- *Corresponding Author:
- Miaomiao Wang
Department of Dermatology, Affiliated Hospital of Qingdao University, Qingdao, Shandong 266005, P. R. China
E-mail: wangmiaomiao@qdu.edu.cn
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “38-43” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of this study was evaluation of effect of heat shock protein 70 on toll-like receptor 4 and toll-like receptor 2 in melanoma cell line, A-375. The melanoma cell lines were stimulated in time course with heat shock protein 70 protein and incubation was performed. After 48 h, the cells were collected and total messenger ribonucleic acid was extracted and the expression of toll-like receptor 2 and toll-like receptor 4 was determined by quantitative reverse transcription polymerase chain reaction. The results showed that the appearance of toll-like receptor 2, toll-like receptor 4 in melanoma cell line, A-375 decreased in exposure to heat shock protein 70. The results showed the expression of nuclear factor kappa B in melanoma cell line. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide evaluation results of melanoma cell death were compared at different times and statistical analysis showed a significant difference (p<0.001). The role of heat shock protein 70 has been identified as a tumor suppressor in melanoma cancer. Heat shock protein 70 is a new treatment for melanoma in the near future. Heat shock protein 70 can be considered as a potential target in gene therapy for patients with melanoma cancer and the advantages of gene therapy by heat shock protein 70 in this type of cancer over chemotherapy can reduce cytotoxic effects and its low invasiveness.
Keywords
Heat shock protein 70, toll-like receptor 2, toll-like receptor 4, melanoma
Cancer is a disorder that regulates cell growth, which causes the rapid and uncontrollable division and growth of abnormal cells in a part of the body that has the ability to grow indefinitely and invade and spread to other parts of the body. And the importance of that initial treatment is increasing day by day. Melanoma is the most serious and deadly type of skin cancer, accounting for 4 % of men and 3 % of women. Early diagnosis is treatable and the probability of death at this stage is 1 % and in case of late diagnosis the possibility of death is 86 %. Melanoma develops in melanocyte cells. These cells make melanin, a skin pigment. A melanocyte mole, which is a benign lesion, also forms in these cells.
Knowledge about skin and melanocytes, how they function and grow, is essential to understand melanoma and what happens when cancer occurs[1]. The number of moles on each person’s body depends on factors such as genetics and sun exposure. People with more moles have a higher risk of developing melanoma.
Melanoma occurs when pigmented cells become cancerous[2-5]. These pigment-producing tumors are present on the surface layer of the skin (epidermis) [6-8]. Melanoma sometimes occurs in the eye and rarely in the meninges, gastrointestinal tract and lymph nodes, etc.[9,10]. Heat Shock Proteins (HSP) are classified according to the molecular weight of their monomers: HSP40, HSP60, HSP70, HSP90 refer to heat-shrinking factors with atomic mass units of 40, 60, 70 and 90 kDa, respectively[11-13]. These proteins are involved in various processes (under stressful conditions) including protein accumulation and transport, peptide transport and antigen processing[14]. Toll-Like Receptors (TLRs) are a protected family of receptors responsible for identifying pathogendependent molecular patterns and are responsible for initiating the activity of immune cells[15-18]. Several TLR genes have been identified in humans today[19-21]. Cell surface TLRs mainly detect microbial membrane components such as lipids, lipoproteins and proteins.
TLR4 detects bacterial Lipopolysaccharides (LPS). To detect the association between HSP70 and TLR4, the results indicated that HSP70 was introduced as a new TLR4 antagonist and that both the Mitogen-Activated Protein Kinase (MAPK) and Nuclear Factor Kappa B (NF-κB) pathways are essential for the TLR-induced immune response[22-24]. In the study of this research, it has shown that serum HSP70 levels were higher in cancer patients than in controls, suggesting that HSP70 may be involved in proliferation, cell cycle, migration and invasion of cancer cells[13]. The purpose of this study was to investigate the effect of HSP70 on the expression of TLR4 and TLR2 in melanoma cell line and to evaluate the expression of NF-κB protein by staining technique. Skin cancer is one of the most common cancers and melanoma is the most dangerous type of skin cancer, which affects 4 % of men and 3 % of women (x, y). The importance of early detection of skin cancer is such that researchers have found that having skin cancer increases the risk of other cancers. Melanoma can be treated early and the chance of death is 1 % at this stage[25] and after increase of disease, the chance of death is 86 %. One of the reasons for the high mortality rate of this type of cancer (melanoma) is that the disease rapidly undergoes a process of malignancy, metastasis and spread to other sensitive organs of the body such as the brain. Because the disease is highly invasive and metastatic, it is essential to identify the malignancy of the disease and its associated processes for accurate prediction, early diagnosis and effective treatment.
Materials and Methods
This research is a basic study. Cells from malignant human melanoma cell line (A-375 and C-6181) of melanoma were purchased from the Pasteur Cell Bank- Iran and cultured in medium Roswell Park Memorial Institute 1640 (RPMI 1640)+10 % Fetal Bovine Serum (FBS). The cells were treated with HSP70 as a time course (2 h, 6 h, 12 h, 24 h and 48 h). The cells were collected and total messenger Ribonucleic Acid (mRNA) was extracted.
The real-time Polymerase Chain Reaction (PCR) method is based on fluorescence produced by the reporter molecule, which increases during the reaction. SYBR green molecules are fluorescent reporters in the form of dyes that bind to double-stranded Deoxyribonucleic Acid (DNA). The primers were summarized in Table 1.
| Primers | Sequences | |
|---|---|---|
| β-actin | F | 5'-CAAGATCATCACCAATGCCT-3' |
| R | 5'-CCCATCACGCCACAGTTTCC-3' | |
| HSP70 | F | 5'-TTTCAAACCGCACGGATA-3' |
| R | 5'-CCCTAATGTTAAGAGGTA-3' | |
| TLR2 | F | 5'-CCCTAATGTTAAGAGGTA-3' |
| R | 5'-CAAATAATTCGGGATAT-3' | |
| TLR4 | F | 5'-AATCACAATTGGATCCTA-3' |
| R | 5'-TTGGTCAGTCCCCCAAAC-3' | |
Table 1: Specific Primers for β-ACTIN, HSP70, TLR2 and TLR4
Western blot:
Western blotting technique in this study was performed to identify the effect of NF-κB pathways of 4 % NF-κB antibodies on cells treated with HSP70. For this purpose, the cell line was electrophoresed on gel after lysis and transferred to Polyvinylidene Difluoride (PVDF) transfer plates and then plates with 5 % skim milk (fermented milk) with Tween 1 % in Phosphate Buffered Saline (PBS) were blocked for 1 h at room temperature for 1 d with NF-κB antibody, it is incubated. This technique is used to measure the amount of effective proteins of inflammatory factors and to evaluate the mediation of the NF-κB pathway. This method is a general method used to detect the presence of a specific protein in a complex mixture of proteins. To obtain a semi-quantitative estimation of a protein it can be based on the size and color intensity of a protein band formed and checked on the gel. It is also possible to use a series of dilutions of a pure protein at known concentrations to obtain a more accurate estimate of the concentration of the unknown protein. Western blotting is commonly used to confirm protein production after the cloning technique. Bleaching methods were used to detect and analyze the proteins. This method is a confirmatory test and examines the presence of type G immunoglobulin antibodies against several types of viral proteins and this test is relatively expensive and is not done as the first test.
Cell line A-375 was purchased from Pasteur Institute of Iran in a vial and the tests were performed in a laboratory and there was no intervention in living organisms. Statistical analysis was done using GraphPad Prism 7.0 software, in order to statistically compare the expression of genes and one way Analysis of Variance (ANOVA) test was used, p value was used for all relationships and p<0.05 was considered significant.
Results and Discussion
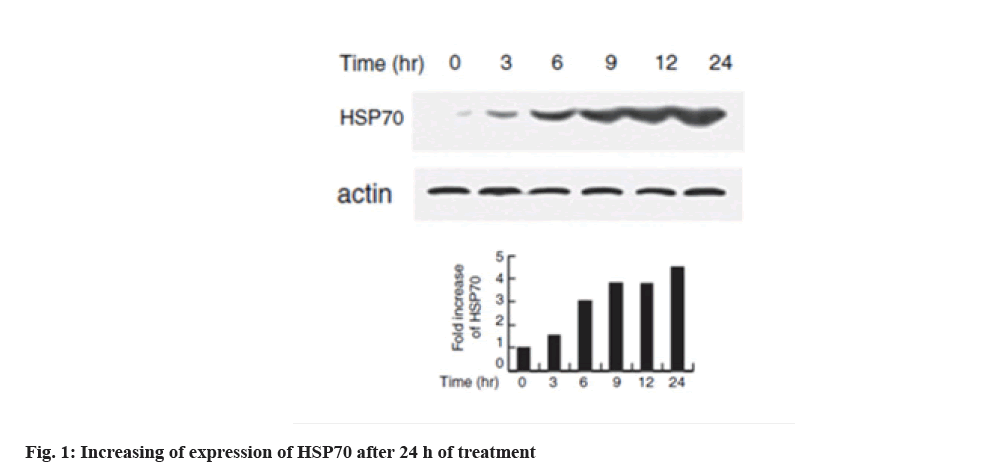
Increased expression of HSP70 in melanoma cell line is shown below. Western blotting technique was used to evaluate the expression of HSPs with heat shock in the cell line. HSP70 expression increased dramatically at 6 h after heat shock which is shown in fig. 1. In the interpretation of fig. 1, the expression of HSP70 can be significantly increased at 6 h after heat shock.
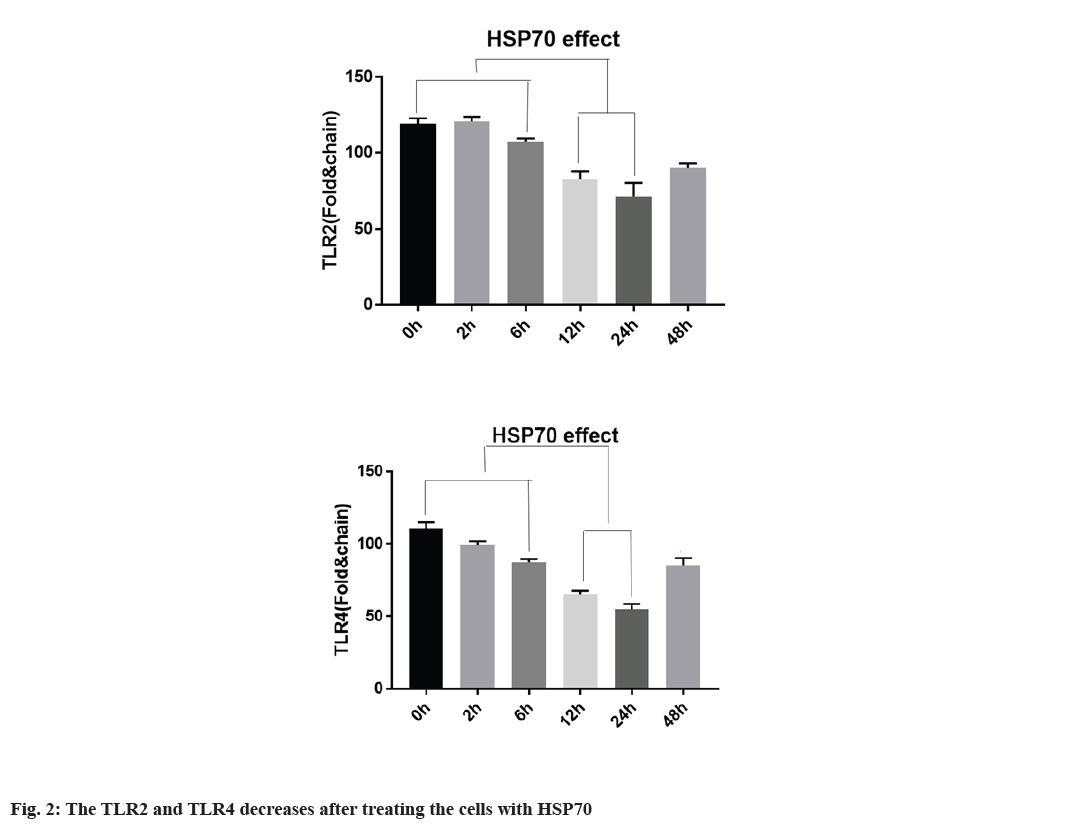
Decreased expression of TLR2, TLR4 with HSP70 in A-375 cell line is explained below. To determine whether TLR2 and TLR4 expression in A-375 cells is regulated by HSP70, the cells were first treated at 42° for 1 h and returned to 37° at the specified time. According to fig. 2, TLR2 and TLR4 mRNA expression is regulated by heat shock for 3 h, continuously, TLR2 and TLR4 protein expression were also regulated after heat shock. TLR2 expression decreased 12 and 24 h after heat shock. TLR4 expression is regulated within 3 h and after 9 h and TLR4 expression begins to decrease it and the expression was decreased after 12 h of treating (fig. 2).
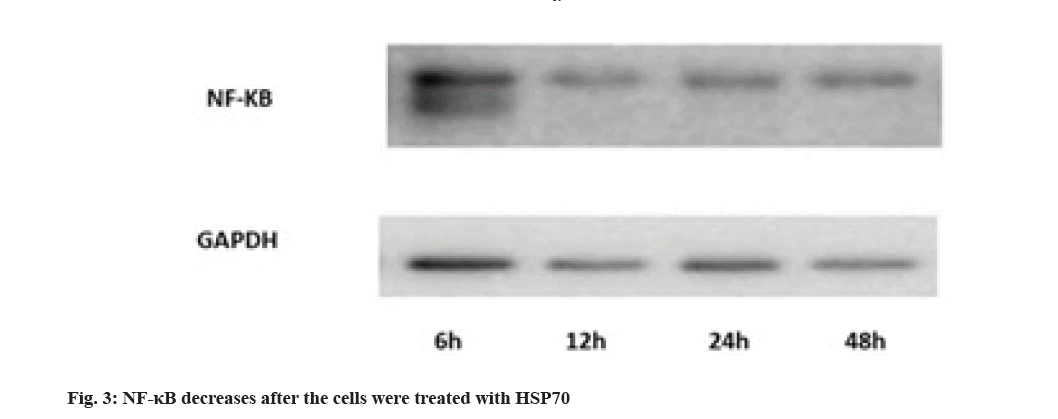
Evaluation of NF-κB protein expression by Western blotting technique is shown here. Activation of NF-κB pathways is involved in LPS-induced TLR4 expression. The results show that the rate decreases in 12 h after treating with HSP70, Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) was used for control (fig. 3).
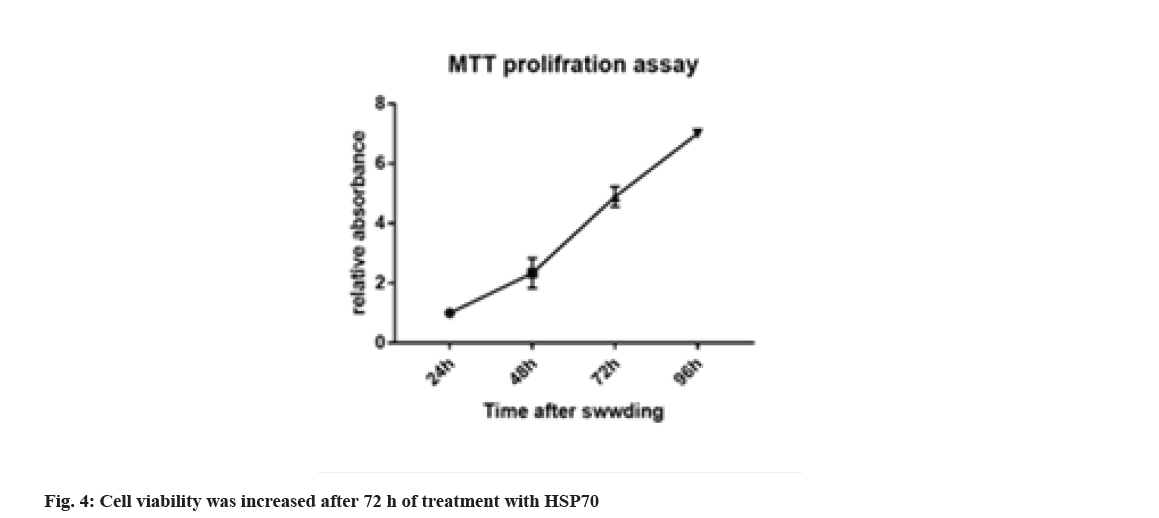
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl-2HTetrazolium Bromide (MTT) is a test method to assess cell viability or to evaluate the toxicity of drugs or other supplements on the cell, which can differentiate between living and dead cells by affecting intracellular organs. The activity and rate of cell proliferation may or may not increase under the influence of hormones, growth factors, cytokines and mitogens. Also, some drugs and cytotoxic agents, such as anticancer drugs, may cause cell necrosis or apoptosis, or slow the rate of proliferation and growth, or loss of cell structure. This method is based on the intensity of the dye produced by the mitochondrial activity of the cells, which is measured at a wavelength of 540 to 630 nm and is directly proportional to the number of living cells so that the number of cells increases or decreases. Living cells are linearly related to the activity of cell mitochondria. In this study, considering that HSP70 cell inhibition is based on concentration and time. For this purpose, melanoma cell death was evaluated at different times with different concentrations and assurance of the concentration and how long it has this feature, and statistical analysis showed a significant difference of p<0.001. Cell viability was increased after 72 h of treating with HSP70 (fig. 4).
Transformation of a normal cell into a cancer cell requires a change in the genes involved in cell growth. The form of treatments over time tends to reduce side effects and make the treatment more specific. Common treatments for melanoma cancer include chemotherapy, radiation therapy and gene therapy in the combined form[26-28]. Among them advantages of gene therapy are the ability to induce extinction in the advanced stages of growth, high strength and high efficiency, transmission of gene extinction to the next generation and its low cost compared to other treatment methods[29]. Gene therapy is used to increase the sensitivity of tumor cells to other therapies[30-32]. Expression of TLRs is related to immune response and our results for the effect of heat shock on the expression of TLR2 and TLR4 in melanoma cell line shows the regulation of TLR2 and TLR4 expression by heat shock and modulation of TLR expression by affecting the immune response[33]. TLRs are inherently linked to intrinsic safety and compatibility which are the basis for the development of vaccines aimed at involving the TLR signal. The presence of a TLR ligand enhances the immunizing effect of a given antigen[34]. TLR activation strategies can be used in induced and laboratory reactions for infectious diseases and cancer treatment. So the choice of TLR agonist and the subsequent effects of the vaccine becomes an important point in the vaccine approach. The results suggest that the HSP70 assay is a useful tool for detecting protein interactions and for identifying new receptors and even receptors[35-38]. Activation of NF-κB pathways is involved in LPS-induced TLR4 expression. The results show that the rate decreases in 12 h. The results also indicate that the heat shock group can reduce the expression of TLRs in the cancer cell line by reducing the signaling pathway of NF-κB and MAPK. Also in the present study, the effect of heat shock inhibition of 70 on the expression of TLR4 and TLR2 has been illustrated. The role of HSP70 has generally been recognized as a tumor suppressor in melanoma cancer. HSP70 is a new treatment for melanoma in the near future. HSP70 can be considered as a potential target in gene therapy for patients with melanoma cancer and the advantages of gene therapy by HSP70 in this type of cancer over chemotherapy can reduce cytotoxic effects and its low invasiveness.
Conflict of interests:
The authors declared no conflict of interest.
References
- Asea A. Mechanisms of HSP72 release. J Biosci 2007;32(3):579-84.
[Crossref] [Google scholar] [PubMed]
- Afzali M, Mirzaei M, Saadati H, Mazloomi-Mahmood-Abadi SS. Epidemiology of skin cancer and changes in its trends in Iran. Feyz J Kashan Univ Med Sci 2013;17(5):501-11.
- Atalay M, Oksala N, Lappalainen J, Laaksonen DE, Sen CK, Roy S. Heat shock proteins in diabetes and wound healing. Curr Protein Pept Sci 2009;10(1):85-95.
[Crossref] [Google scholar] [PubMed]
- Baba CS, Alexander G, Kalyani B, Pandey R, Rastogi S, Pandey A, et al. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J Gastroenterol Hepatol 2006;21(1):191-8.
[Crossref] [Google scholar] [PubMed]
- Basu N, Todgham AE, Ackerman PA, Bibeau MR, Nakano K, Schulte PM, et al. Heat shock protein genes and their functional significance in fish. Gene 2002;295(2):173-83.
[Crossref] [Google scholar] [PubMed]
- Black C, O’Connor P. (197) Short term effects of 2-grams of dietary ginger on muscle pain, inflammation and disability induced by eccentric exercise. J Pain 2008;9(4):25-6.
- Black CD, O’Connor PJ. Acute effects of dietary ginger on quadriceps muscle pain during moderate-intensity cycling exercise. Int J Sport Nutr Exerc Metab 2008;18(6):653-64.
[Crossref] [Google scholar] [PubMed]
- Chiefari E, Foti DP, Sgarra R, Pegoraro S, Arcidiacono B, Brunetti FS, et al. Transcriptional regulation of glucose metabolism: The emerging role of the HMGA1 chromatin factor. Front Endocrinol 2018;9:357.
[Crossref] [Google scholar] [PubMed]
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MS, Mesa JL, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA 2008;105(5):1739-44.
[Crossref] [Google scholar] [PubMed]
- Coenen KR, Gruen ML, Lee-Young RS, Puglisi MJ, Wasserman DH, Hasty AH. Impact of macrophage toll-like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia 2009;52(2):318-28.
[Crossref] [Google scholar] [PubMed]
- Drastichova Z, Skrabalova J, Jedelsky P, Neckar J, Kolar F, Novotny J. Global changes in the rat heart proteome induced by prolonged morphine treatment and withdrawal. PLoS One 2012;7(10):e47167.
[Crossref] [Google scholar] [PubMed]
- Ebrahimi M, Kazemi-Bajestani SM, Ghayour-Mobarhan M, Moohebati M, Paydar R, Azimi-Nezhad M, et al. Metabolic syndrome may not be a good predictor of coronary artery disease in the Iranian population: Population-specific definitions are required. Sci World J 2009;9:86-96.
[Crossref] [Google scholar] [PubMed]
- Gunaldi M, Afsar CU, Okuturlar Y, Gedikbasi A, Kocoglu H, Kural A, et al. Elevated serum levels of heat shock protein 70 are associated with breast cancer. Tohoku J Exp Med 2015;236(2):97-102.
[Crossref] [Google scholar] [PubMed]
- Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci 2014;8:537.
[Crossref] [Google scholar] [PubMed]
- Jacob P, Hirt H, Bendahmane A. The heat?shock protein/chaperone network and multiple stress resistance. Plant Biotechnol J 2017;15(4):405-14.
[Crossref] [Google scholar] [PubMed]
- Jain S, Pise N. Computer aided melanoma skin cancer detection using image processing. Procedia Comput Sci 2015;48:735-40.
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol 2010;11(5):373-84.
[Crossref] [Google scholar] [PubMed]
- Kim JJ, Lee SJ, Toh KY, Lee CU, Lee C, Paik IH. Identification of antibodies to heat shock proteins 90 kDa and 70 kDa in patients with schizophrenia. Schizophr Res 2001;52(1-2):127-35.
[Crossref] [Google scholar] [PubMed]
- Kim JJ, Sears DD. TLR4 and insulin resistance. Gastroenterol Res Pract 2010;2010:1-11.
[Crossref] [Google scholar] [PubMed]
- Kugelberg E. Pattern recognition receptors: Curbing gut inflammation. Nat Rev Immunol 2014;14(9):583.
[Crossref] [Google scholar] [PubMed]
- Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun 2009;388(4):621-5.
[Crossref] [Google scholar] [PubMed]
- Linderoth NA, Popowicz A, Sastry S. Identification of the peptide-binding site in the heat shock chaperone/tumor rejection antigen gp96 (Grp94). J Biol Chem 2000;275(8):5472-7.
[Crossref] [Google scholar] [PubMed]
- Lu HA, Sun TX, Matsuzaki T, Yi XH, Eswara J, Bouley R, et al. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem 2007;282(39):28721-32.
[Crossref] [Google scholar] [PubMed]
- American Cancer Society. Skin cancer prevention and early detection. Atlanta: American Cancer Society; 2012.
- Nicchitta CV, Carrick DM, Baker-LePain JC. The messenger and the message: Gp96 (GRP94)-peptide interactions in cellular immunity. Cell Stress Chaperones 2004;9(4):325-31.
[Crossref] [Google scholar] [PubMed]
- Noori-Daloii MR, Ebadi N. Pharmacogenomics and cancer stem cells. Med Sci J Islamic Azad Univ Tehran Med Branch 2015;25(1):1-5.
- Noori-Daloii MR. Medical molecular genetics in the third millennium. Tehran, Iran: Samer Publication; 2012.
- Noori-Daloii MR, Zekri A. Genetic instability and centrosome abnormality in cancer. Med Sci J Islamic Azad Univ Tehran Med Branch 2014;24(3):125-35.
- Penzo C, Arnoldo L, Pegoraro S, Petrosino S, Ros G, Zanin R, et al. HMGA1 modulates gene transcription sustaining a tumor signalling pathway acting on the epigenetic status of triple-negative breast cancer cells. Cancers 2019;11(8):1105.
[Crossref] [Google scholar] [PubMed]
- Qu P, Ma JH, Zhang XM, Huang XJ, Yang XW, Sui YF. A novel DNA vaccine constructed by heat shock protein 70 and melanoma antigen-encoding gene 3 against tumorigenesis. Indian J Exp Biol 2010;48(5):436-43.
[Google scholar] [PubMed]
- Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, et al. Fatty acid?induced induction of Toll?like receptor?4/nuclear factor?κB pathway in adipocytes links nutritional signalling with innate immunity. Immunology 2009;126(2):233-45.
[Crossref] [Google scholar] [PubMed]
- Shimizu K, Iyatomi H, Celebi ME, Norton KA, Tanaka M. Four-class classification of skin lesions with task decomposition strategy. IEEE Trans Biomed Eng 2015;62(1):274-83.
[Crossref] [Google scholar] [PubMed]
- Shomali N, Hatamnezhad LS, Tarzi S, Tamjidifar R, Xu H, Shotorbani SS. Heat shock proteins regulating toll-like receptors and the immune system could be a novel therapeutic target for melanoma. Curr Mol Med 2021;21(1):15-24.
[Crossref] [Google scholar] [PubMed]
- Wang XF, Wang D, Zhu W, Delrahim KK, Dolnak D, Rapaport MH. Studies characterizing 60 kda autoantibodies in subjects with schizophrenia. Biol Psychiatry 2003;53(5):361-75.
[Crossref] [Google scholar] [PubMed]
- Wu XD, Zeng K, Liu WL, Gao YG, Gong CS, Zhang CX, et al. Effect of aerobic exercise on miRNA-TLR4 signaling in atherosclerosis. Int J Sports Med 2014;35(04):344-50.
[Crossref] [Google scholar] [PubMed]
- Yang T, Wei L, Ma X, Ke H. Columbamine suppresses proliferation and invasion of melanoma cell A375 via HSP90-mediated STAT3 activation. J Recept Signal Transduct 2021;41(1):99-104.
[Crossref] [Google scholar] [PubMed]
- Yan Y. The anti-melanoma effects of heat shock protein inhibitors (Doctoral dissertation); 2017.
- Zarember KA, Godowski PJ. Tissue expression of human toll-like receptors and differential regulation of toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol 2002;168(2):554-61.
[Crossref] [Google scholar] [PubMed]