumor behavior, metastasis and poor prognosis[15,16]. HSP27 is overexpressed in many different types of human cancers, including prostate, ovarian, gastric, breast, and liver cancers. HSP27 accumulation has been shown to reduce the chemosensitivity induced by vincristine and adriamycin in gastric cancer cells[17]. In contrast, a combination of traditional chemotherapeutic agents (cisplatin and gemcitabine) and an HSP27 inhibitor (quercetin) exerts a surprisingly greater chemotherapeutic effect in lung stem-like cells[18]. In addition, a recent study from our laboratory showed that a reduction in HSP27 expression, delivered by an adenoviral vector containing small hairpin HSP27 (shHSP27), can sensitize gemcitabine-induced cell death in pancreatic cancer cells.
In this research, we designed adenoviral vector delivered HSP27 short hairpin Ribonucleic Acid (shRNA) to knock down HSP27 expression, respectively. The subsequent reductions in HSP27 expression result in an increase p38 activity, not only to activate the cellular death signal, but also to synchronously reduce the ability of HSP27 to protect cells, where an increase in HSP27 phosphorylation resulted in cell death cooperatively. When HSP27 shRNA was combined with sorafenib, HCC cells were no longer resistant to sorafenib and the efficiency of sorafenib against advanced HCC was significantly increased.
Materials and Methods
Cell culture:
Hepatitis B virus positive and tumorigenic (Hep-3B), Hepatocyte-derived carcinoma cell line (Huh-7) and Immortal, Human Hepatic adenocarcinoma cell line (SK-Hep-1) cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) with 10 % Fetal Bovine Serum (FBS). Seoul National University (SNU)-182, SNU- 398 and SNU-449 cells were cultured in RPMI with 10 % FBS. Cells were maintained in a 37° humidified atmosphere containing 5 % carbon dioxide.
Construction and recombinant adenoviral vectors:
Construction of adenoviral vectors: For the expression of small interfering RNA (siRNAs) targeting human HSP27, shRNA construct was cloned into a pSP72ΔE3-U6/H1 vector using BamHI/HindIII digestion. These vectors, designated and pSP72ΔE3- H1-shhHSP27 (E3 shuttle vector), were linearized by XmnI digestion and co-transformed into Escherichia coli BJ5183 together with the SpeI-digested adenoviral vector (dl324-IX) for homologous recombination.
Recombinant adenoviral vectors: NC: Ad-IX- ΔE1-ΔE3, control; shHSP27: Ad-IX-ΔE1-ΔE3-H1- shHSP27, expressing shRNA of Human HSP27 (shHSP27)
MTS viability assays:
The CellTiter 96® Aqueous Assay kit (Promega, Madison, WI, USA) is composed of solutions of a novel tetrazolium compound MTS ((3-(4,5-dimethylthiazol- 2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)- 2H-tetrazolium), inner salt; an electron coupling reagent (phenazine ethosulfate; Polyethersulfone (PES)). MTS is bioreduced by cells into a formazan product that is soluble in tissue culture media. Subsequently, HCC cell lines were treated with varying doses of sorafenib (0, 2.5, 5, 10, 15, 20 and 25 μM) to in a 96- well plate for 24 h. The absorbance of formazan at 490 nm can be measured directly from 96-well plates without additional processing. The conversion of MTS into aqueous, soluble formazan is accomplished by dehydrogenase enzymes found in metabolically active cells. The quantity of formazan product measured at 490 nm is directly proportional to the number of living cells in culture.
Western blot analysis:
Cells were lysed with 1x Laemmli sample buffer (62.5 mM Tris, pH 6.8, 2 % sodium dodecyl sulfate, 10 % glycerol, 0.002 % bromophenol blue) and protein concentration was determined using the Bicinchoninic Acid (BCA) Protein Assay Kit (Thermo Scientific, Fremont, CA, USA). Then, protein samples were separated by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS PAGE) and gels were electro-transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). Immunodetection was performed using anti-phosho Protein Kinase B (AKT) (p-AKT), anti-phosho p65 (p-p65), anti-phosho p38 (p-p38), anti-phosho HSP27 (p-HSP27), anti-p38, anti-HSP27 and anti-Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) primary antibodies, with the chemiluminescent and fluorescent image analysis system (Syngene, Cambridge, UK).
Clonogenic assays:
Cells were plated into six-well plates at a density of 1×105 cells/well. Subsequently, HCC cell lines were treated with sorafenib (24 h) at a variety of concentrations (0, 2.5, 5, 10, 15 or 20 μM) or treated with 2.5 μM sorafenib (12 h) followed by a pre-treatment with adenoviral vectors (NC, shHSP27) for 24 h. Cells were trypsinized and plated into six-well plates at densities of 5×103 or 1×104 cells/well. Cells were subsequently monitored daily by microscopy. When cells exhibited colonies, surviving cells were fixed with 4 % paraformaldehyde and stained with 0.5 % crystal violet.
Statistical analysis:
The data are expressed as mean±Standard Error (SE). Statistical comparisons (Students t-test) were made using GraphPad (Systat Software, Inc., Chicago, IL, USA). p<0.05 were considered statistically significant (*p<0.05; **p<0.01;***p<0.001).
Results and Discussion
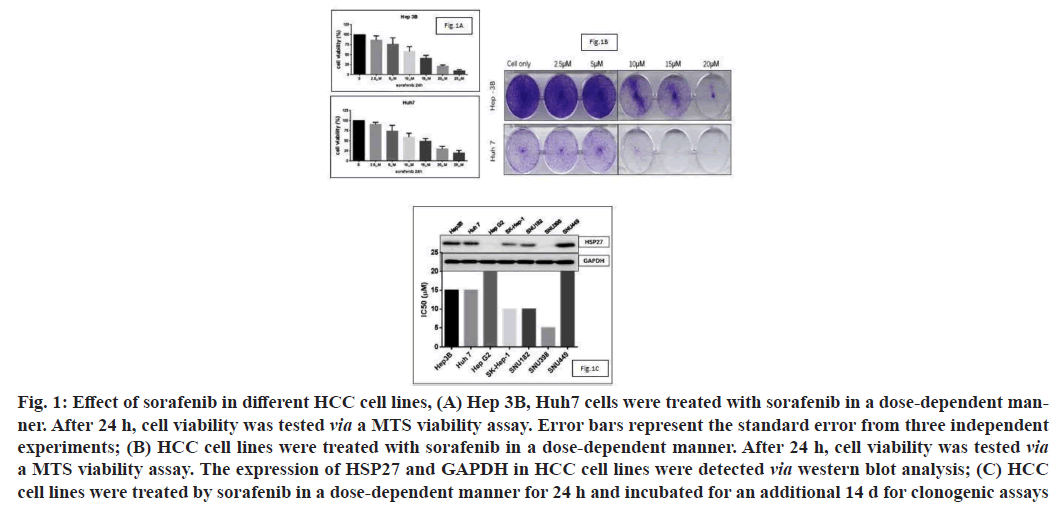
Sorafenib is a standard chemotherapy treatment for advanced HCC patients and can target many different signaling pathways. To evaluate the impact of sorafenib in vitro, we first identified the Half-maximal Inhibitory Concentration (IC50) of sorafenib in different HCC cell lines. As the MTS assay results showed that the IC50 of sorafenib in HCC cell lines ranged from 10 μM to 15 μM (fig. 1A). We then performed a clonogenic assay to confirm the cell viability of HCC cells after dose-dependent treatments with sorafenib (fig. 1B). We screened HSP27 expression in HCC cell lines and analyzed its relationship with the IC50 of sorafenib (fig. 1C). As expected, we observed that cell lines with elevated levels of HSP27 expression usually had low sensitivities to sorafenib, indicating that there is a negative correlation between HSP27 expression and the IC50 of sorafenib. Suggesting that it may play a role in inducing resistance to sorafenib in HCC cell lines.
Fig. 1: Effect of sorafenib in different HCC cell lines, (A) Hep 3B, Huh7 cells were treated with sorafenib in a dose-dependent manner. After 24 h, cell viability was tested via a MTS viability assay. Error bars represent the standard error from three independent experiments; (B) HCC cell lines were treated with sorafenib in a dose-dependent manner. After 24 h, cell viability was tested via a MTS viability assay. The expression of HSP27 and GAPDH in HCC cell lines were detected via western blot analysis; (C) HCC cell lines were treated by sorafenib in a dose-dependent manner for 24 h and incubated for an additional 14 d for clonogenic assays
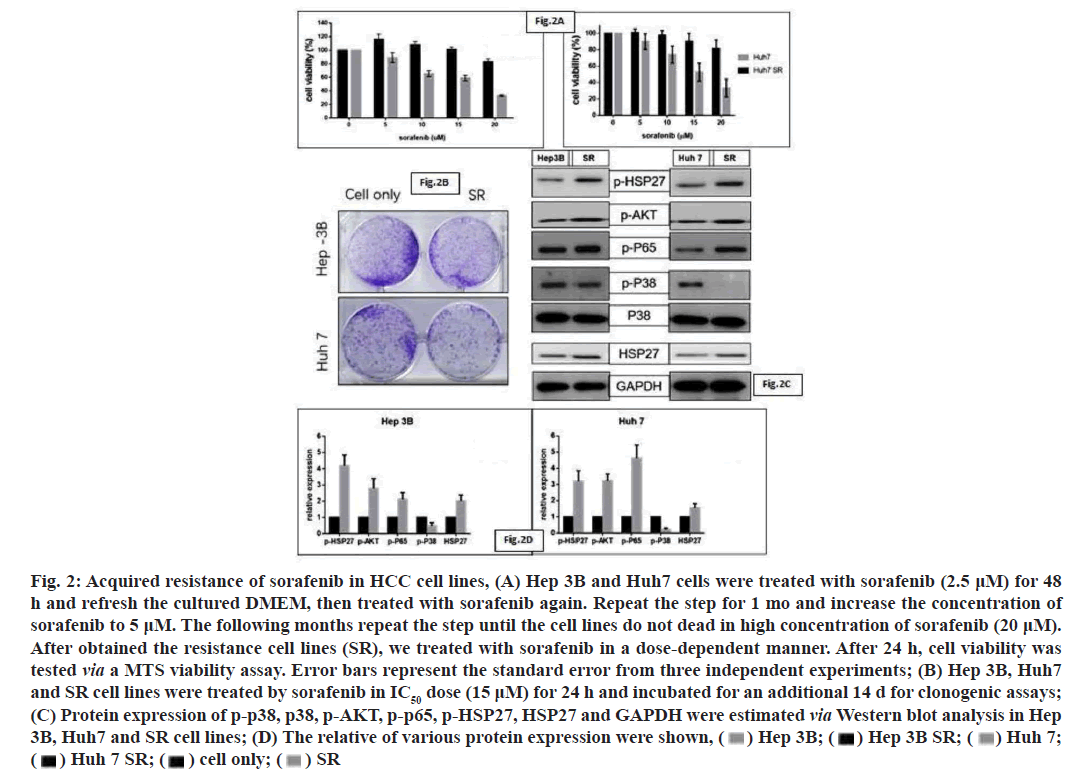
In this study, we treated the HCC cell lines with low dose of sorafenib and increased the concentration of sorafenib per month; finally obtain the cell lines with acquired resistance to sorafenib. As the MTS assay results showed that the IC50 of Sorafenib in Resistance (SR) cell lines were markedly increased than cell lines with no resistance (fig. 2A). We then performed a clonogenic assay to confirm the resistance of sorafenib in HCC cell lines (fig. 2B). Then we also screened the expression of various key signaling pathway molecules, including p38, p-p38, HSP27, p-HSP27, p-p65 and p-AKT via Western blot analyses, in wild type and SR cell lines. We observed that the expression of pAKT, P-p65 and pHSP27 were increased by SR condition (fig. 2C and fig. 2D). The activity of p-p38 was decreased, which may inhibit the death-related signaling pathway (fig. 2C and fig. 2D).
Fig. 2: Acquired resistance of sorafenib in HCC cell lines, (A) Hep 3B and Huh7 cells were treated with sorafenib (2.5 ?M) for 48
h and refresh the cultured DMEM, then treated with sorafenib again. Repeat the step for 1 mo and increase the concentration of
sorafenib to 5 ?M. The following months repeat the step until the cell lines do not dead in high concentration of sorafenib (20 ?M).
After obtained the resistance cell lines (SR), we treated with sorafenib in a dose-dependent manner. After 24 h, cell viability was
tested via a MTS viability assay. Error bars represent the standard error from three independent experiments; (B) Hep 3B, Huh7
and SR cell lines were treated by sorafenib in IC50 dose (15 ?M) for 24 h and incubated for an additional 14 d for clonogenic assays;
(C) Protein expression of p-p38, p38, p-AKT, p-p65, p-HSP27, HSP27 and GAPDH were estimated via Western blot analysis in Hep
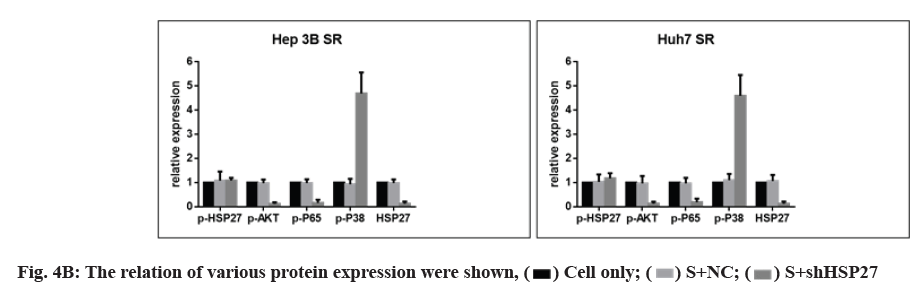
3B, Huh7 and SR cell lines; (D) The relative of various protein expression were shown, 
 SR
SR
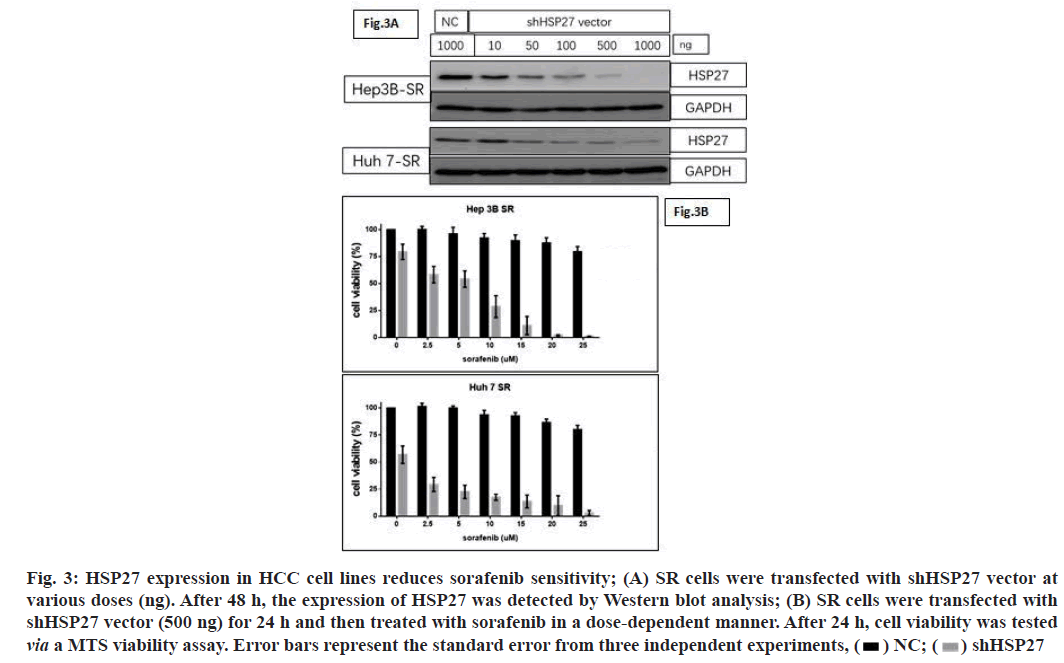
As our previous study reported, the proportion of pHSP27 and HSP27 is a key biological switch, which modulates cell survival and death in pancreatic cancer cell lines. In this study, we designed plasmid vector expressing scrambled sequences, HSP27 shRNA. After confirming the HSP27 downregulation by vector expressing shRNA of HSP27 in dose-dependent manner (fig. 3A). We next observed decreased cell viability when HSP27 was down regulated in SR cells, after sorafenib treatment (fig. 3B). Therefore, HSP27 may have a significant function in intrinsic HCC sorafenib resistance, thus providing a platform for further elucidation of the underlying mechanisms of intrinsic drug resistance.
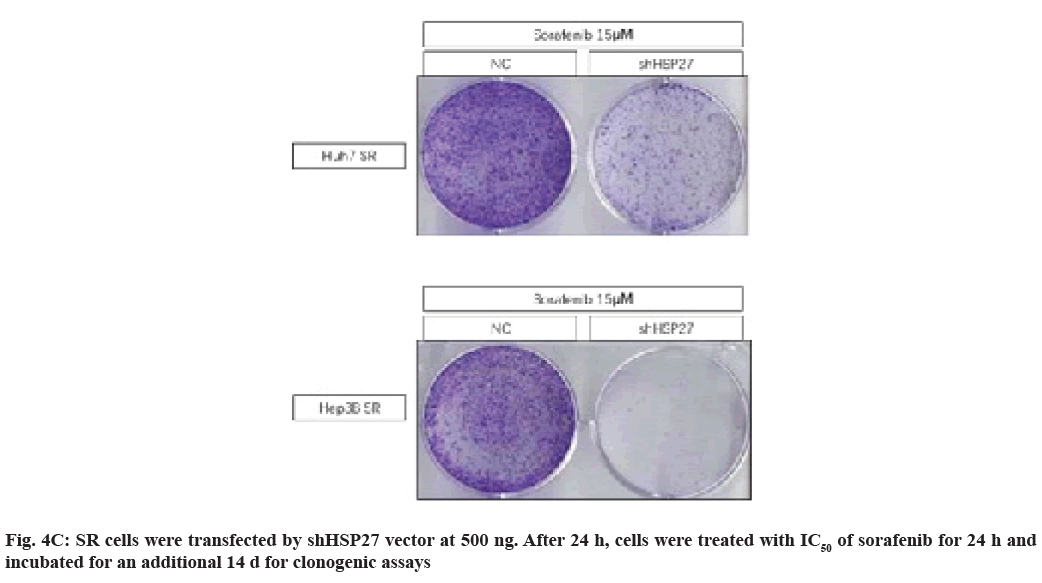
Fig. 3: HSP27 expression in HCC cell lines reduces sorafenib sensitivity; (A) SR cells were transfected with shHSP27 vector at
various doses (ng). After 48 h, the expression of HSP27 was detected by Western blot analysis; (B) SR cells were transfected with
shHSP27 vector (500 ng) for 24 h and then treated with sorafenib in a dose-dependent manner. After 24 h, cell viability was tested
via a MTS viability assay. Error bars represent the standard error from three independent experiments, 
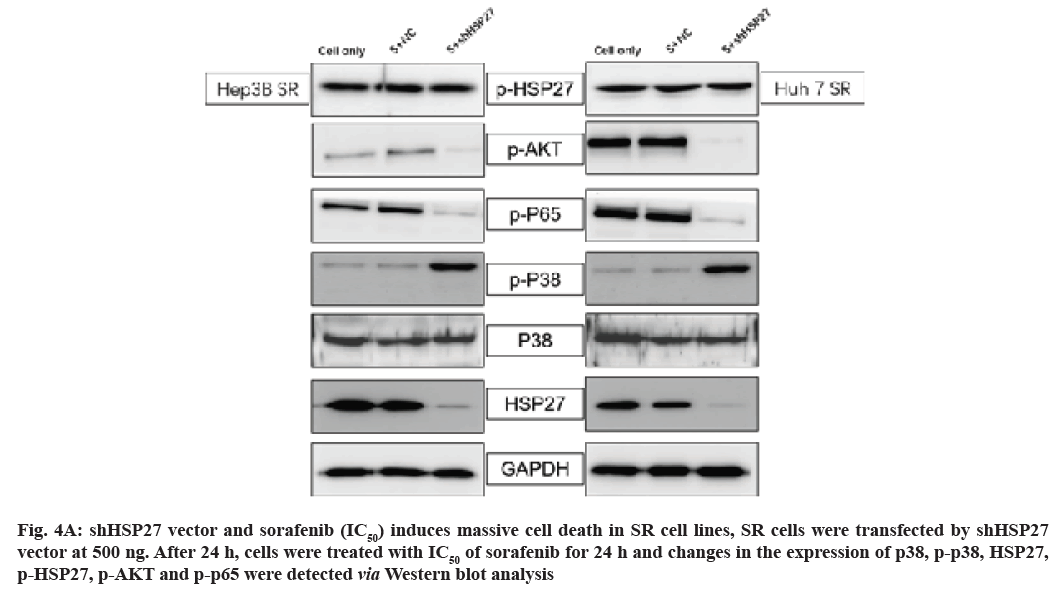
Next, we investigated whether HSP27 down regulation reduced the activity of p-AKT/p-p65 in SR cell lines combined with sorafenib treatment by Western blot. HSP27 down regulation did decrease the phosphorylation of AKT/p65 and the level of p-p38 was increased (fig. 4A and fig. 4B). However, the phosphorylation of HSP27 has no change in HSP27 down regulation condition combined with sorafenib treatment (fig. 4A and fig. 4B).
Fig. 4A: shHSP27 vector and sorafenib (IC50) induces massive cell death in SR cell lines, SR cells were transfected by shHSP27 vector at 500 ng. After 24 h, cells were treated with IC50 of sorafenib for 24 h and changes in the expression of p38, p-p38, HSP27, p-HSP27, p-AKT and p-p65 were detected via Western blot analysis
This suggests that an increase in the activity of p-HSP27 and/or knock down of HSP27 expression could reduce sorafenib resistance and induce effective cell death in HCC cell lines.
We observed that many of the clones originating from HCC cells lines emerged from the NC vector group, while there was complete disappearance of colony formation in the shHSP27 treatment groups (fig. 4C). Interestingly, we found that the down-regulation of HSP27 expression by shHSP27 resulted in an increase in the activity of p-p38 (fig. 4A). We used shHSP27 combined with sorafenib to increase p-p38 activity in order to more effectively induce cell death in HCC cells. As a result, we demonstrated that the increased phosphorylation of HSP27 combined with IC50 concentration of sorafenib did effectively induce increased cell death in comparison to treatment with sorafenib alone.
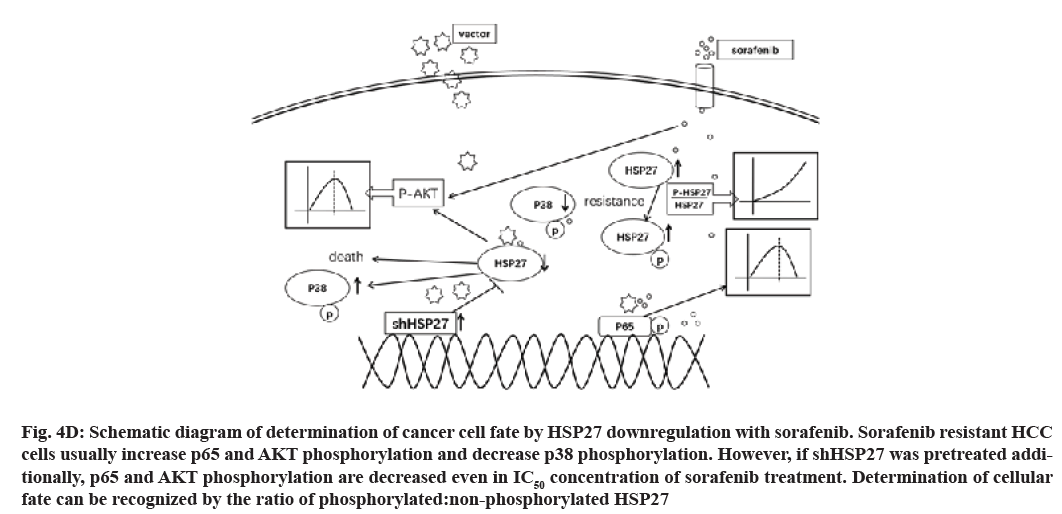
In this study we used the shRNA against HSP27 to knock down HSP27 expression. Our results show p-AKT, p-p65 were decreased while that of HSP27 was reduced, thereby leading to an increase in the activity of p-p38 and p-HSP27 (fig. 4D). Finally, the combination treatment could induce massive cell death in SR cell lines, which could overcome the resistance of sorafenib.
Fig. 4D: Schematic diagram of determination of cancer cell fate by HSP27 downregulation with sorafenib. Sorafenib resistant HCC cells usually increase p65 and AKT phosphorylation and decrease p38 phosphorylation. However, if shHSP27 was pretreated additionally, p65 and AKT phosphorylation are decreased even in IC50 concentration of sorafenib treatment. Determination of cellular fate can be recognized by the ratio of phosphorylated:non-phosphorylated HSP27
Sorafenib is the first-line systemic drug for advanced HCC. For patients in which HCC is diagnosed at advanced stage, sorafenib is the only choice of systemic therapy, as other potentially curative treatments, such as resection and liver transplantation, are mainly only applicable for patients diagnosed at early stage[19]. Recently, the unstable efficacy of sorafenib as a treatment for HCC has raised the concerns of more and more researchers and ‘sorafenib resistance’ has become well-known terminology and is regularly used to describe the impaired efficacy of sorafenib, especially in patients with advanced HCC. However, the exact mechanisms underlying sorafenib resistance in HCC remain unclear and no effective therapy for late-stage HCC has emerged in the wake of the failure of sorafenib as a therapy.
HSP27 is a chaperone protein that protects cells against external stimuli, including anti-tumor drugs. Therefore, HSP27 has become a hot topic in cancer research, where studies involving anti-tumor drug resistance often refer to HSP27 as a drug resistance inducer.
Thus, in this study, we also analyzed the expression of HSP27 in HCC cell lines. Our results show that the expression of HSP27 in HCC cells is negatively correlated with the IC50 of sorafenib and thus the higher expression of HSP27, the lower the drug sensitivity of the cells. Thus, the expression of HSP27 increased under conditions of resistance to sorafenib in HCC cell lines. These results indicate a correlation between HSP27 and sorafenib resistance.
Our previous study of gemcitabine drug resistance in pancreatic cancer cells showed that, the increased activity of p-HSP27 and the decreased expression of HSP27 effectively reduced drug resistance and increased cytotoxicity.
The p38 MAPK pathway is a multifunctional system that cannot only improve cell viability to protect cells, but can also induce apoptosis. The activity of p38 in HCC tissues has been shown to be lower in comparison to p38 activity in normal liver tissues, while the increased the activity of p38 is known to induce cell death[20].
The main purpose of sorafenib is to inhibit the ERK signaling pathway, where the activities of p-AKT and p-p65 displayed signs of increase after long-term treatment with sorafenib, thereby leading to drug resistance[21,22].
In the early study, we have demonstrated that a low concentration of sorafenib (2.5 μM) can inhibit the activity of p-p38. However, no significant cell death appeared in the low-dose sorafenib treatment. In fact, after the low-dose treatment, the activities of p-AKT and p-p65 were increased. Thus, we can infer that a decrease in p-p38 is the underlying mechanism responsible for sorafenib resistance in HCC cell lines.
The role of sorafenib, a type-II kinase inhibitor, in the inhibition of p38 activity is well known[23-25]. As the role of the inhibition of p38 activity in the resistance of HCC cells to sorafenib has become more clear, a therapy that utilizes sorafenib while increasing the activity of p38 could not only greatly improve the rate of cell death, it could also reduce the incidence of drug resistance in HCC tumor cells.
After elucidating the importance of p-p38 activity, we employed shRNA against HSP27, to stimulate an increase in p-p38 activity, thereby overcoming drug resistance.
In the present study, fortunately, as p-HSP27 is a downstream signal of p-p38, the increased the activity of p-p38 induced the activity of p-HSP27. In the results section, we mentioned that a decrease in HSP27 expression induces p-p38 activity, and also the activity of p-HSP27.
The adenoviral vector used in the present study as shRNA delivery tools displayed high efficient. Although adenoviral anti-tumor drugs have been previously used in clinical patients, the effects have been limited. Therefore, improving the antitumor effects of adenovirus-based therapies moved to the forefront of cancer therapy research, where a large number of studies focused on the ideal genes for adenoviral delivery and to induce the most desirable anti-tumor effects. In this study, we expressed two kinds of shRNA simultaneously using an adenovirus, thereby maximizing the functions of the treatment.
In summary, although a series of in vitro experiments demonstrated that the inhibition of p-p38 activity is the underlying mechanism of sorafenib drug resistance. Similarly, expression of shHSP27 can effectively reduce drug resistance and improve the antitumor effect.
Therefore, in the future, it will be necessary to analyze other family of HSPs while responsible for drug resistance and to use gene therapy to improve antitumor effect.
The mechanisms underlying the resistance of HCC cells to sorafenib remain complex. In this study we demonstrate that the inhibition of p38 activity by sorafenib treatment is one of the mechanisms responsible for sorafenib resistance. The increased activity of p38 by shHSP27 effectively induced massive cell death and reduced the resistance of HCC cell lines to sorafenib. Likewise, a reduction in the expression of HSP27 increases the sensitivity of HCC cell lines to sorafenib. In this study, we demonstrate that sorafenib chemotherapy combined with gene therapy, which expressed shRNA against HSP27, effectively overcomes the sorafenib resistance of HCC cells and improves the anti-tumor effect of sorafenib. This combinational therapy will be a new hope for HCC patients who have sorafenib resistance, can be a better therapeutic strategy to control the malignant liver cancer.
Authors’ contributions:
Yongmin Jin and Honghua Sun have contributed equally to this work.
Acknowledgement:
This research was supported by the Department of science and technology of Jilin province (20180520121JH, China).
Conflict of interests:
The authors declared no conflicts of interest.
References
- Joo M, Chi JG, Lee H. Expressions of HSP70 and HSP27 in hepatocellular carcinoma. J Korean Med Sci 2005;20(5):829-34.
[Crossref ] [Google Scholar] [PubMed]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362(9399):1907-17.
- Spinzi G, Paggi S. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359(23):2497-8.
[Crossref ] [Google Scholar]
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42(5):1208-36.
[Crossref ] [Google Scholar]
- Zhai B, Hu F, Jiang X, Xu J, Zhao D, Liu B, et al. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol Cancer Ther 2014;13(6):1589-98.
[Crossref ] [Google Scholar] [PubMed]
- Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64(19):7099-109.
[Crossref ] [Google Scholar] [PubMed]
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10(1):25-34.
[Crossref ] [Google Scholar] [PubMed]
- Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 2008;7(10):3129-40.
[Crossref ] [Google Scholar] [PubMed]
- Zhai B, Sun XY. Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma. World J Hepatol 2013;5(7):345-52.
[Crossref ] [Google Scholar] [PubMed]
- Berasain C. Hepatocellular carcinoma and sorafenib: Too many resistance mechanisms? Gut 2013;62(12):1674-5.
[Crossref ] [Google Scholar] [PubMed]
- English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci 2002;23(1):40-5.
[Crossref ] [Google Scholar] [PubMed]
- Iyoda K, Sasaki Y, Horimoto M, Toyama T, Yakushijin T, Sakakibara M, et al. Involvement of the p38 mitogen‐activated protein kinase cascade in hepatocellular carcinoma. Cancer 2003;97(12):3017-26.
[Crossref ] [Google Scholar] [PubMed]
- Koul HK, Pal M, Koul S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer 2013;4(9-10):342-59.
[Crossref ] [Google Scholar]
- Yang YX, Sun XF, Cheng AL, Zhang GY, Yi H, Sun Y, et al. Increased expression of HSP27 linked to vincristine resistance in human gastric cancer cell line. J Cancer Res Clin Oncol 2009;135(2):181-9.
- Hsu HS, Lin JH, Huang WC, Hsu TW, Su K, Chiou SH, et al. Chemoresistance of lung cancer stem like cells depends on activation of Hsp27. Cancer 2011;117(7):1516-28.
[Crossref ] [Google Scholar] [PubMed]
- Kang D, Choi HJ, Kang S, Kim SY, Hwang YS, Je S, et al. Ratio of phosphorylated HSP27 to nonphosphorylated HSP27 biphasically acts as a determinant of cellular fate in gemcitabine-resistant pancreatic cancer cells. Cell Signal 2015;27(4):807-17.
[Crossref ] [Google Scholar] [PubMed]
- Massague J. TGFbeta in Cancer. Cell 2008;134(2):215-30.
[Crossref ] [Google Scholar] [PubMed]
- Lin TH, Shao YY, Chan SY, Huang CY, Hsu CH, Cheng AL. High serum transforming growth factor-β1 levels predict outcome in hepatocellular carcinoma patients treated with sorafenib. Clin Cancer Res 2015;21(16):3678-84.
[Crossref ] [Google Scholar] [PubMed]
- Lo J, Lau EY, Ching RH, Cheng BY, Ma MK, Ng IO, et al. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology 2015;62(2):534-45.
[Crossref ] [Google Scholar] [PubMed]
- Namboodiri HV, Bukhtiyarova M, Ramcharan J, Karpusas M, Lee Y, Springman EB. Analysis of imatinib and sorafenib binding to p38α compared with c-Abl and b-Raf provides structural insights for understanding the selectivity of inhibitors targeting the DFG-out form of protein kinases. Biochemistry 2010;49(17):3611-8.
[Crossref ] [Google Scholar] [PubMed]
- He C, Dong X, Zhai B, Jiang X, Dong D, Li B, et al. miR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget 2015;6(30):28867-81.
[Crossref ] [Google Scholar] [PubMed]
- Grossi V, Liuzzi M, Murzilli S, Martelli N, Napoli A, Ingravallo G, et al. Sorafenib inhibits p38α activity in colorectal cancer cells and synergizes with the DFG-in inhibitor SB202190 to increase apoptotic response. Cancer Biol Ther 2012;13(14):1471-81.
[Crossref ] [Google Scholar] [PubMed]
- Zhang Y, Tao X, Jin G, Jin H, Wang N, Hu F, et al. A targetable molecular chaperone Hsp27 confers aggressiveness in hepatocellular carcinoma. Theranostics 2016;6(4):558-70.
[Crossref ] [Google Scholar] [PubMed]
- Kang S, Elf S, Lythgoe K, Hitosugi T, Taunton J, Zhou W, et al. p90 ribosomal S6 kinase 2 promotes invasion and metastasis of human head and neck squamous cell carcinoma cells. J Clin Invest 2010;120(4):1165-77.
[Crossref ] [Google Scholar] [PubMed]
- Yang F, Yin Y, Wang F, Wang Y, Zhang L, Tang Y, et al. miR-17-5p promotes migration of human hepatocellular carcinoma cells through the p38 mitogen-activated protein kinase-heat shock protein 27 pathway. Hepatology 2010;51(5):1614-23.
[Crossref] [Google Scholar] [PubMed]
 S+shHSP27
S+shHSP27