- *Corresponding Author:
- B. Mathalaimuthu
Department of Zoology, Annamalai University, Annamalai Nagar, Tamil Nadu 608002,India
E-mail: bharanitharan2011@gmail.com
| Date of Received | 12 August 2021 |
| Date of Revision | 10 March 2022 |
| Date of Acceptance | 02 September 2022 |
| Indian J Pharm Sci 2022;84(5):1116-1132 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Liver is an important part in human beings and plays a very important and major role in metabolism and excretion of xenobiotics from the body. Further, hepatotoxicity is caused by different types of toxic chemicals, such as antibiotics and chemotherapeutic agents, paracetamol (C8H9NO2), thioacetamide (C2H5NS), carbon tetrachloride (CCl4), silymarin (C25H22O10), ethanol (C2H5OH) and excessive alcohol intake and microbes is well researched. The markedly available synthetic drugs to treat liver sickness in this condition also cause further damage to the liver. Therefore, herbal medicines have become increasingly famous and their utilization is wide-spread. In medicinal plant derived drugs have been utilized in the treatment of liver diseases for a long time the protection of a healthy liver has been essential for the overall well-being of an individual. Liver injury induced by toxins is more common now-a-days. Herbal remedies are focused in the pharmaceutical industry to evolve a safe route for liver disorders and it is very low cost, no side effects compare with synthetic drugs. Therefore, hepatoprotective plants such as Avicennia alba, Anisochilus carnosus, Baliospermum montanum, Centella asiatica, Clitoria ternatea, Eclipta alba, Justicia adhatada, Phyllanthus emblica, Pisonia Grandis and Syzgium cuminiare were reviewed. The present review is aimed at compiling data on promising phytochemicals from medicinal plants that have tested in hepatotoxicity models using modern scientific system.
Keywords
Liver diseases, hepatotoxicity, hepatoprotective, medicinal plants, xenobiotics
Liver disease has a strong position as one of the chief health troubles in the world, with cirrhosis being the most drug-stimulated liver injury, according to the 9th most common cause of death in modern and developing countries[1]. However, it is caused by infectious agents or ingestion of toxic foods, chemical, over dose of drugs and chemicals that causes liver damage are called hepatotoxins[2,3]. It may have possible side effects of chronic medications or can be caused by chemicals, such as microcystins, as well as artificial chemicals like antibiotics, tetrachloride, chemotherapeutic agents, dimethyl nitrosamine, aflatoxin, Carbon tetrachloride (CCl4), pyrrolizidine alkaloids, allyl alcohol, Thioacetamide (C2H5NS), biomobenzene[4,5].
Susceptibility of the liver to chemical attacks, which comes in close contact with many harmful substances, environmental pollutants, xenobiotics and chemotherapeutic agents could repress. However,maintaining a healthy liver is a challenge for overall health and well human being, and the treatment of such diseases by using artificial pharmaceuticals or by using separated main compounds or importance parts of indigenous medicinal plants utilized in popular medicine[6,7]. In spite of this, there are nevertheless few drugs used to treat liver diseases, with possible effects on humans[8,9]. Thus, important medicinal plants with hepatoprotective or curative process utilized for the therapy of hepatic disorders become important; mostly important subjects of studies to explain their mechanism of action and characterize the compounds that can be utilized for the increased of new hepatoprotective drugs[10-13]. Some experimental models are utilized to show the hepatoprotective action of certain medicinal plants, especially against C2H5NS stimulated liver damage[14,15].
Hepatotoxicity Agents
Several chemicals have been known to induce hepatotoxicity and CCl4, C2H5NS, C8H9NO2, C2H5OH and C25H22O10 are used to induce experimental hepatotoxicity in laboratory animals.
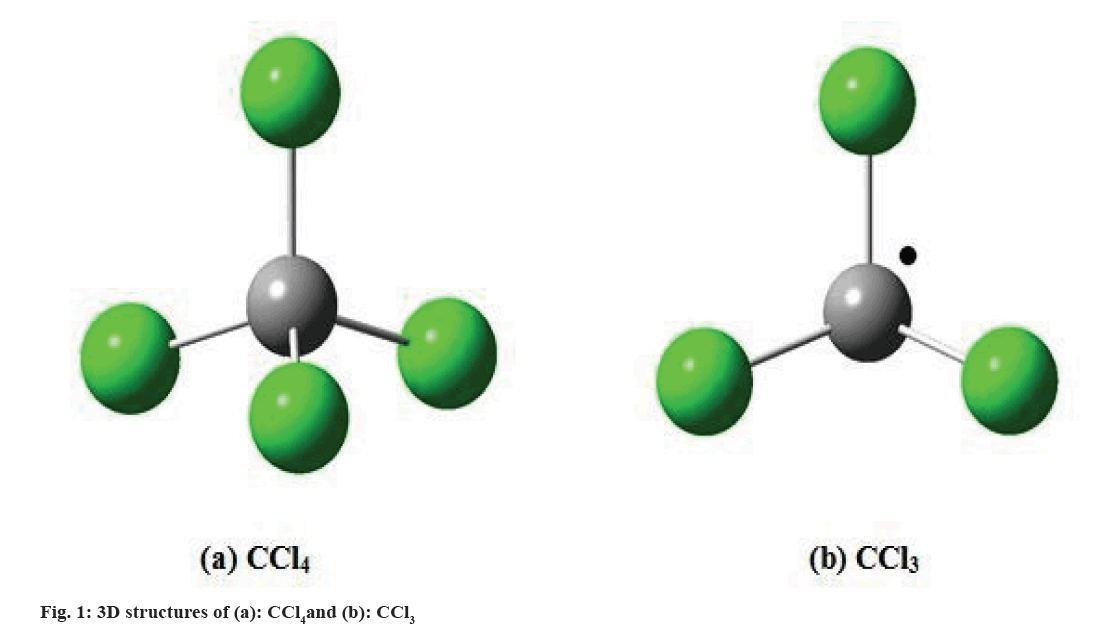
CCl4:
Liver injury due to CCl4 (fig. 1a) in rats was first reported in 1936 and broadly utilized by so many researchers[16,17]. CCl4 toxicity depends on dosage and the duration of exposure. In low dose, effects like loss of Ca2+ homeostasis, lipid peroxidation and release of cytokines are produced, and apoptotic events may be generated, followed by cellular regeneration. Further, in high doses or if there is a longer exposure, the effects are more severe and the damage occurs during a longer period of time, the patient may develop fibrosis, cirrhosis, or even cancer[18], is metabolized by the cytochrome P450 dependent of monooxygenases, mainly through the CYP2E1 isoform in the endoplasmic reticulum and mitochondria[19]. Hepatotoxicity is produced by the formation of the trichloromethyl radical (CCl3) (fig. 1b), which is highly reactive. These radicals may saturate the organism’s antioxidant defense system, react with proteins, attack unsaturated fatty acids, generating lipid peroxidation, reduce the amount of cytochrome P450, which leads to a functional failure with the consequent lowering of protein and accumulation of triglycerides (fatty liver), and alter water and electrolyte equilibrium with an increase of hepatic enzymes in plasma[20]. Lipid peroxidation leads to a cascade of reactions, such as the destruction of membrane lipids, the generation of endogenous toxic substances, which originate more hepatic complications and functional anomalies. For this reason, lipid peroxidation is considered a critical factor in the pathogenesis of liver injuries induced by CCl4[21]. The inhibition of the radical CCl3 generation is a key point in the protection against the damage generated. Because of this, model is widely utilized for the evaluation of pharmaceuticals and natural products with hepatoprotective and antioxidant activity[22,23].
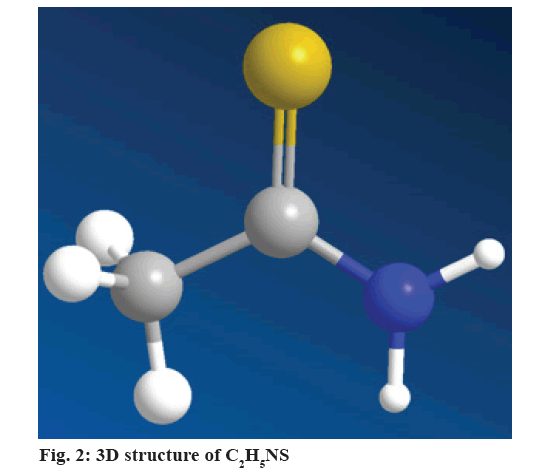
C2H5NS:
C2H5NS was particularly utilized as a fungicide to maintain agricultural citrus materials, later it was denied that is a strong potent hepatotoxin and carcinogen due to organo-sulfur-containing compound enriched with liver damaging and carcinogenic activities[24,9]. Currently, it is focused as a carcinogen, and very speedily metabolized into freebie radical derivatives such as C2H5NS sulfoxide, TAA-S-S-dioxide, even though it leads to lipid peroxidation, thus eventually culminates in centrilobular damages and liver injuries[15]. Earlier studies have also demonstrated that, rodents intoxicated with C2H5NS (fig. 2) was caused such as fibrosis, liver injury, cirrhosis and steatosis in test animals of this disease with etiology, and pathology comparable equal to the one seen in humans[25-27].
However, C2H5NS was recognized as an exemplary of liver fibrosis in rats. Though in the present scenario, the broadly utilized treatment of liver fibrosis and cirrhosis is inadequate; thus there is no effectively broadly utilized therapy that can prevent the improvement of hepatic diseases is explained. Despite, newly improved drugs have been utilized to heal liver diseases; presently these drugs have abundant side effects. There is an urgent need for alternative deputing remedies or drugs, to the treatment of chronic liver disorders to change current drugs of uncertain safety and non-effectiveness[28]. Liver markers are found of Aspartate Aminotransferase (AST), Transaminases, APT, Gamma (γ)-Glutamyl Transferase (GGT), Alanine Transaminase (ALT), lipids, bilirubin, cholesterol and proteins are discharged in the blood. As a result of cell leakage and the measurement of the serum markers of the liver could be utilized for diagnosis of injuries[29]. Many products available commercially are from herbal origin, and herbal elements and dietary supplements have power as possible choice medicines for the therapy of chronic liver diseases and associated metabolic derailments[30,31].
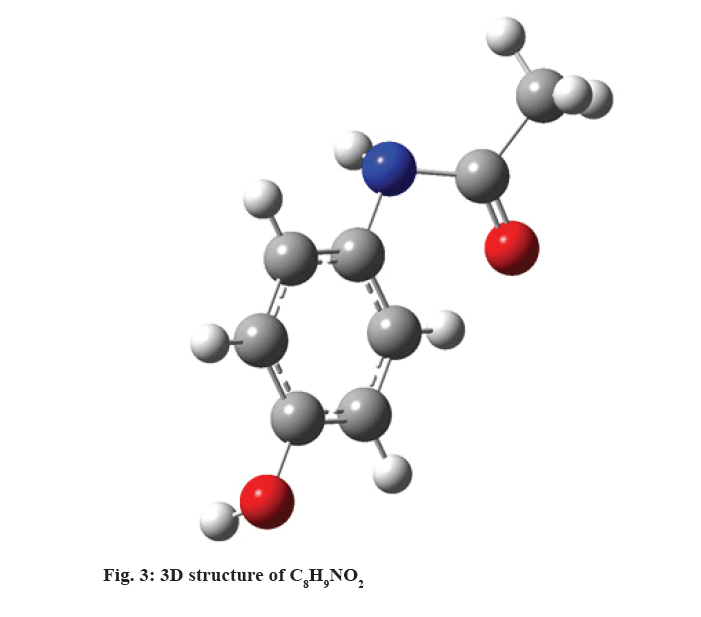
C8H9NO2:
C8H9NO2, (fig. 3) is a widely used analgesic, antipyretic drug and hepatocellular injury through three mechanisms, independently or in association. It produces acute liver damage in high doses[5] and is a widely used experimental model of clinical importance as an example of drug-induced liver damage[20]. At therapeutic doses, it is mainly metabolized to glucuronic or sulfated and excreted derivatives, the rest metabolizes to intermediate reactives, which are eliminated by conjugation with glutathione. The 1st and most common mechanisms is ingestion of doses higher than 10 g by adults and up to 150 mg/kg by children, popularly known as “overdose” and 2nd is the cytochrome P450 at N-acetyl-p-benzoquinone (NAPQI), which quickly attaches to glutathione, resulting from the use of enzyme inducing drugs and chronic alcohol abuse, 3rd occurs with glucagon depletion in hepatocytes through alcohol intake or malnutrition[32]. Under excessive conditions of NAPQI and glutathione depletion, a covalent bond of metabolite to proteins, adduct formation, mitochondrial dysfunction and oxidative stress occurs. The result is necrosis or hepatocellular death[33].
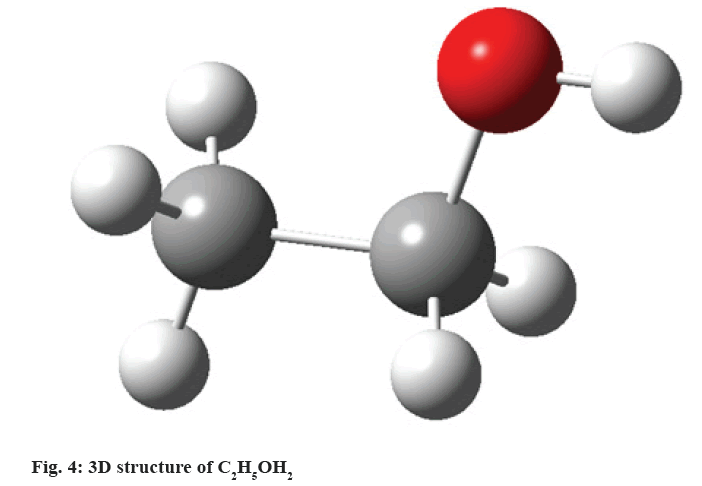
C2H5OH:
The liver is the most susceptible organ to the toxic effects of C2H5OH (fig. 4). Damage mechanism is due to the metabolism of ethanol by the CYP2E1 isoform of the cytochrome P450 producing oxidative stress with the generation of reactive species of oxygen and the increase of lipid peroxidation, leading to the alteration of the compositions of phospholipids of the cellular membrane[34]. Membrane lipid peroxidation results in the loss of its structure and integrity, elevating serum levels of glutamyl-transpeptidase, a membrane bonding enzyme. C2H5OH inhibits glutathione peroxidase; it reduces the activity of catalase and superoxide dismutase[20]. The decrease in the activity of antioxidant enzymes, superoxide dismutase and peroxidase glutathione is believed to come as a result of the harmful effects of free radicals produced after exposure to C2H5OH or alternatively, they could be a direct effect of acetaldehyde, a product of C2H5OH oxidation[35].
C25H22O10:
C25H22O10 (fig. 5) is an important component of Silybum marianum. Thus, it has been evidenced to be mostly hepatoprotective and has been utilized for the therapy of abundant liver disorders such as cirrhosis, fatty acid infiltration due to alcohol and toxic chemicals, and hepatitis, it’s specifically characterized by functional impairment or deterioration of necrosis[36]. However, it’s mechanisms of the process is not entirely understood, it appears that it acts in various ways, including anti-inflammatory activities and antioxidant, membrane stabilizer, cell permeability regulator, inhibiting the deposition of collagen fibers and stimulating liver regeneration, which may lead to cirrhosis[37].
Liver function markers:
Functions performed by the liver, there is a wide range of markers through which we are able to determine the functionality or damage generated by this organ or its cells[38]. Although there is no biochemical marker specific to liver damage, the combination of several of these and knowing the correlation they have with the liver, will help to better interpret the results of the hepatoprotective models. Markers can be divided into tests related to the liver’s excretory function (bilirubin), tests related to synthetic function (albumin and prothrombin time) and tests related to the integrity of hepatocytes (APT, Alkaline Phosphatase and GGT).
Hepatoproctive Plants
The medicinal plant plays a key role in the human health care. About 80 % of the world population relies on the use of traditional medicine which is predominantly based on plant materials[39]. Traditional medicine refers to a wide range of ancient natural health care practices including folk/tribal practices as well as Ayurveda, Siddha, Amchi and Unani. These medicinal plant practices originated from time immemorial and developed gradually, to a large extent, by relying or based on practical experiences without significant references to modern scientific principles. This estimated that about 7500 plants are used in local health traditional in, mostly, rural and tribal villages of India. Out of these, the real medicinal plant value of over 4000 plants is either little known or hitherto unknown to the mainstream populations. This is classical system of medicine such as Ayurveda, Siddha, Amchi, Unani and Tibetan use about 1200 plants[40,41]. Plants based therapeutics for liver diseases has been used in India for a long time and has been popularized world over by leading pharmaceuticals. The despite their important popularity several plant medicines in general and for liver diseases in particular they are still unacceptable treatment modalities for the liver diseases. Medicinal plant remedies are focused in the pharmaceutical industry to evolve a safe route for liver disease (Table 1). Hence, in this review we focused on some medicinal plants such as Avicennia alba, Anisochilus carnosus, Baliospermum montanum, Centella asiatica, Clitoria ternatea, Eclipta alba, Justicia adhatada, Phyllanthus emblica, Pisonia grandis, Syzgium cumini.
| S.No. | Plant/Tamil name | Family | Part used | Constituents | Hepatotoxicity inducing agents |
|---|---|---|---|---|---|
| 1 | Aegle marmelos (Tamil name-Vilvam) | Rutaceae | Leaves | Saponins, flavonoids, glycosides, alkaloids and tannins | C8H9NO2 |
| 2 | Agrimonia eupatoria | Rosaceae | Whole plants | β-sitosterol, betalain andneoandrographolide | C2H5OH |
| 3 | Aerva lanata Linn (Serupeelai) | Amaranthaceae | Coarse powder plant material | Alkaloids-β-carboline-1 -propionic acid, 6-methoxy-β carboline-1-propionic acid, 6-methoxy-β-carbolin-l-ylpropionic acid (ervolanine) and aervolanine (3-(6-methyoxy-β-carbolin-1-yl) propionic acid) Flavanoids-Kaempferol, quercetin, isorhamnetin, isorhamnetin 3-O-β-[4-p-coumaroyl-α-rhamnosyl, galactoside and flavanone glucoside persinol | C8H9NO2 |
| 4 | Acacia confusa | Leguminosae | Bark | Flavonoids, phenolic acids, tannins and phenolic diterpenes | CCl4 |
| 5 | Agrimonia eupatoria | Rosaceae | Whole plants | β-sitosterol, betalain andneoandrographolide | C2H5OH |
| 6 | Aloe barbadensis Mill. (Kattalai) | Liliaceae | Aerial part | Flavanoids, hydroxyanthraquinones and coumarin | CCl4 |
| 7 | Alchornea cordifolia | Euphorbiaceae | Leaves | Saponins, alkaloids, carbohydrates, reducing sugar, tannins and flavonoids | C8H9NO2 |
| 8 | Andrographis paniculata (Tamil name-Nilavembu) | Acanthaceae | Leaf, aerial parts | Andrographolide, bicyclic diterpene, lactone, kalmegh, andrograholide | C8H9NO2 |
| 9 | Artemisia absinthiumL. (Tamil name-Masipathiri) | Asteraceae | Aerial parts,leaf | Tricyclene, α-thujene, α-pinene, sabinene, 6-methyl-5-hepten-2-one, α-phellandrene | CCl4 |
| 10 | Artemisia sacrorum Ledeb. | Compositae | Aerial parts | 1,8-cineole, chrysanthenone, chrysanthenol (and its acetate), α/β-thujones and camphor | C8H9NO2 |
| 11 | Astragalus polysaccharides | Magnoliaceae | Dried fruits | Flavonoids, non-protein, amino acid, saponins, alkaloids, nitro chemically compounds, mucilage, sterols, proline content and phenolics | CCl4 |
| 12 | Asteracantha longifolia L. (Neermulli) | Acanthaceae | Leaved axil, flower, root, seed | Andrographolide | C6H13NO5 |
| 13 | Azadirachta indica (Vembu) | Meliaceae | Whole parts | Azadirachtin, margolone, mono-, di-, sesqui- and triterpenoids, coumarins, chromones, lignans, flavonoids and other phenolics | C8H9NO2 |
| 14 | Baliospermum montanum (Tamil name-Nakatanti) | Euphorbiaceae | Root | Alkaloids, phenols, carbohydrates, tannins, steroids, saponins, flavonoids, cardiac glycosides, proteins, terpenoids, resinsand glycosides | C8H9NO2 |
| 15 | Byrsocarpus coccineus Schum | Connaraceae | Leaf | Alkaloids, tannins, cardiac glycosides, steroids, terpenoids, flavonoids, anthraquinones, phlobatannins, reducing sugars and saponins | CCl4 |
| 16 | Bauhinia variegata L. | Leguminosae | Stem bark | Terpenoids, flavonoids, tannins, saponins, reducing sugars, steroids and cardiac glycosides | CCl4 |
| 17 | Cassia toraL. (Thangarai) | Caesalpiniaceae | Leaves, seeds | Alkaloids, steroids and phlobatannins, phenolics and flavonoids, saponins and cardiac glycosides and tannins | CCl4 |
| 18 | Citrus limonL. Burm. (Elumichai) | Rutaceae | Fruits | Coumarins, flavonoids, carotenes, terpenes and linalool | CCl4 |
| 19 | Cleome viscoseLinn | Capparidaceae | Leaf powder | Alkaloids, flavonoids and fatty acids are the major active constituents of this genus, six main flavonoid gycosides such as kaempferol, chrysoeriol, isorhamnetin, chrysoeriol-7-O-xylosoid, kaempferol-3-galactorhamnoside and isorhamnetin 3-O-β-dapio-furanosyl and β-D-galactopyranoside | C8H9NO2 |
| 20 | Curcuma longa | Zingiberaceae | Rhizome | Curcumin, turmerone, monoterpenes, 5% curcuminoids, minerals, carotene and vitamin C | C8H9NO2, C14H11Cl2NO2 |
| 21 | Chamomile capitula | Compositae | Whole parts | α-bisabolol, α-bisabolol oxide A and B, chamazulene, sesquiterpenes; coumarins: umbelliferone; flavonoids: luteolin, apigenin, quercetin and spiroethers: en-yn dicycloether | C8H9NO2 |
| 22 | Cuscuta reflexaRoxb | Cuscutaceae | Whole plant | Scoparone, melanettin, quercetin hyperoside, luteolin, dulcitol, luteolin and glycoside | C8H9NO2 |
| 23 | Cassia occidentalis | Caesalpinaceae | Whole plant | Alkaloids, aaponins, carbohydrates, glycosides, fixed oils and fats, aminoacids, flavanoids, anthraquinones, tannins and phenolic compounds | C8H9NO2 |
| 24 | Capparis spinosa | Capparidaceae | Root, bark | Isorhamnitine-3-O rutinoside, 1 tetradecanol, p-hydroxybenzaldehyde, 6,10,14-trimethyl-2-pentadecanone, ursolic acid, glycerol monotetracostanoate, 4-coumaric acid, nicotinamide, methyl hexadecanoate, sitosterol, sitosterylglucoside, cadabicine, octadecanoic acid, rutin and stachydrine | CCl4 |
| 25 | Clerodendrum inerme | Verbenaceae | Leaves | Phenylpropanoid and phenylethanoid glycosides, flavonoids, diterpenoids and iridoids | CCl4 |
| 26 | Decalepis hamiltoniiWight. | Asclepiadaceae | Root | 4-Omethylresorcylaldehyde, benzyl alcohol, β-caryophyllene and α-atlantone. Aromatic aldehydes, monoterpene, hydrocarbons, alcohols and ketones, β-phellandrene and trans-anethole | CCl4 |
| 27 | Diospyros malabaricaKostel. | Ebenaceae | Bark | Tannins, Triterpenoid compounds such as α-amyrin, uvaol, ursolic acid, 19α-hydroxyursolic acid and 19α, 24-dihydroxyursolic acid | CCl4 |
| 28 | Diplotaxis acris Boiss. | Compositae | Seeds | Tannins, saponins, sterols and/or triterpenes, alkaloids, anthraquinones, flavonoids, lactones/esters, protein and/or amino acids and carbohydrates and/or glycosides | CCl4 |
| 29 | Equisetum arvense | Equisetaceae | Aerial parts | Phenolic petrosins, onitin and onitin-9-O-glucoside, flavonoids, apigenin, luteolin, kaempferol-3-O-glucoside and quercetin-3-O-glucoside | CCl4 |
| 30 | Embelia ribes | Myrsinaceae | Fruits | Reducing sugars, non-reducing polysaccharides, rides, gums, mucilage, proteins, amino acids, fats and oils, steroids, glycosides, saponin, flavonoids, alkaloids, tannins and volatile oil | C8H9NO2 |
| 31 | Garcinia mangostana | Clusiaceae | Whole plant | Methylparaben, methyl 3,4,5-trihydroxybenzoate, parvifoliol A1, methyl 2,3-dihydroxybenzoate, 4-hydroxybenzoic acid, epicatechin and xanthone, mangostin | C8H9NO2 |
| 32 | Gundelia tourenfortii | Asteraceae | Fresh edible stalk | Steroid and triterpenoids, phenolic and tannins, flavonoids, saponin, alkaloid, anthraquinone, glycoside and protein | CCl4 |
| 33 | Glycyrrhiza glabraL. | Leguminosae | Glycyrrhizin from root | Saponin, flavonoids, alkaloids, steroids, terpenoids, tannins and glycosides, carbohydrates, proteins, phlobatannins and phenolic compounds | CCl4 |
| 34 | Grewia tiliaefoliaVahl. | Tiliaceae | γ-lactones from stem bark | Triterpenoids, steroids, glycosides, flavones, lignanes, phenolics, alkaloids, lactones and organic acids | CCl4 |
| 35 | Halenia elliptica | Gentianaceae | Whole plant | Xanthones, xanthone glycosides, chromones flavonoids, secoiridoid glycosides, triterpenoid alkaloids | CCl4 |
| 36 | Hygrophila auriculataHeine. | Acanthaceae | Root | Seed contain yellow colour oil, diastase, lipase, protease, salts of potassium and mucilage | CCl4 |
| 37 | Indigophora tinctorea (Avuri) | Fabaceae | Whole plant | Inorganic salts of nitrogen, phosphoric acid, lime, potash along with apigenin, kaempferol, luteolin, quercetin, seed-galactomannan, galactoss, mannose | C8H9NO2 |
| 38 | Justicia simplexD. Don. | Acanthaceae | Whole plant | Alkaloids, proteins, flavonoids, amino acids, tannins, carbohydrates, saponins, terpenoid and steroids | CCl4 |
| 39 | Juncus subulatus | Juncaceae | Powdered tubers | Flavonoids, coumarines, terpenes, stilbenes, sterols, phenolic acids, carotenes, phenanthrenes derivatives. | C8H9NO2 |
| 40 | Kyllinga nemoralis L. | Cyperaceae | Rhizome | Alkaloids, flavonoids, carbohydrates, phenols, tannins and steroids | CCl4 |
| 41 | Kalanchoe pinnataPers (Runa kalli) | Crassulaceae | Leaves | Alkaloids, phenols, flavonoids, tannins, anthocyanins, glycosides, bufadienolides, saponins, coumarins, sitosterols, quinines, carotenoids, tocopherol and lectins | CCl4 |
| 42 | Kigelia africana | Bignoniaceae | Leaves | Flavanoids, steroidal saponins, napthoquinones and volatile constituents | C8H9NO2 |
| 43 | Laggera alata(D. Don) | Sch.-Bip. | Whole plant | Triterpenes, flavonoids, alkaloids, polyphenols, sterols and saponins | CCl4 |
| 44 | Ligustrum robustumRoxb. | Oleaceae | Leaves | Terpenoids, saponins, polyphenols (especially flavonoids), glycosides and many other compounds | CCl4 |
| 45 | Luffa echinata | Cucurbitaceae | Fruits | Lucosides C, E, F, H, a mixture of alpha-spinasterol, alpha-spnisteryl glucoside, stigmasteryl-beta-D-glucoside and methyl ester | CCl4 |
| 46 | Lactuca sativa | Asteraceae | Whole plants | Ursolic acid , stigmasterol, sitosterol, b-sitosterol galactoside, herniarin and 2,4,6-trihydroxyethylbenzoate | CCl4 |
| 47 | Macrotyloma uniflorum | Seeds | Flavanoids and tannins | C6H13NO5, C8H9NO2, C25H22O10 | |
| 48 | Moringa oleiferaLam. (Murungai maram) | Moringeaceae | Seed | Hydrocarbons, hexacosane, pentacosane, heptacosane, pentacosane hexacosane, (E)-phytol, thymol, hexanoic acid, acetic acid, nonacosane, 1,2,4-trimethyl-benzene | CCl4 |
| 49 | Myrtus communis Linn | Myrtaceae | Leaves | Flavonoids, terpenoids, steroids | C8H9NO2 |
| 50 | Momordica dioica | Cucurbitaceae | Leaves | Saponins, tannins, flavonoids, steroids, triterpenes, coumarins, quinones, organic acids and alkaloids | CCl4 |
| 51 | Nelumbo nucifera Gaertn. | Nelumbonaceae | Leaves | Glucose, tannin, fat, resin, metarbin, alkaloid nelumbine | CCl4 |
| 52 | Ocimum snctum(Thulasi) | Lamiaceae | Leaves | Alkaloids, tannin, saponin, steroid phlobatannin, terpenoid, flavonoid, cardiac, glyceride | C8H9NO2 |
| 53 | Ptrospermum acerifolium | Sterculiaceae | Leaves | Alkaloid, tannin, saponin, flavonoid, cardiacglycosides, sterols, anthroquinone, glycosides, carbohydrates and protein | CCl4 |
| 54 | Petroselinum Crispum(Mill.) | Umbelliferae | Leaves | Alkaloid, carbohydrate, phenolic compound, tannins, flavonoids, proteins, amino acids and saponins | CCl4 |
| 55 | Pergularia daemia Forsk. | Asclepiadaceae | Aerial part | Cardenolides, alkaloid, saponins and steroidal compounds, fixed oil, volatile oil, resin, alkaloid, triterpenoid, carissol, carissic acid and ursolic acid | CCl4 |
| 56 | Phyllanthus niruriL. | Euphorbiaceae | Aerial parts | Phyllanthin, niranthin, hypophyllanthin, alkaloid, lignas, vitamin-C, quercetin, astrogaln, querscitrin, rutin, glucoflavon, linoleic, linolenic, acid Coumarins, tannins and polyphenols, gallic acid, ellagic acid, brevifolin, carboxylic acid, ethyl brevifolin, carboxylate, methyl brevifolin, carboxylate, lizuka, geraniin, corilagin, phyllanthusiin D amariin, amariinic acid, elaeocarpusin, geraniinic acid B, repandusinic acid, Amarulone, Furosin, 1,6-Digalloyl glucopyranoside, catechin, Epicatechin, gallocatechin,epigallocatechin, epicatechin 3-o-gallate, epigallocatechin 3-o-gallate | C6H13NO5, C8H9NO2 |
| 57 | Plantago majorL. | Plantaginaceae | Seeds | Total phenol, flavonoid and tannin | CCl4 |
| 58 | Platycodon grandiflorum A. DC. | Campanulaceae | Saponins derived from root | Steroidal saponins, flavonoids, polyacetylenes, sterols, phenolics and other bioactive compounds | CCl4 |
| 59 | Pracparatum mungo | Fermented product | Essential oils, saponins, carotenoids, lectins, vitamins, fiber and fatty acids | CCl4 | |
| 60 | Pterocarpus marsupiumRoxb. | Papilionaceae | Stem bark | Protein, pentosan, mucilage, pterosupin, pseudobaptigenin, liquiritigenin, garbanzol, beta-cudesmol, pterostil-bene, marsupol, carpusin, proterol, marrsupinol, parsupin, oleanolic, tannins and ksinotanic acid, quercetin, kaempferol, epicatechin, and rutin, phytol, 1H-indene, 1-ethylideneoctahydro-7 a-methyl-, (1E,3a.alpha.,7a.beta.), 2H-1-Benzopyran,6,7-dimethoxy-2,2-dimethyl, Inositol,1-deoxy, 2-Methoxy-4-vinylphenol, 2-methoxy-3-2-propenylphenol-, 2-Ethylacridine, Delta-selinene and Fatty acids | CCl4 |
| 61 | Punica granatum Linn. (Maathulai) | Punicaceae | Whole plant | triterpenoids, steroids, glycosides, saponins, alkaloids, flavonoids, tannins, carbohydrates and vitamin C | CCl4 |
| 62 | Plumbago zeylanica | Plumbaginaceae | Volatile oils, chitranone, alpha and beta amyrin, lupeol, taraxasterol, fructose, glucose, invertase, protease, chloroplumbagin, droserone, ellipticine, zeylanone, zeylone, meritone, catechol, tannin, amino acids, plumbagic acid | C8H9NO2 | |
| 63 | Physalis minima | Solanaceae | Whole plant | Alkaloids, anthraquinones, flavonoids, cardiac glycosides, phenols, quinones, reducing sugars, saponins, steroids, starch, tannins and terpenoids | C8H9NO2 |
| 64 | Pseudarthria vicida | Fabacea | Roots | Leucopelargonidin | C8H9NO2 |
| 65 | Phyllanthus emblica(Perunelli) | Euphorbiaceae | Whole plant | Protein, fats, fibres, carbohyderates, vitamin-C, nicotinic acid, tannins, gallic acid, ellagic acid, flavin and glucose, linolenic acid, oleic acid | C8H9NO2 |
| 66 | Quercus aliena Blum. | Fagaceae | Whole plant | Tannins, polyphenols, abscisic acid and indoleacetic acid | CCl4 |
| 67 | Rhodococcum vitisIdaea Linn | Ericaceae | Leaves | Amyrin acetate, mixture of amyrins, β-sitosterol, scopoletin, iridoids, isoplumericin, plumieride, plumieride coumarate, plumieride coumarate glucoside | C6H13NO5 |
| 68 | Rhoicissus tridentate Wild. | Vitaceae | Root | Phenols, alkaloids, flavonoids, tannins and saponins | CCl4 |
| 69 | Rheum emodi Wall (Reval senni) | Polygonaceae | Whole plants | Anthraquinones, anthrones, stilbenes, oxanthrone ethers and esters, flavonoids, lignans, phenols, carbohydrates, oxalic acids, anthraquinones includes rhein, chrysophanol, Aloe-emodin, emodin, physcion (emodin monomethyl ether), chrysophanein and emodin glycoside. Stilbene includes picetannol, resveratrol and their glycosides | CCl4 |
| 70 | Ricinus Communis (Aamanakku) | Euphorbiaceae | Leaves | Steroids, saponins, alkaloids, flavonoids and glycosides. Dried leaves: Alkaloids, ricinine and N-demethylricinine, flavones glycosides, kaempferol-3-O, kaempferol-3-O-β-D-glucopyranoside, quercetin xylopyranoside, quercetin-3-O-β-D-lucopyranoside, kaempferol, O-β-rutinoside, quercetin-3-O-β- monoterpenoids, gallic acid, quercetin, gentisic acid, rutin, epicatechin, ellagic acid, indole-3-acetic acid, ricinoleic, isoricinoleic, stearic and dihydroxystearic acids and also lipases and aricinine | CCl4 |
| 71 | Saururus chinensis | Saururaceae | Whole plant | Isoflavons, saponins, phytosterols and phenols | CCl4 |
| 72 | Spondias pinnata | Anacardiaceae | Stem heart wood | Flavonoids, tannins, saponins and terpenoids, essential oils from the pulp yielded carboxylic acids and esters, alcohols, aromatic hydrocarbons, 9, 12, 15-octadecatrien-1-ol, hexadecanoic acid, furfural, 24-methylene cycloartanone, stigma-4en-3one, lignoceric acid, β-sitosterol and its β-D-glucoside, ß-amyrin, oeanolic acid, glycine, cystine, Serine, alanine and leucine, lignoceric acid, ß-sitosterol, glucoside | CCl4 |
| 73 | Sarcostemma brevistigma | Asclepiadaceae | Stem | Bergenin, brevine, brevinine, sarcogenin, sarcobiose and flavonoids | CCl4 |
| 74 | Sesbania grandiflora L. | Fabaceae | Whole plant | Sterols, saponins, and tannins | C2H5NS and C13H23ClN4O3S |
| 75 | Sesbania sesbanMers | Fabaceae | Leaf, Bark, Seed | Alkaloids, carbohydrates, protein, phytosterol, flavonoids, fixed oil cholesterol, campesterol, galactomannan, D-galactopyranoside | C2H5 |
| 76 | Schisandra chinensis | Schisandraceae | Leaves | Lignans, schizandrin, deoxyschizandrin. | C6H13NO5 |
| 77 | Schouwia thebaica | Arecaceae | Aerial parts | Tannins, saponins, sterols, triterpenes, alkaloids, anthraquinones, flavonoids, lactones/esters, protein, amino acids and carbohydrates, glycosides | CCl4 |
| 78 | Scoparia dulcis | Scrophulariaceae | Whole plant | Alkaloids, flavonoids, phenols, terpenoids, tannins and saponins | CCl4 |
| 79 | Solanum nigrum (Manathakkali ) | Solanaceae | Fruits, leaves | Steroidal components, withanolides, Flavonoids, terpenoids | C2H5NS, CCl4 |
| 80 | Strychnos potatorum Linn. | Loganiaceae | Seed | Norharmane, akuammidine, Nor-C-fluroiocuraine, ochrolifuanine, Bis nor Dihydro toxiferine, 11-Methoxy- Henningsamine, 11-methoxy-12 hydroxydiabolin and 11-Methoxydiabolin | CCl4 |
| 81 | Swertia chirata | Gentianaceae | Whole plants | Carbohydrates, glycosides, alkaloids, phenols, flavonoids and tannins | C6H13NO5, C8H9NO2 |
| 82 | Syzygium cuminiL. | Myrtaceae | Leaves | Friedelin, kaempferol, tannins, quercetin, beta-sitosterol, betullinic acid, anthocyanin acid, eugin, ellagic acid, oxalic acid, citric acid, glycolic acid, glucose, fructose, gallic acid, glycine, alanin, leucin, tyrosin | CCl4 |
| 83 | Spermacoce hispida | Rubiaceae | Seed | Borreline, β-sitosterol, ursolic acid and isorhamntin | CCl4 |
| 84 | Taraxacum officinale | Asteraceae | Root | Alkaloids, tannins, flavonoids and phenolic compounds | CCl4 |
| 85 | Tecomella undulata | Bignoniaceae | Stem, Bark | Alkaloids, steroids, volatile oil, fat, tannin, carbohydrate, saponin and flavonoids | C2H5OH and C8H9NO2 |
| 86 | Terminalia arjunaRoxb | Combretaceae | Bark | Beta-sitosterol, arjunic acid, friedlene, glucoside, tannins, sugars, sodium, magnessium, aluminium, calcium carbonate | CCl4 |
| 87 | Terminalia catappaL. (Combretaceae) | Combretaceae | Leaves | Tannins, sugars, sodium, magnesium, aluminium, calcium carbonate | CCl4 |
| 88 | Thunbergia laurifoliaLinn. | Acanthaceae | Leaves, aerial part | Benzyl alcohol glucosides , Iridoid glucoside, two aliphatic alcohol glucosides and two flavonoid C-glucosides | C2H5OH |
| 89 | Trigonella foenumgraecum (Venthayam) | Fabaceae | Leaves, seeds | Fibers, flavonoids, polysaccharides, saponins, flavonoids and polysaccharides fixed oils alkaloids | C22H19Br2NO3 |
| 90 | Tridax procumbensLin (Vettukaaya poondu) | Asteraceae | Leaves | Steroid like saponin, coumarins, alkaloids, amino acids, diterpenes, phenol whereas Flavonoids like tannin, anthocyanin, emodins, proteins, phytosterol, phlobatannin, | C6H13NO5 |
| 91 | Trichosanthes cucumerinaL. | Cucurbitaceae | Whole plant | Cucurbitacin B, Cucurbitacin E, Isocucurbitacin B, 23,24-Dihydroisocucurbitacin B, 23,24-Dihydrocucurbitacin E, Sterols 2 β-sitosterol Stigmasterol | CCl4 |
| 92 | Vernonia amygdalina | Astereaceae | Leaves | Alkaloids, flavonoids, glycosides, saponins, tannins, phenols, β-carotenoids, cyanogenic glycosides and steroids | CCl4 |
| 93 | Vigna unguiculataL.Walp (Karamani in tamil) | Fabaceae | Seeds | Carotene, thiamine. riboflavin, niacin, folic acid, vitamin C, tripsin inhibitors as A2a,A2b,A2c,A2d,A2e; phytohemagglutinin, α-cedrene,1,8-cineole, hexanal, limonene, nonanal, α-pinene and β-pinane. | C8H9NO2 |
| 94 | Vitis viniferaL. (Thirachai) | Vitaceae | Leaves | Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, sugars, sterols, amino acids and minerals | CCl4 |
| 95 | Vitex trifolia (Moovilai nochi) | Verbenaceae | Leaves | Alkaloids, saponin, tannin, phenols, terpenoids, flavonoids, steroids | CCl4 |
| 96 | Wedelia calendulacea | Asteraceae | Whole plant | Flavonoids, wedelolactone | C6H13NO5 |
| 97 | Woodfordia fruticosa Kurz | Lythraceae | Flowers | Malvidin, pentose, glycosides, quercetin, Kaempferol-3-Glycoside, hecogenin, carotene, carbohydrates, insulin, 3 mannitol, lawsone, aspartic acid, protein, riboflavin, citric acid, punicaline, estrone | CCl4 |
| 98 | Xylopia aethiopica | Annonaceae | Fruit | Mono and sesqui terpenes,a-pinene, myrcene, p-cymene, limonene, linalool, terpinen-4-ol , R-terpineol, and 1,8-cineole are the most predominant. | C8H9NO2 |
| 99 | Zanthoxylum ArmatumDC. | Rutaceae | Bark | Nitidine, dihydronitidine, oxynitidine, fagaronine, dihydroavicine, chelerythrine, ihydrochelerythrine, methoxychelerythrine, norchelerythrine, oxychelerythrine, decarine and fagaridine), furoquinolines carbazoles , aporphines , canthinones, acridones and aromatic and aliphatic amides. | CCl4 |
| 100 | Zingiber officinaleRos. (Inchi) | Zingiberaceae | Rhizome | Fibres, proteins, starch, carbohydrates, resin, glutamine, thrionin, free aminoacid, zingiberol, zingiberin, glutamic acid, aspartic acid | C8H9NO2 |
| 101 | Ziziphus mauritianaL. (Ilanthai) | Rhamnaceae | Leaves, fruits, bark | Sugars, mucilage | CCl4 |
Table 1: Hepatoprotective Plants with Chemical Constituents and Hepatotoxic Agents
Avicennia alba (Blume):
Avicennia alba, (Avicenniaceae family), is used in Indian system of medicine for the treatment of several types of conditions such as scabies, rheumatism, paralysis, asthma and snake-bites, skin disease and ulcer[42]. The plant is rich source of steroids, triterpenes, saponins, flavonoids, alkaloids and tannins[43]. Recently, find the three naphthoquinones and their analogues, named avicequinone-A, avicequinone-B, avicequinone-C and avicenol-A, avicenol-B, avicenol-C respectively[44]. These are compounds isolated from the stem bark and isolated a new flavonoid, 2-[3'-(3"-(hydroxymethyl) oxiran-2"-yl)-2'-methoxy-4'-(methoxymethyl) phenyl]-4Hchromen-4-one from the aerial parts. Hepatotoxicity was induced by C8H9NO2 and this experiment was assessment by biochemical parameters such as AST, Alkaline Phosphatase (ALP), ALT and total bilirubin (serum bilirubin). The in vivo antioxidant such as superoxide dismutase, catalase, Glutathione, vitamin C and E, and thiobarbituric acid reactive substances, and histopathological changes in liver were studied along with C25H22O10 as standard hepatoprotective agent[45]. Results of this study showed preliminary phytochemical analysis of the ethanolic extract shows the presence of alkaloids, flavonoids, tannins, terpenoids, proteins and steroids. Treatment with plant extract to C8H9NO2 administered rats caused a significant reduction in the values of AST, ALP, ALT and total bilirubin almost comparable to standard drug C25H22O10. Hepatoprotective activity was confirmed by histopathological assessment of the liver tissue of control and treated animals. In this research, it can be concluded that C2H5OH extract of leaves possess hepatoprotective effect[46].
Anisochilus carnosus (L) Wall.:
Anisochilus carnosus (Lamiaceae family) “karppura-valli” is an annual herb and has been traditionally used for the treatment of gastrointestinal disorders, respiratory disorders, cough, cold and fever[47]. Its popular herbal preparation together with Ocimum basilicum, Mentha piperita and Alpinia galanga is used against the symptoms of influenza, dermatitis and the slight illness that derives from the bites of bugs[48]. Essential oils have been extracted by hydro distillation from the leaves and have been reported to be antimicrobial in nature[49]. A pharmacological activity of this plant shows anti-inflammatory activity[50], antiulcer activity[51], antifungal property[52] and anticancer property[53]. Previously reported that, this plant shows phytochemicals active compounds such as saponins, tannins, flavonoids (apigenin and luteolin), phytosterols, triterpenoids and essential oil components (carvacrol, β-selinene, camphor, α-cis-bergamotene and caryophyllene) etc.,[54]. Analysis of leaf and leaf callus extracts was done by qualitative analysis and was used for hepatotoxicity induced by alcohol. This research results revealed that C2H5OH leaf extract pretreated HepG2-Human liver cancer cell line show 94 % cell viability compared to the standard C25H22O10 pretreated HepG2 cells which showed 81 % cell viability. This plant leaf callus extracts also showed significant hepatoprotective activity where C2H5OH callus extract pretreated HepG2 cells showed 86 % viability after intoxication with alcohol. Results revealed that HepG2 cell viability percentage is dose dependent. Phytochemical studies revealed the presence of different secondary metabolites in leaf and leaf callus extracts that shows hepetoprotective activities[55].
Baliospermum montanum (Willd) Muell. Arg:
Baliospermum montanum (Euphorbiaceae family) “pey-amanakku” is one of the very important plant of Ayurveda being used for millennia as a purgative along with its wide-ranging health benefits and is useful against many more disorders. Danti has been explained in various classics as a major as well as minor ingredient of various formulations used in different diseases.
Single-handed information on the external application of usage of Danti is not available[56]. C2H5OH leaf extract gas chromatography mass spectrometric spectrum showed various phyto-constituents like Olean-12-ene, 3β-methoxy, α-amyrin, lanosterol, Lup-20 (29)-en-3-ol, acetate, betulin etc.,[57]. On the other hand, hepatoprotective activity of methanol extract from the roots of Baliospermum montanum and its methanol fraction were carried out using C2H5NS induced liver damage in albino rats. This study was assessed by glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, alkaline phosphatase, total bilirubin, total cholesterol, total protein and albumin in serum. At the same time analyzed histopathology of liver sections confirmed that, pre-treatment with methanol extract and methanol fraction prevented hepatic damage induced by C2H5NS. It is suggested that, the presence of flavonoids in methanol extract and its methanol fraction may be responsible for hepatoprotective properties. HPTLC profile of flavonoids of bio-active extracts was developed using quercetin-3-O-galactosyl-7-O-rhamnoside as a marker. Methanolic extract of Baliospermum montanum has shown strong hepatoprotective activity[58].
Centella asiatica L.:
Centella asiatica (Apiaceae family), which is a slender, prostrate, glabrous, perennial creeping herb rooting at the nodes, with simple petiolate, palmately lobed leaves and it has various pharmacological activities like memory enhancing, anti-inflammatory, antioxidant, wound healing, and immune-stimulant, anti-anxiety (anti-hypertensive), anti-stress and anti-epilepsy. Various health benefits of Centella asiatica have led to the amplified usage of this plant in food and beverages[59]. It has been extensively used for the treatment of ailments like inflammation, syphilis, mental illness, skin diseases, rheumatism, epilepsy, hysteria, diarrhea, wounds, dehydration and ulcers[60]. Aqueous extract of the plant aerial parts extracted from essential oil. Around 64 volatile compounds were identified from the essential oil p-cymene (35 %) is the predominant compound in the leaf essential oil, such as α-thujene, α-pinene, camphen, γ-2-carene, α-terpene, t-cymene, limonene, p-menth, 3,8-diene, c-terpinens, linalool, allo-ocimene, 3-non-2-one, menthone, methyl cavacrol, trans myrtenol, bornyl acetate, myrtenyl acetate, α-elemene, bicyoloelemens, nonanal, E-caryophyllene, guaiene, B-caryophyllene etc.,[61]. The protective effect of Centella asiatica is against C8H9NO2 liver injury which may be attributed to its hepatoprotective activity[62].
Clitoria ternatea L.:
Clitoria ternatea (Fabaceae family) “Kannikkodi” is a medicinal plant native to tropical equatorial Asia is commonly used in folk medicine to treat various diseases[63]. The leaves and roots are used in the treatment of a number of ailments including body aches, infections, urinogenital disorders, and as an anthelmintic and antidote activity to animal stings. The young shoots, leaves, flowers and tender pods are eaten as a vegetable in Kerala (India) and in the Philippines. In Malaysia, the leaves impart a green color to food and the flowers to impart a bright blue color to rice cakes. It’s commonly used in Ayurvedic medicine to treat various types of ailments including memory enhancer, notropic, anti-stress, anxiolytic, antidepressant, anticonvulsant, tranquilizing and sedative agent. Various secondary metabolites such as polyphenolic flavonoids, anthocyanin glycosides, pentacyclic triterpenoids and phytosterols have been reported from this plant. Flavonoils i.e., kaempherols, quercetin and myricetin and their glycosides were also isolated from this plant[64]. Mass spectral analysis of leaf methanolic extract compounds, such as Butyl-2-methylpropylphthalate, Pentadecanoic acid ME, Decyloctylphthalate, 3-methylhexane, Cyclotetradecane, 2-methylpentane, Decyloctylphthalate, 3-methylhexane, Butyl-2-ethylhexylphthalate, Isopropylbenzene etc.,[65] was carried out. Rats treated with Clitoria ternatea leaf extracts showed positive results in protecting themselves against damage caused by C8H9NO2. Interestingly, the treated group with Clitoria ternatea extracts was observed to possess a reduced level of enzymes such as AST, ALT and bilirubin compared to a raised level in AST, ALT, and bilirubin in C8H9NO2-treated group[66].
Eclipta alba (Linn):
The plant Eclipta alba (Family: Asteraceae) having important role in the traditional Ayurvedic, “Karisilanganni” Unani systems of holistic health and herbal medicine of the east, have reported to possess Hepatoprotective, antimicrobial, anti-inflammatory, analgesic, immune modulatory, antiviral and promoter for blackening and growth of hair. Important source of chemicals is wedelolactone, dimethyl wedelolactone exhibit antihepatotoxic activities. The traditional knowledge with its holistic and systematic approach supported through experimental base can serve as an innovative and powerful discovery of natural 5α-reductase inhibitor[67]. Eclipta alba having important role in the traditional Ayurvedic and Unani systems of holistic health and herbal medicine of the east. The principal constituents of Eclipta alba are coumestan derivatives like wedololactone (1.6 %), dimethyl wedelolactone, desmethyl-wedelolactone-7 glucoside and other constituents are ecliptal, ß-amyrin, luteolin-7-O-glucoside, hentriacontanol, heptacosanol, stigmasterol. All the parts of Eclipta alba and chemical constituents are used as anticancer, anti-leprotic, analgesic, antioxidant, anti-cytotoxic, anti-haemorrhagic, anti-hepatotoxic, antiviral, antibacterial, spasmogenic, hypotensive, hepatoprotective ovicidal, promoter for blackening and growth of hair[68]. Therefore, this plant plays a momentous role in medicinal field and it has promising cosmetic as well as therapeutic application and hence its extraction is essential. Root are analyzed by mass spectral analysis, and exhibit various phyto-constituents such as 2-Thiophenecarbaldehyde, 5-[5-(thien-2-yl)thien-2-yl]-Benzyl-beta-d-glucoside, Octadeca-9,12-dienoic acid methyl ester, 2-Propenoic acid, 3-(4-hydroxy-3- methoxyphenyl)-,methyl ester, Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester, Dodecanoic acid, Benzenepropanoic acid, 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol, Retinol[69]. It’s significantly counteracted CCl4-induced inhibition of the hepatic microsomal drug metabolizing enzymes. Further, the loss of hepatic is lysosomal acid, phosphatase and alkaline phosphatase by CCl4. The study shows the hepatoprotective activity[70].
Justicia adhatoda (L) Willd.:
Justicia adhatoda (Family: Acanthaceae) with the common name ‘‘Adathoda” is a perennial shrub, and mainly consist of quinazoline alkaloids like visicine, vasicinone, vasicol, pregnane along with other minor constituents like adhatonine, vasicinol and vasicinolone[71]. Extracts have been used for the treatment of various diseases and disorders in Ayurveda and tuberculosis[72]. Justicia adhatoda leaf extract is a known antioxidant and has also been reported to possess hepatoprotective activity[73]. The present study has been undertaken to explore the hepatoprotective action of isolated vasicinone from the leaves in mice. Preliminary phytochemical analysis shows alkaloids, carbohydrates, glycosides, cardiac glycosides, saponins, hydroxyanthraquinones, phlobatannins, proteins, xanthoprotein, amino acids, steroids, terpenoids, phenols, volatile oil, fatty acid, emodins[52]. Justicia adhatoda leaf showed significant hepatoprotective effect at doses of 50 to 100 mg/kg on liver damage induced by D-galactosamine in rats[74].
Phyllanthus emblica (Linn.):
Phyllanthus emblica (Family: Euphorbiaceae). All parts of the plant are used for medicinal purposes; especially the fruits are found having tremendous pharmacological applications. They are used both as a medicine and as a tonic to build up lost vitality and vigor, and it is highly nutritious, important dietary source of vitamin C, amino acids and minerals. In traditional medicine, the fruits are used for the treatment of diarrhea, jaundice and inflammation. Further, they also showed antidiabetic, hypolipidemic, antibacterial, antioxidant, antiulcerogenic, hepatoprotective, gastroprotective, and chemopreventive properties[75]. Phenolic components were find out from Phyllanthus emblica leaf, flower, fruit by column chromatography and associated with Nuclear Magnetic Resonance (NMR) spectrum. It is acknowledged that gallotannins are the major phenolic constituents of leaf, flower and fruit. The NMR data with the literature led to identification of compounds such as mucic acid 1,4-lactone-5-O-gallate, 2-keto-glucono-lactone, 6-methyl ester[76]. The study also confirms the hepatoprotective and antioxidant activities of leaves of Phyllanthus emblica[77].
Pisonia grandis R.Br:
Pisonia grandis (Family: Nyctaginaceae). Leaves, stems and roots of this species are extensively used by the tribes in the preparation of several folk medicines and is traditionally used as anti-rheumatic and antifungal. It is also pharmacologically studied for its anti-fungal, anti-oxidant, anti-microbial, anti-inflammatory, anti-diabetic, diuretic, analgesic and wound healing properties[78], then phytoconstituents such as protein, carbohydrate, sterols, alkaloids, flavanoids, quinones, fatty acids, tannins, terpenoids, phenols, saponins, glycosides, coumarin, xanthoproteic acid etc.,[79] from the C2H5OH extract. The C2H5OH and aqueous extracts of leaves are screened for its hepatoprotective potential against liver injury induced by CCl4, C8H9NO2 or C2H5NS and chronic liver damage induced by CCl4 in rats. Pretreatment of animals with the extract reduced inflammation and degenerative changes. Histological examination of liver tissues supported the hapatoprotection by both the extracts and thus the C2H5OH and aqueous extracts showed significant hepatoprotective activity in CCl4 induced acute and chronic liver damage[75].
Syzygium cumini (L.) Naval:
Syzgium cumini (Family: Myrtaceae), gives the authority of due to the presence of the various phytochemical constituents such as alkaloids, fatty acids, steroids and tannins. Biochemical analysis and histopathology were achieved by collecting the blood samples and liver tissues. The methanol extracts of plant seed shows significantly increase the serum protein and decrease the enzyme level in control and treated groups as compared to that of the CCl4 treated group. The hepatic tissues protected by the extract of seeds in both the doses and C25H22O10 from CCl4 induced stress which indicates by histological examination of liver tissues. It was concluded that extract of seed has hepatoprotective activity[52].
Some studies were carries out for the presence of anti-diabetic, hepatoprotective, anti-inflammatory, antioxidant, anti-ulcers, anti-diarrheal and anti-microbial activities. It contains anthocyanins, glucoside, ellagic acid, isoquercetin, kaemferol and myrecetin[16]. Photochemical analysis of this plant identified gallic acid, cyanidin glycoside, glycoside jambolin, triterpenoids, tannins, gallotanins, essential oils, myricetin, β-sitosterol, myricyl alcohol etc.,[80]. Leaves and seeds from aqueous extracts (LASc, SASc, respectively) as well as their effect in a 2,2 azobis-2-amidinopropane dihydrochloride (AAPH) induced model of oxidative damage in human lymphocytes, in vitro[79].
Conclusion
This review results exhibit Syzgium cumini has protective and immune-modulatory effects on AAPH-induced damage in lymphocytes, assessed by in vitro studies. The protective effect of these indigenous medicinal plant extracts against CC14, C8H9NO2, and C2H5NS may be related to polyphenolic compounds, terpenoids, alkaloids, coumarines, phytosterols. Polyphenolic compounds such as flavonoids can protect the cells against emptying reduced glutathione via increasing the capability of antioxidant enzymes, and shows antioxidant activity, free radical scavenging and anti-lipoperoxidant agent is helpful for hepatoprotection. Furthermore, these phytocompounds with antioxidant properties can counteract free radicals in the environment and therefore avoid their destructive effects. Terpenoids such as carotenoids with anti-hepatotoxic activity are also known as antioxidants. Ursolic acid is a triterpene, with potential hepatoprotective effects. Therefore, herbal medications should be recommended within the setting of more finely-conducted clinical trials, in spite of, better training of both patients and physicians about herbal preparations seems necessary.
Acknowledgments:
The authors are would like to acknowledge the help and support stretched by the Department of Zoology, Annamalai University, Chidambaram in Tamil Nadu, India.
Conflict of interest:
The authors report no conflict of interest in this work.
References
- Al-Snafi AE, Thuwaini MM. Arabian Medicinal plants with hepatoprotective activity. Res J Pharm Biol Chem Sci 2018;9(5):1469-97.
- Sanghvi MM, Hotez PJ, Fenwick A. Neglected tropical diseases as a cause of chronic liver disease: The case of schistosomiasis and hepatitis C co-infections in Egypt. J Liver Int 2013;33(2):165-8.
- Das S, Bandyopadhyay S, Ramasamy A, Mondal S. Evaluation of hepatoprotective activity of aqueous extracts of leaves of Basella alba in albino rats. Nat Prod Res 2015;29(11):1059-64.
[Crossref] [Google Scholar] [PubMed]
- Holt MP, Ju C. Mechanisms of drug-induced liver injury. AAPS J 2006;8(1):E48-54.
[Crossref] [Google Scholar] [PubMed]
- Domitrović R, Potočnjak I. A comprehensive overview of hepatoprotective natural compounds: Mechanism of action and clinical perspectives. Arch Toxicol 2016;90(1):39-79.
[Crossref] [Google Scholar] [PubMed]
- Aktay G, Deliorman D, Ergun E, Ergun F, Yeşilada E, Cevik C. Hepatoprotective effects of Turkish folk remedies on experimental liver injury. Ethnopharmacology 2000;73(1):121-9.
[Crossref] [Google Scholar] [PubMed]
- Dhiman RK, Chawla YK. Herbal medicines for liver diseases. Dig Dis Sci 2005;50(10):1807-12.
[Crossref] [Google Scholar] [PubMed]
- Muriel P, Rivera‐Espinoza Y. Beneficial drugs for liver diseases. J Appl Toxicol 2008;28(2):93-103.
[Crossref] [Google Scholar] [PubMed]
- Al-Attar M, Al-Rethea A. Chemoprotective effect of omega-3 fatty acids on thioacetamide induced hepatic fibrosis in male rats. Saudi J Biol Sci 2017;24(4):956-65.
[Crossref] [Google Scholar] [PubMed]
- Girish C, Koner BC, Jayanthi S, Ramachandra Rao K, Rajesh B, Pradhan SC. Hepatoprotective activity of picroliv, curcumin and ellagic acid compared to silymarin on paracetamol induced liver toxicity in mice. Fundam Clin Pharmacol 2009;23(6):735-45.
[Crossref] [Google Scholar] [PubMed]
- Ganesan K, Sukalingam K, Xu B. Solanum trilobatum L. ameliorate thioacetamide-induced oxidative stress and hepatic damage in albino rats. Antioxidants 2017;6(3):68.
[Crossref] [Google Scholar] [PubMed]
- Hussain A, Ali AA, Ayaz S, Akram M. Hepatoprotective effects of various medicinal plants: A systematic review. J Pharmacog Phytochem 2021;10(3):109-21.
- Ielciu I, Sevastre B, Olah NK, Turdean A, Chișe E, Marica R, et al. Evaluation of hepatoprotective activity and oxidative stress reduction of Rosmarinus officinalis L. shoots tincture in rats with experimentally induced hepatotoxicity. Molecules 2021;26(6):1737.
[Crossref] [Google Scholar] [PubMed]
- Amin ZA, Bilgen M, Alshawsh MA, Ali HM, Hadi AH, Abdulla MA. Protective role of Phyllanthus niruri extract against thioacetamide-induced liver cirrhosis in rat model. Evid Based Complement Alternat Med 2012;2012:241583.
[Crossref] [Google Scholar] [PubMed]
- Sukalingam K, Ganesan K, Xu B. Protective effect of aqueous extract from the leaves of Justicia tranquebariesis against thioacetamide-induced oxidative stress and hepatic fibrosis in rats. Antioxidants 2018;7(7):78.
[Crossref] [Google Scholar] [PubMed]
- Raj VP, Chandrasekhar RH, Vijayan P, Dhanaraj SA, Rao MC, Rao VJ, et al.In vitro and in vivo hepatoprotective effects of the total alkaloid fraction of Hygrophila auriculata leaves. Indian J Pharmacol 2010;42(2):99.
[Crossref] [Google Scholar] [PubMed]
- Ai G, Liu Q, Hua W, Huang Z, Wang D. Hepatoprotective evaluation of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) Medic: In vitro and invivo studies. J Ethnopharmacol 2013;146(3):794-802.
[Crossref] [Google Scholar] [PubMed]
- Shailajan S, Joshi M, Tiwari B. Hepatoprotective activity of Parmelia perlata (Huds.) Ach. against CCl4 induced liver toxicity in albino Wistar rats. J Appl Pharm Sci 2014;4(2):70-4.
- Zhou G, Chen Y, Liu S, Yao X, Wang Y. In vitro and in vivo hepatoprotective and antioxidant activity of ethanolic extract from Meconopsis integrifolia (Maxim.) Franch. J Ethnopharmacol 2013;148(2):664-70.
[Crossref] [Google Scholar] [PubMed]
- Robin S, Sunil K, Rana AC, Nidhi S. Different models of hepatotoxicity and related liver diseases: A review. Int Res J Pharm 2012;3(7):86-95.
- Binduja S, Visen PK, Dayal R, Agarwal DP, Patnaik GK. Protective action of ursolic acid against chemical induced hepato-toxicity in rats. Indian J Pharmacol 1996;28(4):232-9.
- Huang B, Ban X, He J, Tong J, Tian J, Wang Y. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem 2010;120(3):873-8.
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest 2012;122(4):1574-83.
[Crossref] [Google Scholar] [PubMed]
- Al-Bader A, Omu AE, Dashti H. Chronic cadmium toxicity to sperm of heavy cigarette smokers: Immunomodulation by zinc. Arch Androl 1999;43(2):135-40.
[Crossref] [Google Scholar] [PubMed]
- Yeh CN, Maitra A, Lee KF, Jan YY, Chen MF. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: An animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis 2004;25(4):631-6.
[Crossref] [Google Scholar] [PubMed]
- Kaur V, Kumar M, Kaur P, Kaur S, Singh AP, Kaur S. Hepatoprotective activity of Butea monosperma bark against thioacetamide-induced liver injury in rats. Biomed Pharmacother 2017;89:332-41.
[Crossref] [Google Scholar] [PubMed]
- Bashandy SA, Alaamer A, Moussa SA, Omara EA. Role of zinc oxide nanoparticles in alleviating hepatic fibrosis and nephrotoxicity induced by thioacetamide in rats. Can J Physiol Pharmacol 2018;96(4):337-44.
[Crossref] [Google Scholar] [PubMed]
- Feng YM, Wang X, Wang L, Ma XW, Wu H, Bu HR, et al. Efficacy and safety of combination therapy of chemoembolization and radiofrequency ablation with different time intervals for hepatocellular carcinoma patients. Surg Oncol 2017;26(3):236-41.
[Crossref] [Google Scholar] [PubMed]
- Sunderman FW, Sunderman FW. Association of clinical scientists. Laboratory diagnosis of liver diseases. St Louis 1968;542.
- Njouendou AJ, Nkeng-Efouet AP, Assob Nguedia JC, Chouna JR, Veerapur V, Thippeswamy BS, et al. Protective effect of Autranella congolensis and Sapiumellipticum stem bark extracts against hepatotoxicity induced by thioacetamide. Pharmacology 2014;2:38-47.
- Rehman J, Akhtar N, Asif HM, Sultana S, Ahmad M. Hepatoprotective evaluation of aqueous-ethanolic extract of Capparis decidua (Stems) in paracetamol induced hepatotoxicity in experimental rabbits. Pak J Pharm Sci 2017;30(2):507-11.
[Google Scholar] [PubMed]
- Ahmad F, Tabassum N. Experimental models used for the study of antihepatotoxic agents. J Acute Dis 2012;1(2):85-9.
- Pushpangadan P, Iyengar PK, Damodaran VK. Role of traditional medicine in primary health care. Science for Health. Published By State Committee On Science, Technology And Environment, Govt. Of Kerala. 1995.
- Kumar CH, Ramesh A, Kumar JS, Ishaq BM. A review on hepatoprotective activity of medicinal plants. Int J Pharm Sci Res 2011;2(3):501-15.
- Dhiman RK, Chawla YK. Herbal medicines for liver diseases. Dig Dis Sci 2005;50(10):1807-12.
[Crossref] [Google Scholar] [PubMed]
- Ball KR, Kowdley KV. A review of Silybum marianum (milk thistle) as a treatment for alcoholic liver disease. J Clin Gastroenterol 2005;39(6):520-8.
[Crossref] [Google Scholar] [PubMed]
- Jayaraj R, Deb U, Bhaskar AS, Prasad GB, Rao PL. Hepatoprotective efficacy of certain flavonoids against microcystin induced toxicity in mice. Environ Toxicol 2007;22(5):472-9.
[Crossref] [Google Scholar] [PubMed]
- Burkill IH. Ministry of agriculture and co-operatives. 2nd ed. Malaysia: Kuala Lumpur; 1966. p. 274-9.
- Kar DR, Farhad MS, Sahu PK. A review on pharmacological profiles of ethno-medicinal plant: Avicennia AlbaBlume. Int J Pharm Tech Res 2015;7:370-3.
- Bandaranayake W. Survey of mangrove plants from Northern Australia for phytochemical constituents and UV-absorbing compounds. Curr Topic Phytochem 1995;14:69-78.
- Ito C, Katsuno S, Kondo Y, Tan HT, Furukawa H. Chemical constituents of Avicenniaalba. Isolation and structural elucidation of new naphthoquinones and their analogues. Chem Pharm Bull 2000;48(3):339-43.
[Crossref] [Google Scholar] [PubMed]
- Kamble SY, More TN, Patil SR, Pawar SG, Bindurani R, Bodhankar SL. Plants used by the tribes of Northwest Maharashtra for the treatment of gastrointestinal disorders. Indian J Tradit Knowl 2008;7:321-5.
- Subramanian SS, Nair AG. Flavonoids of the leaves of Mentha spicata and Anisochilus carnosus. Phytochemistry 1972;11(1):452-4.
- Senatore F, Lentini F, Venza F, Bruno M, Napolitano F. Composition and antibacterial activity of the essential oil of Anisochilus carnosus (Linn. fil.) Benth, a Tamil plant acclimatized in Sicily. Flav Frag J 2003;18(3):202-4.
- Grover JK, Adiga G, Vata V, Rathi SS. Extracts of Anisochilus carnosusprevents development of experimental inflammation. J Ethanopharm 2001;7(8):159-64.
- Mohammed A, Kumar RJ, Santosh HY, Nagashruthi MH. Antiulcer activity of Anisochilus carnosusleaf extracts in pylorus ligation rats. Indian Drugs 2008;45(12):979.
- Malathi R, Kaviyarasan D, Chandrasekar S. Preliminary phytochemical analysis of Justicia adhatoda leaves extract using different solvents. Int J Pharm Drug Anal 2018;6(2):186-90.
- Muthuraman MS, Santharam L, Ariraman S, Pemaiah B. Studies on anticancer and antimicrobial efficacy of Anisochilus carnosus wallich-extract. Int J Pharm Pharm Sci 2012;4:132-5.
- Shetty V, Lobo R, Kumar N, Lingadakai R, Pai GC, Balla M. Antimicrobial activity of Anisochilus carnosus (LF) wall against the human gastric pathogen Helicobacter pylori. Asian J Pharm Clin Res 2017;10(10):292-5.
- Reshi NA, Shankarasingh SM, Hodiyala GV. Evaluation of hepatoprotective potential of leaf and leaf callus extracts of Anisochilus carnosus(L) wall. Int J Phytomed 2018;10(3):156-61.
- Gupta A, Gupta V, Goyal A, Kak A, Pandey C. Standardization of the tetrazolium test in Baliospermum montanum(Willd.) Muell.-Arg. Seed Sci Technol 2010;38(2):513-6.
- Tripathi YC, Prabhu VV, Pal RS, Mishra RN. Medicinal plants of Rajasthan in Indian system of medicine. Anc Sci Life 1996;15(3):190-212.
[Google Scholar] [PubMed]
- Singh PB, Aswal BS. Medicinal plants of Himachal Pradesh used in Indian pharmaceutical industry. Bull Med Ethnobot Res 1992;13:172-208.
- Gopakumar K, Yoganarasimhan SN, Nair KV, Murthy KR, Shantha TR, Vijayalakshmi B. Plants used in Ayurveda from Chikmagalur district, Karnataka. J Econ Taxon Bot 1991;15:379-89.
- Rout SP. Danti (Baliospermum montanumWilld.) and its external applications reported in various Ayurvedic pharmacopoeias: An evidence based review. Int J Res Ayurveda Pharm 2017;8(3):52-9.
- Sushen U, Chouhan A. Chemical composition of essential oil of Centella asiaticaL. by GC-MS analysis. Eur J Pharm Med Res 2018;5:544-8.
- Sivakumar V, Sadiq AM, Bharathi SD. Hepatoprotective activity of Centella asiatica linn. against paracetamol induced liver damage in experimental animals. Emergent Life Sci Res 2018;4(1):19-26.
- Mukherjee PK, Kumar V, Kumar NS, Heinrich M. The Ayurvedic medicine Clitoriaternateafrom traditional use to scientific assessment. J Ethnopharmacol 2008;120(3):291-301.
[Crossref] [Google Scholar] [PubMed]
- Mukherjee PK, Kumar V, Houghton PJ. Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phytother Res 2007;21(12):1142-5.
[Crossref] [Google Scholar] [PubMed]
- Thakur AV, Ambwani S, Ambwani TK, Ahmad AH, Rawat DS. Evaluation of phytochemicals in the leaf extract of Clitoria ternatea willd. through GC-MS analysis. Trop Plant Res 2018;5(2):200-6.
- Nithianantham K, Shyamala M, Chen Y, Latha LY, Jothy SL, Sasidharan S. Hepatoprotective potential of Clitoria ternatea leaf extract against paracetamol induced damage in mice. Molecules 2011;16(12):10134-45.
[Crossref] [Google Scholar] [PubMed]
- Jadhav VM, Thorat RM, Kadam VJ, Sathe NS. Eclipta alba Linn-‘‘kesharaja’’: A review. J Pharm Res 2009;2(8):1236-41.
- Mukhopadhyay G, Kundu S, Sarkar A, Sarkar P, Sengupta R, Kumar C. A review on physicochemical and pharmacological activity of Eclipta alba. Pharm Innov J 2018;7(9):78-83.
- Naik SK, Gurushanthaiah M, Raju NG, Johnson WM, Mahesh GM. Extraction of Bio-active compounds of Eclipta albathrough GC-MS analysis. Res J Pharm Biol Chem Sci 2018;9(2):297-302.
- Tabassum N, Agrawal SS. Hepatoprotective activity of Eclipta alba Hassk. against paracetamol induced hepatocellular damage in mice. JK Pract 2004;11(4):278-80.
- Anjara J, Bhatt G. A glossary of selected indigenous medicinal plant of India, Nature Heals, SRISTI, Ahmedabad; 1995. p. 11.
- Chowdhury BK, Bhattacharyya P. A further quinazoline alkaloid from Adhatodavasica. Phytochemistry 1985;24(12):3080-2.
- Amin AH, Mehta DR. A bronchodilator alkaloid (vasicinone) from Adhatoda vasica Nees. Nature 1959;184(17):1317.
[Crossref] [Google Scholar] [PubMed]
- Soni S, Anandjiwala S, Patel G, Rajani M. Validation of different methods of preparation of Adhatoda vasica leaf juice by quantification of total alkaloids and vasicine. Indian J Pharm Sci 2008;70(1):36-42.
[Crossref] [Google Scholar] [PubMed]
- Bhattacharyya D, Pandit S, Jana U, Sen S, Sur TK. Hepatoprotective activity of Adhatoda vasica aqueous leaf extract on D-galactosamine-induced liver damage in rats. Fitoterapia 2005;76(2):223-5.
[Crossref] [Google Scholar] [PubMed]
- Majeed M, Bhat B, Jadhav AN, Srivastava JS, Nagabhushanam K. Ascorbic acid and tannins from Emblica officinalis Gaertn. fruits-A revisit. J Agric Food Chem 2009;57(1):220-5.
[Crossref] [Google Scholar] [PubMed]
- Srirama R, Deepak HB, Senthilkumar U, Ravikanth G, Gurumurthy BR, Shivanna MB, et al. Hepatoprotective activity of Indian Phyllanthus. Pharm Biol 2012;50(8):948-53.
[Crossref] [Google Scholar] [PubMed]
- Elumalai A, Eswaraiah MC, Rahman HA. Pisonia grandis R. Br-A medicinal plant: A review. Int J Pharm Bio Sci. 2012;3(1):76-80.
- Sudharameshwari K, Suganya M, Salini R. Studies on phytochemical and antimicrobial activity in Pisonia grandis R. Br. Int J Pharma Bio Sci 2018;9(4):213-20.
[Crossref]
- Thenmozhi S, Kameshwaran S, Subasini U, Sathyamurthy D, Dhanalakshmi M. Hepatoprotective constituents from the leaves of Pisonia grandisR. Br. Pharmacologia 2013;4(5):383-90.
- Islam M, Hussain K, Latif A, Hashmi FK, Saeed H, Bukhari NI, et al. Evaluation of extracts of seeds of Syzygium cuminiL. for hepatoprotective activity using CCl4-induced stressed rats. Pak Vet J 2015;35(2):197-200.
- Arunpandiyan J, Roselin RB, Chitra RS, Kaniga P. Review on Syzygium cumini(L.). World J Pharm Pharm Sci 2018;7:499-507.
- Ajeet S, Navneet V. Ethnobotanical uses antimicrobial potential, pharmacological properties and phytochemistry of Syzygium cuminiLinn (syn. Eugenia Jambolana(Jamun)-A review. Int J Innov Pharm Sci Res 2018;6:32-47.
- Borges RM, Bitencourt PE, Stein CS, Bochi GV, Boligon A, Moresco RN, et al. Leaves and seeds of Syzygium cumini extracts produce significant attenuation of 2,2 azobis-2-amidinopropane dihydrochloride-induced toxicity via modulation of ectoenzymes and antioxidant activities. J Appl Pharm Sci 2017;7(6):37-48.
- Ward FM, Daly MJ. Hepatic Disease. In: Walker R, Edwards C, editors. Clinical Pharmacy and Therapeutics. 5thed. New York: Churchill Livingstone; 1999. p. 195-212.