- *Corresponding Author:
- J. Zhou
Department of Pharmaceutics, China Pharmaceutical University, China
E-mail: zhoujianping_vip@outlook.com
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3) Spl Issue “294-307” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Tamsulosin hydrochloride sustained-release capsules contained core-shell structure based pellets with tamsulosin hydrochloride as middle layer and the weight of excipients was 800 times more than tamsulosin hydrochloride. Therefore, the extraction should release the related substances of tamsulosin hydrochloride from the pellets and minimize the degradation of tamsulosin hydrochloride during the extraction. And the chromatographic methods should be specific to separate the excipients and their degradation products with the main related substances of tamsulosin hydrochloride. In present study, tamsulosin hydrochloride and its related substances were extracted with sonication after incubated at 50° for 10 min in methanolic sodium hydroxide solution and the dissolved excipients was then precipitate with acetonitrile. The extraction was further separated on a C18 column with gradient elution. Forced degradation proved that 5 possible related substances (retinoschisin 1 to retinoschisin 5) from tamsulosin hydrochloride capsules could be separated with the degradation products from excipients. These related substances were identified with electrospray ionization mass spectrometry/mass spectrometry. The method was also validated and results showed the resolution of main related substances of tamsulosin hydrochloride and the degradation products of excipients was above 1.5. The limit of detection of retinoschisin 4 and retinoschisin 5 equals to 0.1 % of tamsulosin hydrochloride in capsules. The inter-day precisions and recovery were 1.14 %-2.18 % (n=12), 100.0 %-100.8 % (n=9), respectively. With the established method, related substances in tamsulosin hydrochloride sustained-release capsules proved <0.1 % to 0.28 %, with retinoschisin 4 as main related substance. This method could be used for the evaluation of related substances in tamsulosin hydrochloride sustained-release capsules and for its routine quality control and the extraction could be applied to other core-shell structure pellets contained capsules.
Keywords
Tamsulosin hydrochloride, sustained-release capsule, extraction, liquid chromatography-mass spectrometry/mass spectrometry, high performance liquid chromatography
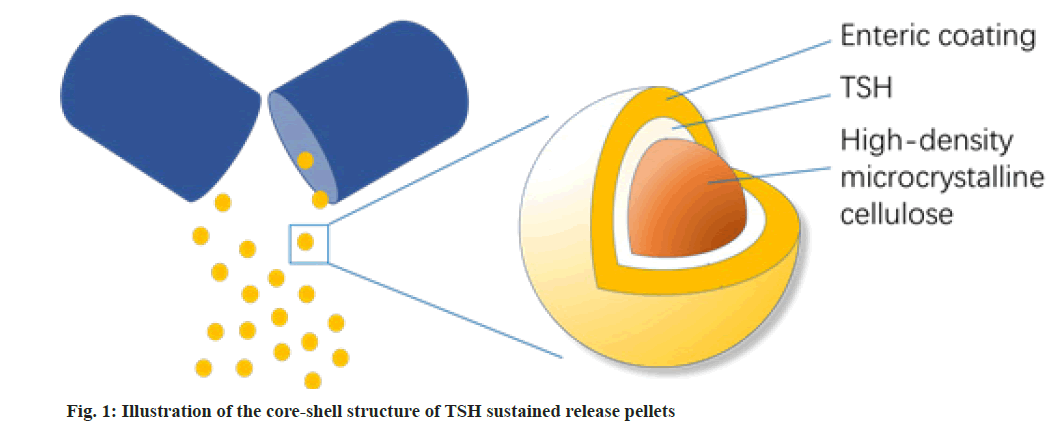
Tamsulosin Hydrochloride (TSH) is a third-generation selective postsynaptic alpha (α)1A-adrenergic receptor antagonist, which has a greater affinity for α1A-receptors than for α1B-receptors[1]. It was widely applied for the treatment of benign prostatic hyperplasia by relaxing smooth muscle in the prostate and bladder neck, thereby enhancing bladder emptying[2]. Sustained-release capsules are one of the main dosage forms of TSH. The TSH sustained-release capsules possessed coreshell structure based pellets, with TSH in the middle, a dense enteric coating and high-density microcrystalline cellulose as the out layer and inner core, respectively as shown in fig. 1.
Therefore, to identify and determine the related substances in TSH sustained-release capsules, the extraction should be optimized to efficiently release the related substances of TSH from the pellets and minimize the degradation of TSH during the extraction. Furthermore, the dosage of TSH is as low as 0.2 mg/capsule, with the weight of excipients is 800 times to that of TSH, the excipients and their degradation products could not only affect the extraction, but also the chromatographic analysis of related substances of TSH.
Most reports applied High Performance Liquid Chromatography (HPLC)[3,4], or Liquid Chromatography- Mass Spectrometry (LC-MS) methods[5,6], to determine and identify the related substances in TSH bulk drugs[7,8] and tablets[9-12]. Present study aimed to study the extraction of TSH and its related substances in TSH sustainedrelease capsules. An LC-MS/MS method based on the extraction was applied to identify the main possible related substances in TSH sustained-release capsules and an appropriate HPLC method specific enough to avoid the interfering of high level excipients in the analysis was also studied and validated. The established method could be used to evaluate the quality of TSH sustainedrelease capsule and provide fundamental information for the analysis of similar preparations with dense core-shell structure pellets.
Materials and Methods
Materials and reagents:
TSH (Batch No. YK1305002) was supplied by Zhejiang Jinghua Conba Bio-pharm. Co., Ltd. (Zhejiang, China). TSH sustained-release capsules (Batch Nos. 20160824, 20160905, 20160915) were supplied by Jinling Pharmaceutical Co., Ltd. (Nanjing, China). Impurity D (2-methoxyl substituted TSH, Batch No. Y0000651, purity 99.86 %) and impurity H (desulfonamided TSH, Batch No. Y0000652, purity 99.32 %) were obtained from European medicines quality administration. Hydrochloric acid (HCl), Sodium hydroxide (NaOH) and Hydrogen peroxide (H2O2) were all of analytical grade from Sinopharm Chemical Reagent Co., Ltd. HPLC grade acetonitrile and methanol were obtained from Tedia (Ohio, United States of America (USA)). Water was purified with a Millipore Milli Q-Plus purification system (Massachusetts, USA).
Instruments:
An Agilent 1260 series HPLC system (California, USA) with ChemStation workstation was used for the analysis of related substances. The LC-MS identification of Related Substances (RSs) was performed on an Agilent 1260 series HPLC and Agilent 6224 Time-Of-Flight (TOF) LC/MS (California, USA). Data acquisition and analysis were controlled by mass hunter work station. The MS/MS spectra were collected on Thermo-Finnigan Surveyor (TSQ) quantum ultra-Accurate Mass (AM) spectrometer equipped with Electrospray Ionization (ESI) source (California, USA) and controlled by Xcalibur™ software (Version 1.2).
Extraction of TSH and its related substances:
Sustained-release capsules containing 1.0 mg of TSH was weighed accurately and transferred into a 50 ml glass tube, 20 ml NaOH solution (0.02 mol/l) was accurately added and then heated at 50° for 10 min to break down the coating of pellets. The mixture was then sonicated for 10 min to extract TSH and 10.0 ml of acetonitrile was added to precipitate the excipients dissolved. Then 5.0 ml of HCl solution (0.2 mol/l) was added drop wisely to neutralize NaOH. The mixture was then diluted with 15.0 ml of 30 % acetonitrile and centrifuged at 10 000 rpm for 15 min. The supernatant was collected for HPLC analysis, with the final concentration of TSH at 200 μg/ml.
HPLC system for the analysis of related substances in TSH capsules:
The separation was achieved on a Phenomenex C18 column (4.6×250 mm, 5.0 μm) by gradient elution with a mobile phase consisting of ammonium acetate solution (15 mM) and acetonitrile-methanol (50:50, v/v) at a flow rate of 1.0 ml/min. The gradient elution program is shown in Table 1. Column oven temperature was set at 30°. The injection volume was 20 μl and the analytes were monitored at 225 nm.
| Time (min) | Mobile phase A (%) | Mobile phase B (%) |
|---|---|---|
| 0 | 92 | 8 |
| 5 | 92 | 8 |
| 10 | 85 | 15 |
| 20 | 47 | 53 |
| 50 | 47 | 53 |
| 51 | 92 | 8 |
| 60 | 92 | 8 |
Table 1: Gradient Elution Program
LC-MS and LC-MS/MS systems for the identification of related substances:
For both LC-MS and LC-MS/MS determination, positive ESI mode was applied and the spray voltage was 4.5 kV, heated capillary temperature was 350° and the pressures of the nitrogen sheath and auxiliary gas were set at 310 kPa and 69 kPa, respectively. The massto- charge ratio (m/z) scanning range was 120-1000 for MS and 50-800 for MS/MS determination. The argon gas of 0.2 Pa was employed for collision-induced dissociation in MS/MS mode.
Forced degradation:
Stress studies of TSH sustained-release capsules were performed with aliquots of 10 mg of TSH under stressing conditions. The optimized stressing conditions were 2 M HCl acidic hydrolysis (25°, 36 h), 2 M NaOH alkaline hydrolysis (25°, 36 h), 10 % H2O2 oxidation (60°, 40 min), water solution thermal hydrolysis (80°, 72 h) and deuterium lamp photolysis (254 nm, 72 h). All degradation samples were prepared by the addition of 30 % acetonitrile (50 ml), sonication (10 min) and centrifugation at 10 000 rpm for 15 min. TSH and excipients were also carried out in parallel.
Results and Discussion
In present study, the TSH sustained-release capsules contained pellets with core shell structure. Therefore, the sustained-release coating layer should be destroyed to release the TSH and its related substances in the extraction process. As both the TSH and its related substances were in the middle layer, the extraction efficiency of TSH was studied to evaluate the extraction of the related substances.
HCl, HCl-Methyl alcohol (MeOH) solution, Ammonium acetate (NH4Ac) buffer (pH 6.5) and NaOH-MeOH solution were studied. As shown in Table 2, both HCl and NaOH hydrolysis can extract more than 90 % of TSH while NH4Ac buffer (pH 6.5) has the lowest extraction efficiency.
| Solvent | Treatment | Extraction efficiency of TSH (%, n=3) |
|---|---|---|
| HCl (1.0 M) | Sonicate 15 min | 90.6 |
| HCl-MeOH (1.0 M) | Sonicate 15 min | 91.3 |
| NH4Ac (15 mM, pH 6.5) | Sonicate 15 min | 62.4 |
| NaOH-MeOH (0.02 M) | Sonicate 15 min | 95.4 |
| NaOH-MeOH (0.02 M) | Shake with glass beads for 10 min | 97 |
| NaOH-MeOH (0.02 M) | Shake with glass beads at 50° for 10 min | 98.3 |
| NaOH-MeOH (0.02 M) | 50° for 10 min and sonicated for 10 min | 99 |
Table 2: Extraction Efficiency of TSH with Different Treatments
The outer layer of the pellets is a dense enteric coating, which could be dissolved at pH 6.5 and higher pH. However, after the breakdown of outer layer, the solubility of TSH in pH 6.5 buffer solution is lower than in MeOH, so the extraction efficiency in pH 6.5 buffer solutions is significantly lower than NaOH-MeOH solution. The dense enteric coating of the pellets is insoluble in acid, but the sonication may help to destroy the coating and TSH is more likely to be extracted with HCl in comparison with NaOH. Therefore, the HCl-MeOH solution had similar extraction efficiency as NaOH-MeOH solution. As NaOH-MeOH solution had the highest extraction efficiency, it was used as the extraction solvent for further study.
Higher temperature and physical treatments such as ultrasonic and shake with glass beads may accelerate the extraction of TSH and its related substances, but these may also cause the degradation of TSH in the extraction and increase the degradation of excipients. Therefore, extraction at room temperature to 50°, with different physical treatments was studied, with NaOHMeOH solution as extraction solvent.
As shown in Table 2, both extract at 50° could improve the extraction efficiency, with extraction efficiency >97.0 % for both ultrasonic and shake with glass beads. Ultrasonic treatment had similar extraction efficiency with shake with glass beads, but the operation of ultrasonic treatment is easier to perform, water bath at 50° for 10 min was used in further study. Extended extraction time could not cause degradation of TSH but the degradation of excipients was increased and could interfere with the chromatographic measurement.
As the labelled content of TSH sustained-release capsule is 0.2 mg and the amount of excipient is 800 times to TSH. The extraction solution contained high concentration of excipients and their degradation products in addition to TSH and related substances. The addition of acetonitrile to the NaOH extraction could precipitate most of the excipients and reduce the interference of excipients.
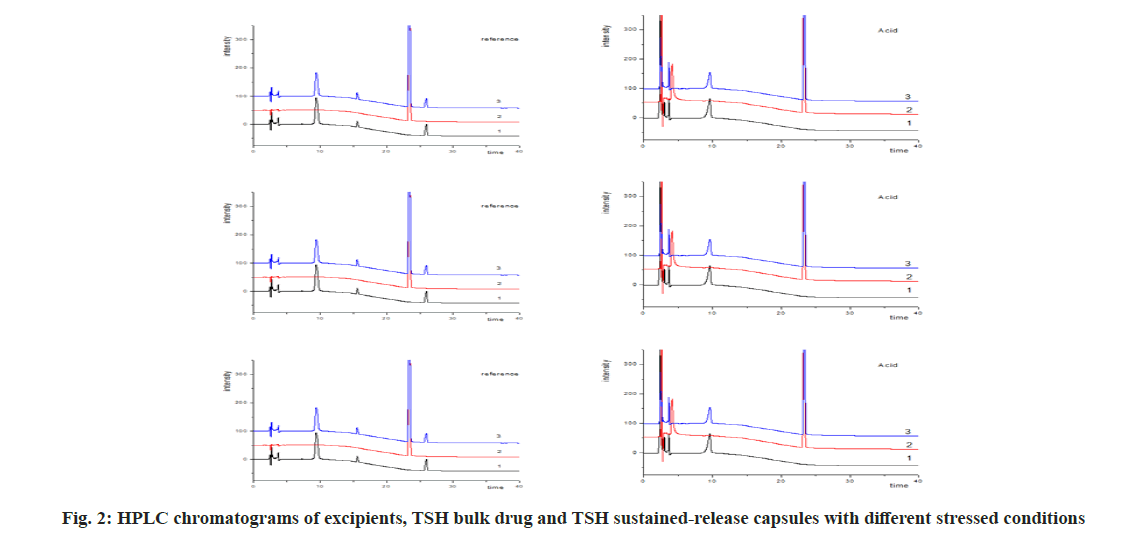
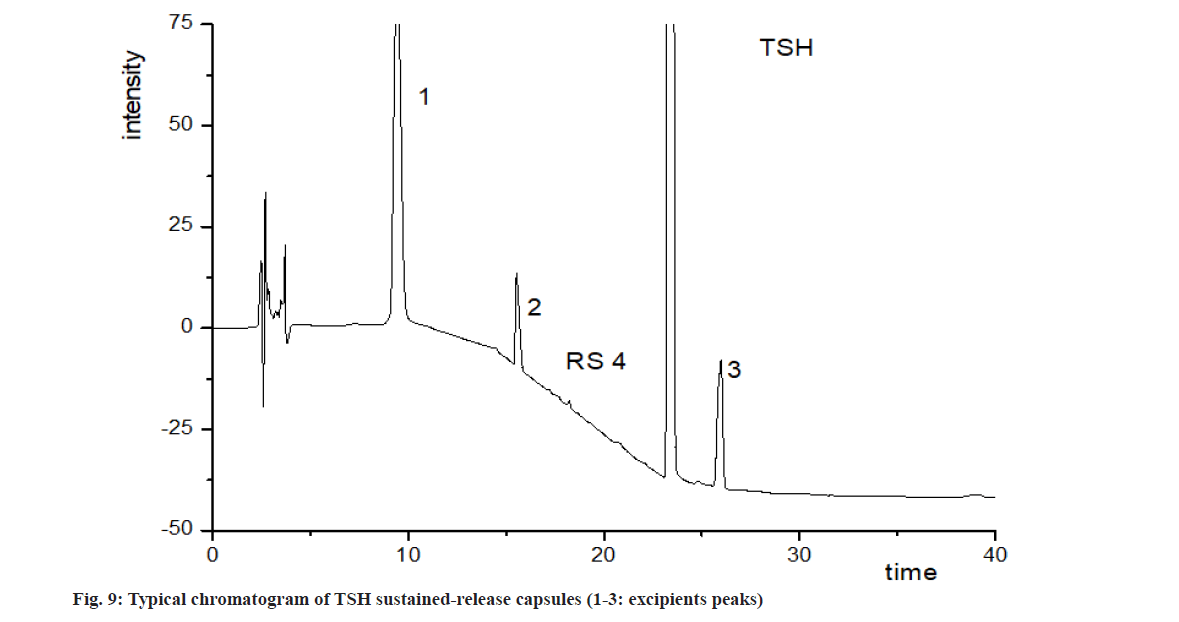
To estimate the main degradation impurities in TSH products, the capsules were treated with stressed conditions. As shown in fig. 2, the retention time of TSH was 23.4 min and its main related substances at 11.3 to 29.0 min. Although the excipients showed several peaks at 7.7, 9.4, 15.6 and 26.0 min, these peaks could be well separated (resolution, R>1.5) with TSH and its related substances. Significant degradations of TSH were observed with stress conditions of oxidation and photolysis, while degradation in acidic, alkaline and thermal treatments was limited. According to Table 3, the five degradation products with the content of about 0.1 % or more, named as Retinoschisin (RS) 1 to RS5, were further identified with LC-MS and LC-MS/MS.
| Related Substance | ""tR (min)" |
Relative Retention Time | "[M+H]+ (m/z)" |
"Product ions (m/z)" |
Structure | Source of RS |
|---|---|---|---|---|---|---|
| RS1 | 8.2 | 0.35 | 289.1422 | 271, 228, 200, 168,148 | 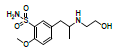 |
Oxidation |
| RS2 (impurity F) | 15.1 | 0.64 | 182.1543 | 137, 121, 110, 93, 65 | 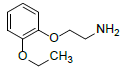 |
Oxidation/photolysis |
| RS3 | 17.2 | 0.74 | 381.1522 | 271, 228, 200, 148 | 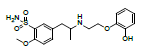 |
Oxidation/photolysis |
| RS4 (impurity D) | 18.9 | 0.81 | 395.1680 | 271, 228, 200, 168, 148 | 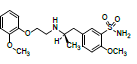 |
Oxidation |
| TSH | 23.4 | 1.00 | 409.1825 | 271, 228, 200, 148 | 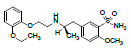 |
- |
| RS5 (impurity H) | 28.9 | 1.21 | 330.2246 | 192, 149, 121, 91 | 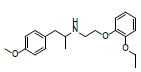 |
Photolysis |
Table 3: Identification of Related Substances from Forced Degradation of TSH Sustained-Release Capsules
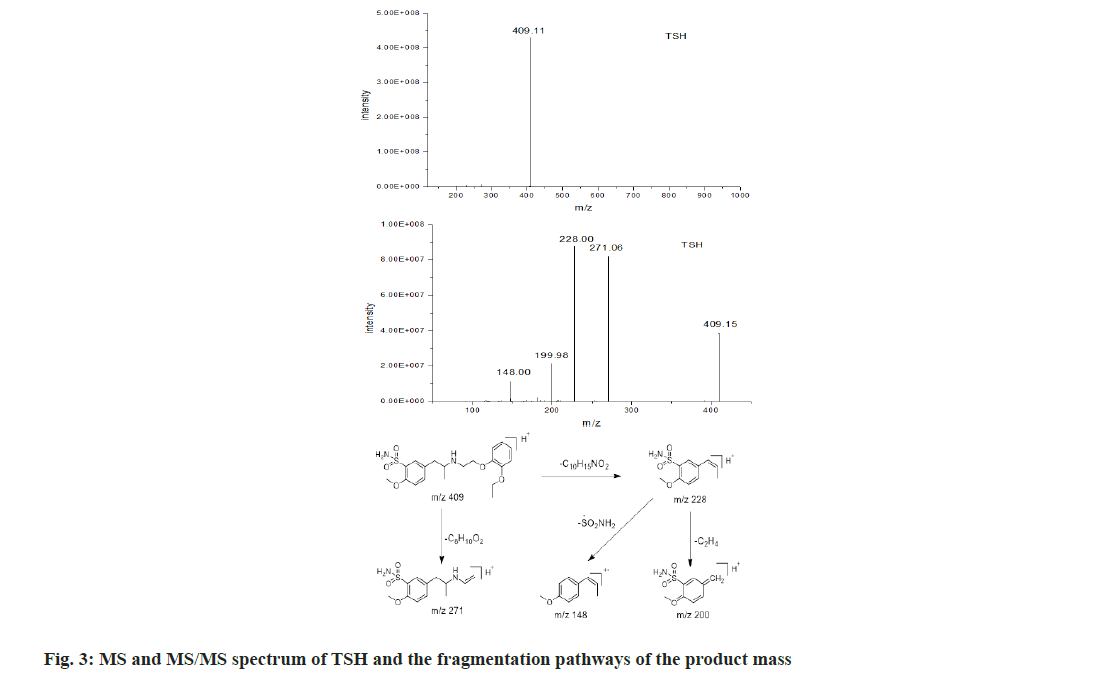
Five main related substances, RS1 to RS5, were characterized with TOF-MS for the information of molecular weight and triple quadrupole mass spectrometer for their product mass spectra to elucidate their structures. TSH was also studied in comparison. The chromatographic and mass spectrometric data were summarized in Table 3 and the fragmentation patterns of the protonated molecular ions [M+H]+ are proposed in fig. 3.
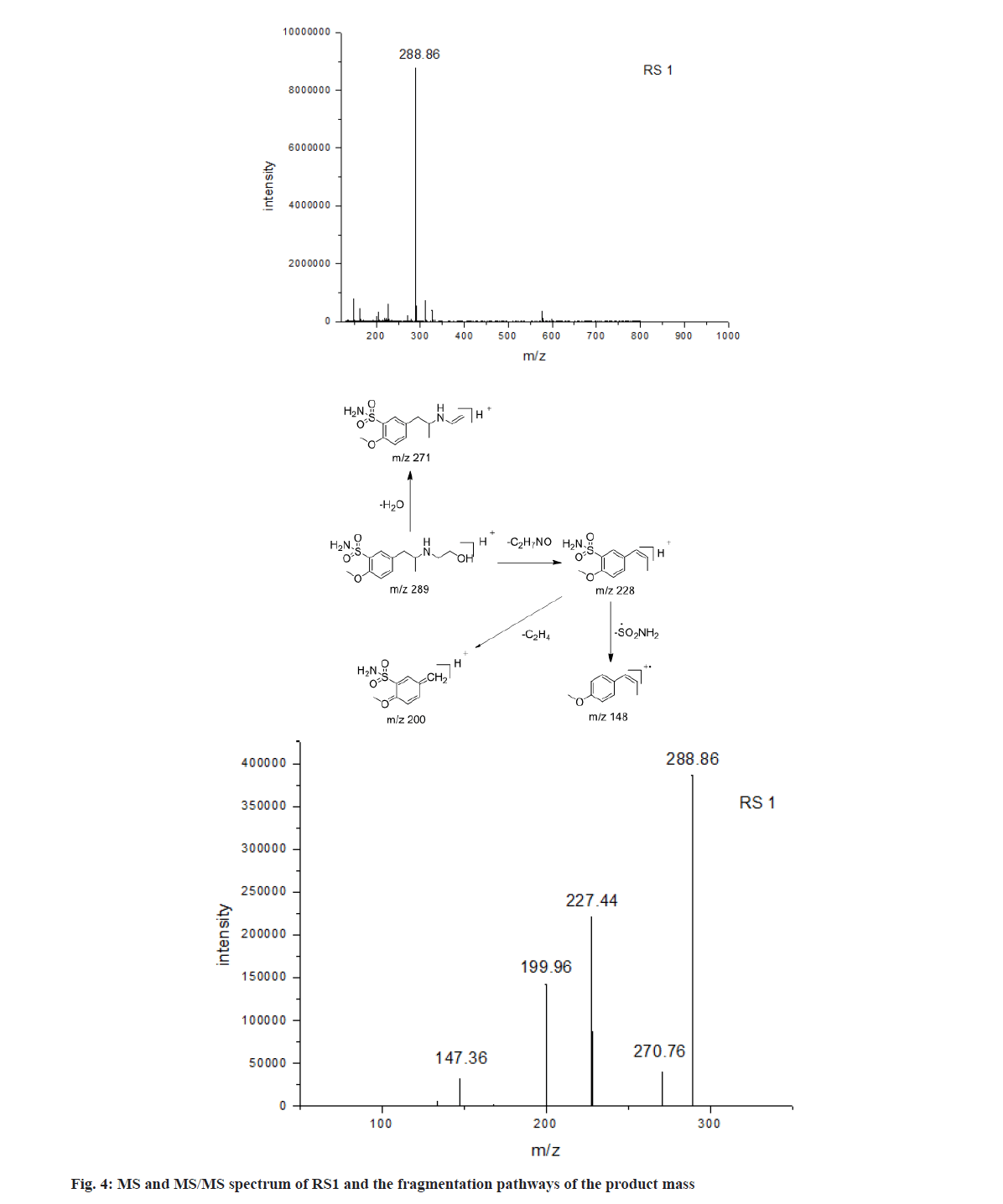
As shown in fig. 3, the protonated molecular ion of tamsulosin produced a base peak with m/z 409.11, corresponding to its ion formula of [C20H29N2O5S]+. Meanwhile, the product mass spectrum of the [M+H]+ ion displayed major fragments at m/z 271 and 228 resulting from breakages of C-O and C-N linkage, respectively, as shown in fig. 3. The fragments with relatively low intensity at m/z 148 and 200, were due to the losses of sulfonamide group (80) and Ethylene (C2H4) (28) from m/z 228, respectively. As shown in fig. 4, the m/z of RS1 was 288.86, corresponding to ion formula [C12H21N2O4S]+, which was 120 Da lower than the protonated molecule ion of tamsulosin [C20H29N2O5S]+ due to the loss of a small molecule. The characteristic fragment at m/z 271 indicated [M+H-H2O]+ and proved the presence of a free hydroxyl group in RS1. Other fragment ions of RS1, m/z 228, m/z 200 and m/z 148, were similar to that of TSH. These proved that RS1 was formed through the breakage of C-O linkage of TSH, which was similar with previous report[6].
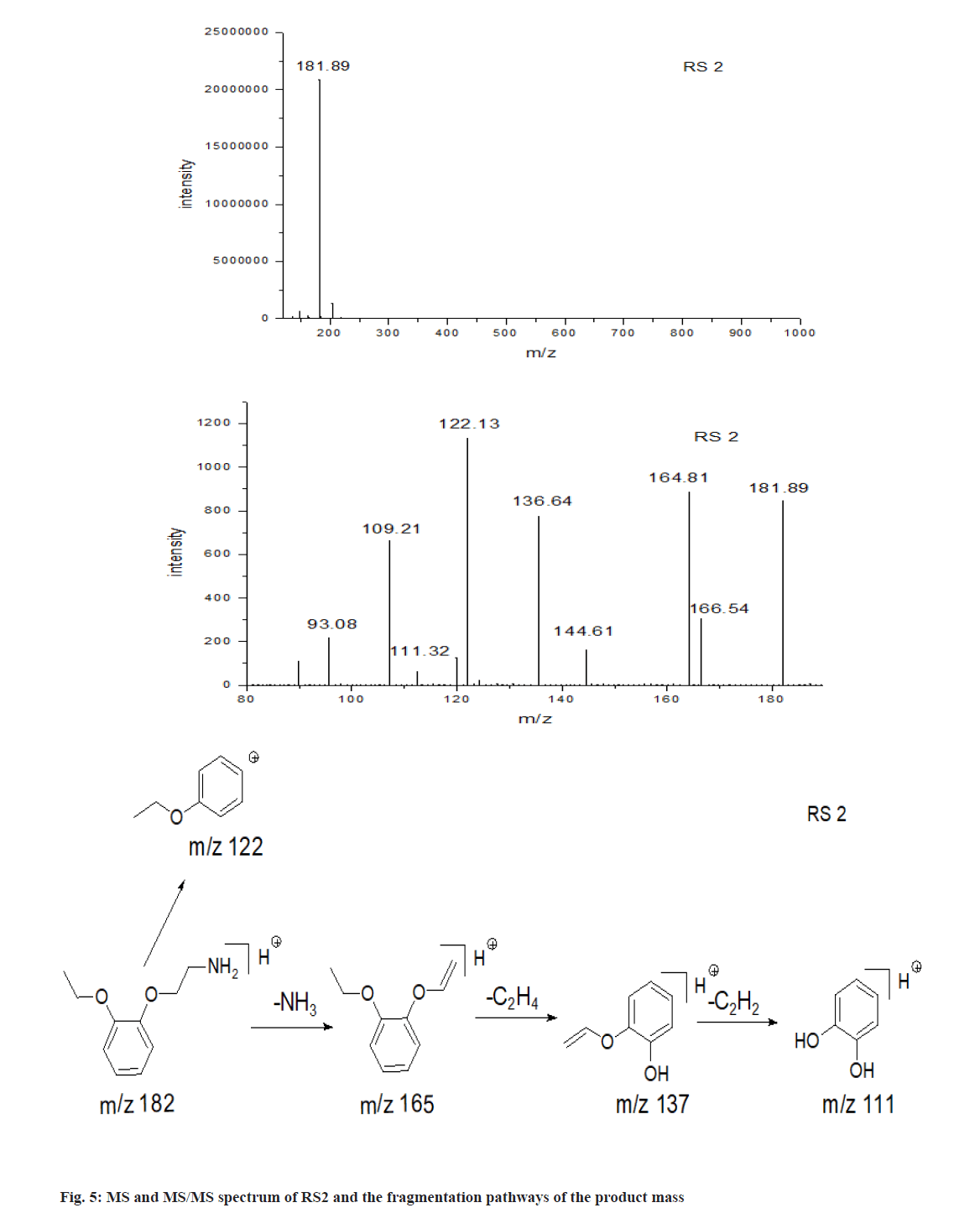
As shown in fig. 5, the m/z of RS2 was 181.89, corresponding to ion formula of [C10H16NO2]+, which was 227 Da lower than the protonated molecule ion of tamsulosin [C20H29N2O5S]+. RS2 was observed to have a characteristic fragment [M+H-NH3]+ at m/z 165, which suggests a free amino occur in the structure. Because amino in sulfonamide is stable and the linkage of S-N is difficult to cleave, it can be concluded that RS2 was produced by the breakage of C-N from tamsulosin. Other product mass spectrum of RS2 contained ions at m/z 137, 121 and 111 due to the neutral losses of C2H4 (28) form daughter ion at m/z 165, C2H6ON (60) from parent ion [M+H]+ and Acetylene (C2H2) (26) from ion at m/z 137, respectively. The formation of m/z 111 was indicative of the presence of 1,2-dihydroxyl phenyl group. Thus, RS2 was identified as the degradation by the breakage of C-N of tamsulosin with oxidation and photolysis conditions.
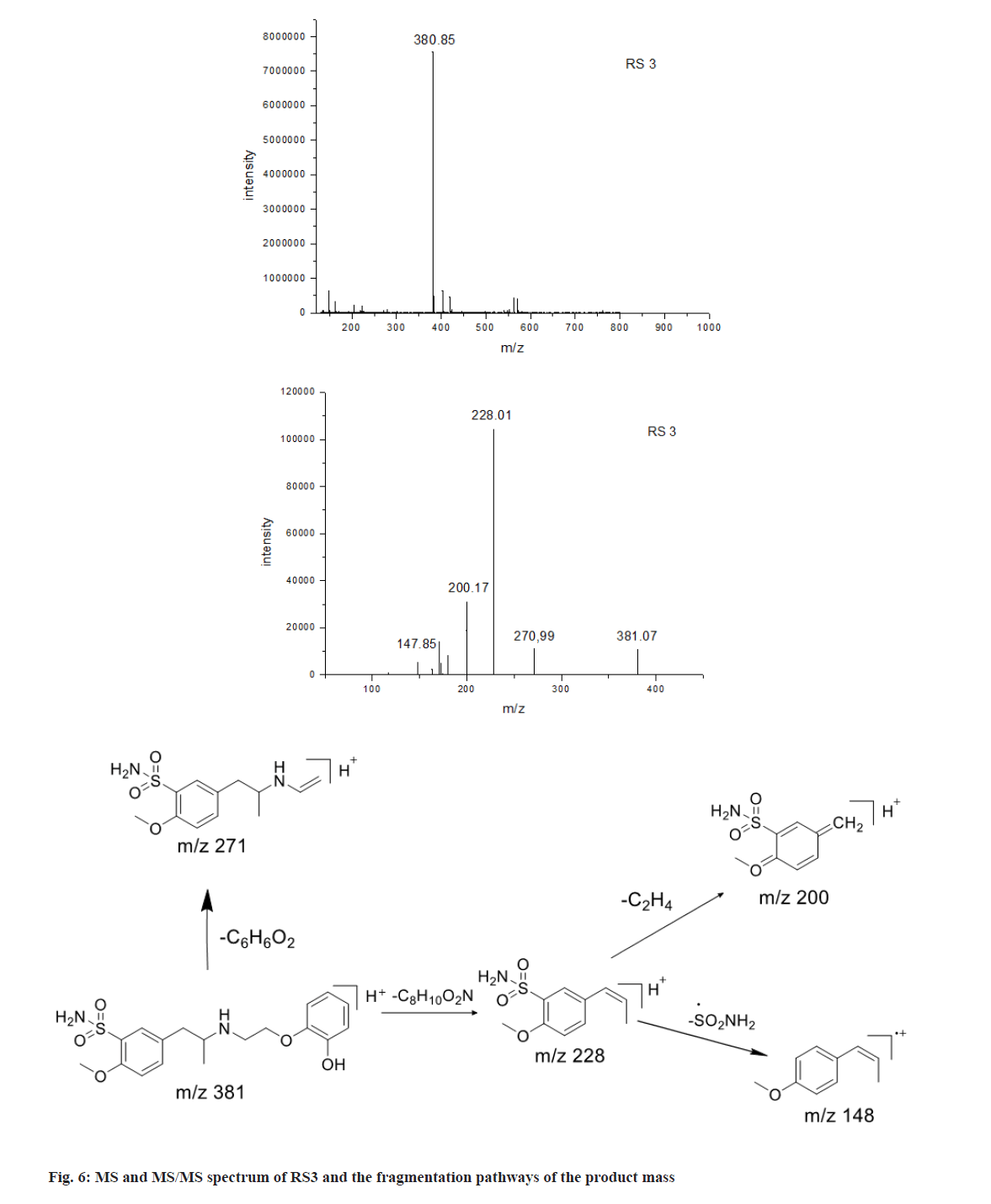
As shown in fig. 6, the ESI-MS spectrum of RS3 revealed a set of correlated adduct ions at m/z 380.85,402.83 and 418.85. Judging from their mass differences, it can be concluded that they were the [M+H]+, [M+Na]+ and [M+K]+ adduct ions of the analyte, respectively. Thus, the corresponding protonated molecule ion formula should be [C18H25N2O5S]+, which was 28 Da lower than that of tamsulosin [C20H29N2O5S]+. RS3 was observed to have the same fragment ions of m/z 271, 228 and 148 as tamsulosin, which suggests RS3 is similar to tamsulosin structure. It was supposed that RS3 was formed by the cleavage of C-O bond of tamsulosin in oxidation.
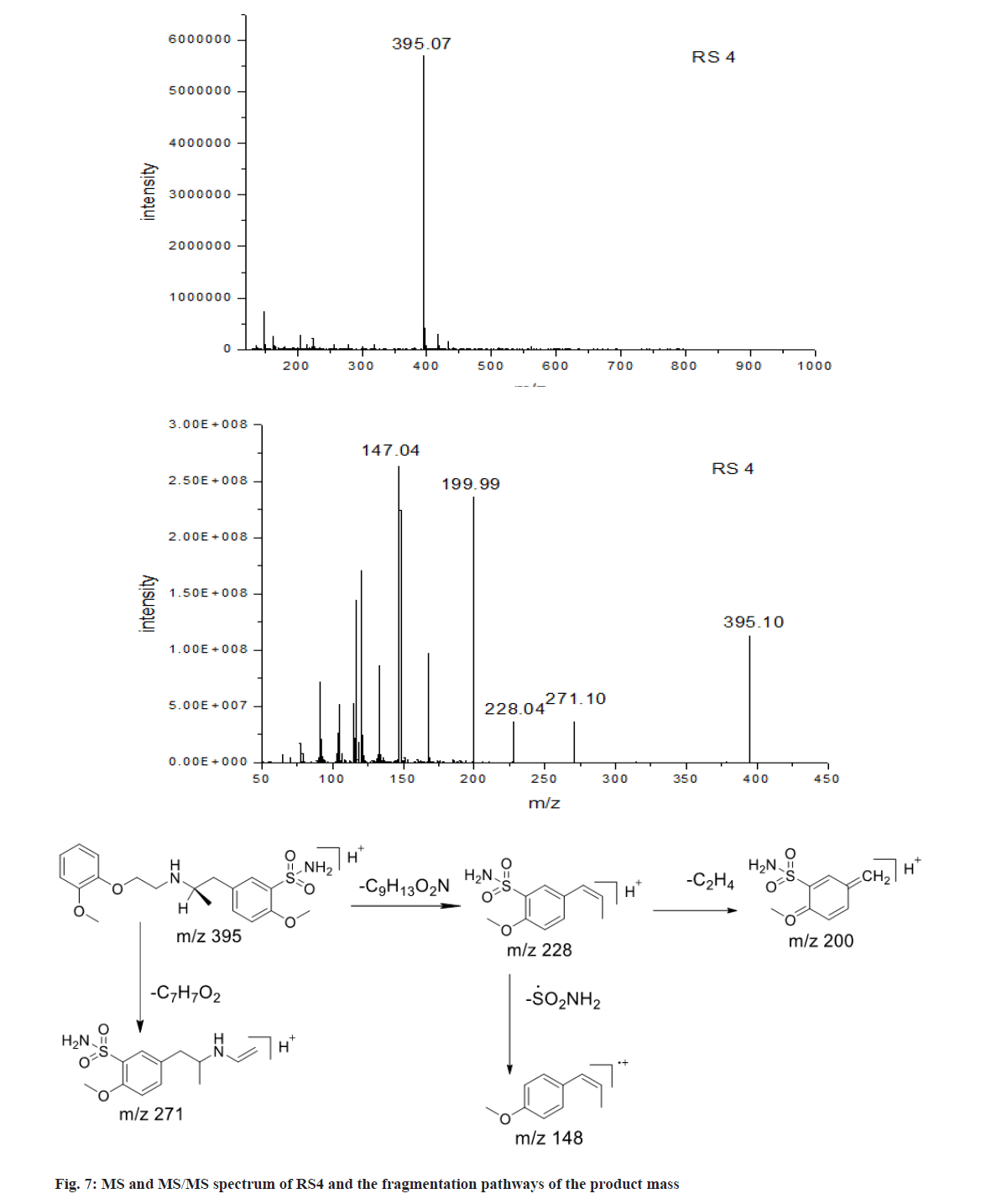
As shown in fig. 7, the [M+H]+ ions of RS4 was m/z 395.07, 14 Da lower than TSH and corresponding to loss of Methylene (CH2) in comparison with TSH ([C19H27N2O5S]+). Its fragment ions had similar pattern as that of TSH. These proved that RS4 possessed methoxyl instead of ethoxyl group. Chromatographic study showed that the retention time of RS4 was same as impurity D, 2-methoxyl substituted TSH. This further proved RS4 was the impurity D of TSH.
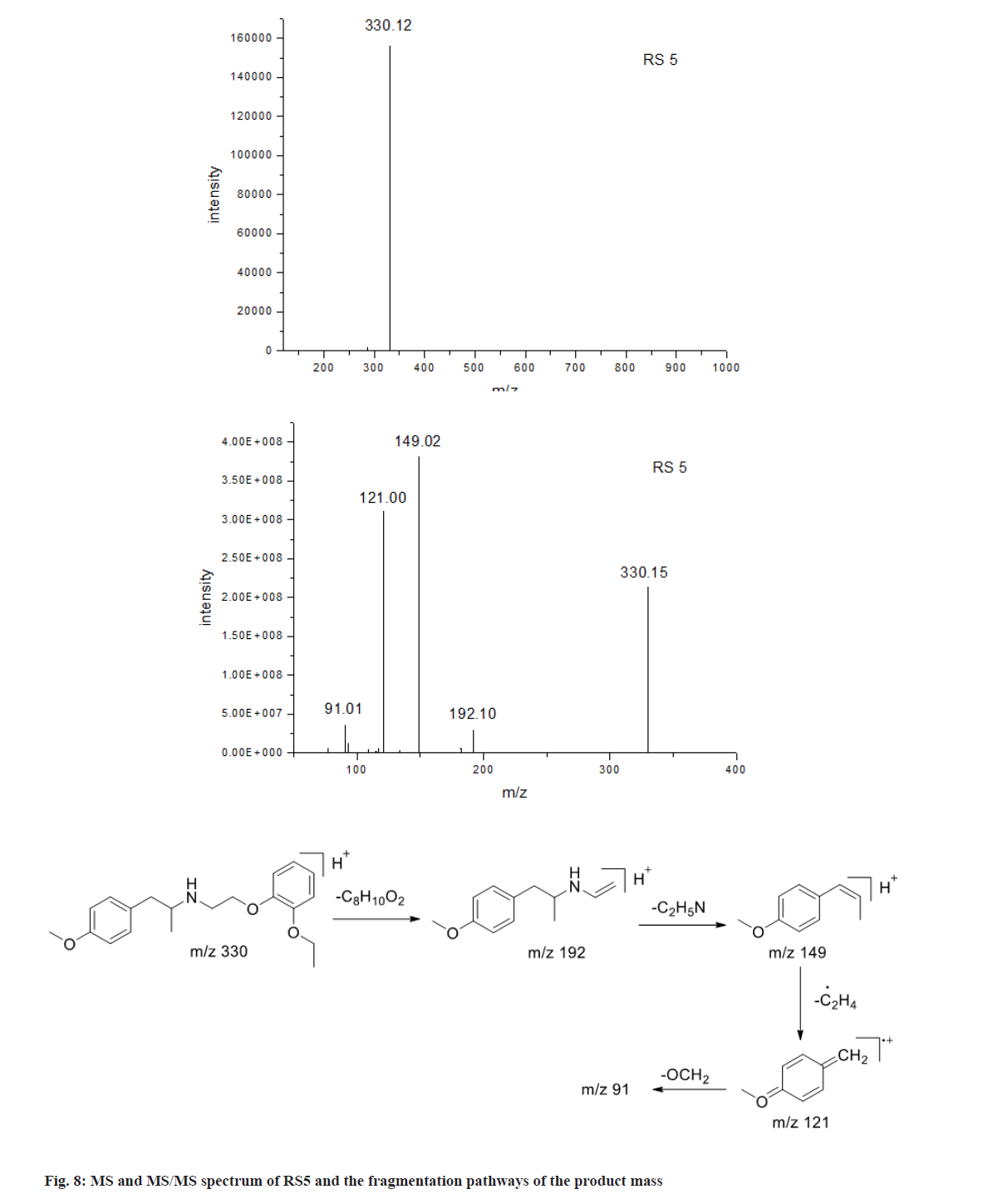
As shown in fig. 8, the [M+H]+ ions of RS5 was m/z 330.12, corresponding to its ion formula of [C20H28NO3]+. Its fragment ion of m/z 192 resulted from breakage of C-O linkage, and m/z 149 was formed from the cleavage of C-N linkage from m/z 192 ion. In the terms of degradation pathway, RS5 was differentiated from TSH by losing molecular weight of sulfonamide group (M-79). Chromatographic study also proved that the retention time of RS5 was same as impurity H, desulfonamide TSH. These showed that RS5 was the impurity H of TSH.
As the reference materials of RS4 and RS5 were available, these 2 impurities were validated with respect to specificity, Limit of Detection (LOD), Limit of Quantification (LOQ), precision (inter-day and intraday), accuracy, stability and robustness as International Council on Harmonisation (ICH) guidelines.
Specificity of the method was evaluated using the forced degradation samples prepared as described in section 2.6 above. As shown in fig. 2, no interfering co-eluting peaks in blank solutions were observed. Additionally, the degradation products of TSH and its degradation products, and the degradation products of excipients were all well-separated and resolutions of higher than 1.5 were obtained. Simulated samples spiked with RS4 and RS5 also proved well separation of related substances to both TSH and the degradation products of excipients. These demonstrated a high specificity of present HPLC method.
LOD and LOQ of RS4 and RS5 were determined by diluting the standard solutions of RS4 and RS5 to a series of concentrations and determined with the established method. Results proved that the LOD of RS4 and RS5 were 0.23 and 0.27 μg/ml (signal-tonoise, S/N >3), respectively, corresponding to 0.1 % of TSH in capsules. The LOQ of RS4 and RS5 were 0.46 and 0.54 μg/ml (S/N>10), respectively, corresponding to 0.2 % of TSH in capsules.
Intra-day precision was evaluated by injecting 6 times of simulated sample solution and impurity reference solutions. The Relative Standard Deviations (RSDs) of all the determinations were calculated and found below 1.80 % (n=6). Inter-day precision was studied with different analysts and different instruments at different days. The RSDs of RS4 and RS5 were 1.14 % and 2.18 % respectively (n=12). These proved that the precision of the method was good.
Test sample was spiked with RS4 and RS5 reference reserve solutions to 0.5 to 4.5 μg/ml, corresponding to 0.2 % to 1.8 % of TSH in capsules. These solutions were analyzed and the recovery percent was calculated. As shown in Table 4, the average recovery percent of RS4 and RS5 were 100.6 % and 100.0 %, respectively, with the RSD 2.0 % and 4.0 % (n=9). These indicated good accuracy of the established method.
| RS4 | RS5 | |||||
|---|---|---|---|---|---|---|
| Added (μg/ml) | Found (μg/ml) | Recovery (%) | Added (μg/ml) | Found (μg/ml) | Recovery (%) | |
| 80 | 1.53 | 1.52 | 99.1 | 1.6 | 1.66 | 103.9 |
| 1.53 | 1.52 | 98.9 | 1.6 | 1.69 | 105.5 | |
| 1.53 | 1.52 | 99 | 1.6 | 1.67 | 104.1 | |
| 100 | 1.92 | 2 | 104.4 | 2.06 | 2.07 | 100.3 |
| 1.92 | 1.93 | 100.6 | 2.06 | 2.07 | 100.6 | |
| 1.92 | 1.96 | 102.3 | 2.06 | 2 | 97.3 | |
| 120 | 2.49 | 2.47 | 99.1 | 2.52 | 2.38 | 94.4 |
| 2.49 | 2.47 | 99 | 2.52 | 2.39 | 95 | |
| 2.49 | 2.57 | 103 | 2.52 | 2.5 | 99.4 | |
| Average (%) | 100.6 | 100 | ||||
| RSD (%) | 2.1 | 4 | ||||
Table 4: Recovery of RS4 and RS5 in Simulated Samples
The simulated sample solutions were determined at control, 4, 6, 8, 12, 16, 20 and 24 h after preparation. The RSD values of RS4 and RS5 were 0.84 % and 1.50 % respectively. This indicated that the solution is stable for 24 h (Table 5).
| Time/h | 0 | 4 | 6 | 8 | 12 | 16 | 20 | 24 | Average (%) | RSD (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Area (RS4) | 78.8 | 79.3 | 78.4 | 79.1 | 78.9 | 80.6 | 79.2 | 79.7 | 79.3 | 0.84 |
| Area (RS5) | 109.8 | 112 | 110.9 | 114 | 113 | 112 | 113.9 | 115 | 112.5 | 1.5 |
Table 5: Stability of RS4 and RS5 in Simulated Samples
The robustness of the method was studied by altering flow rate (±0.2 ml), pH of the buffer (±0.5 units), column temperature (±5°), detection wavelength (±5 nm) and initial proportion in mobile phase. The result was tabulated in Table 6. Changes of wavelength and pH induced errors in the results of RS5, but other changes didn’t influence in determination of RS4 and RS5. This demonstrated the pH and wavelength of the method should be strictly controlled to ensure the results.
| RS4 | RS5 | ||||||
|---|---|---|---|---|---|---|---|
| Content (%) | Average (%) | RSD (%) | Content (%) | Average (%) | RSD (%) | ||
| Flow rate (ml/min) | 0.8 | 0.99 | 0.97 | 2.99 | 0.96 | 0.96 | 3.67 |
| 1 | 0.99 | 0.92 | |||||
| 1.2 | 0.93 | 0.99 | |||||
| Column temperature (°) | 30 | 1.01 | 0.98 | 2.26 | 1.06 | 1.04 | 2.41 |
| 35 | 0.96 | 1.06 | |||||
| 40 | 0.98 | 1.01 | |||||
| Wavelength (nm) | 220 | 0.95 | 0.96 | 1.17 | 0.92 | 0.87 | 7.46 |
| 225 | 0.97 | 0.91 | |||||
| 230 | 0.96 | 0.79 | |||||
| Initial mobile phase | 90:10 | 0.95 | 0.96 | 1.28 | 0.96 | 0.95 | 2.49 |
| 92:08 | 0.97 | 0.92 | |||||
| 94:06 | 0.95 | 0.96 | |||||
| pH | 6 | 0.98 | 0.97 | 1.71 | 0.93 | 0.98 | 8.15 |
| 6.5 | 0.97 | 0.93 | |||||
| 7 | 0.95 | 1.07 | |||||
Table 6: Robustness of RS4 And RS5 in Simulated Sample Solution
Related substances of TSH sustained-release capsules were determined with the established method. Typical chromatograms were shown in fig. 9. The related substances in TSH samples were lower than 0.1 % for all the batches. After kept at 40° for 3 mo, total related substances increased to 0.28 %, with RS4 increased most significantly. Therefore, the results proved that RS4 is the main related substance in TSH capsules and it is more likely to accumulate during storage in comparison with other impurities.
This indicated a high selectivity of method applied in TSH sustained-release capsules for the quantification of related substances without interference from excipients and the established method could be applied to evaluate the quality of TSH sustained-release capsules.
Benign prostatic hyperplasia is a common urological disease in elderly men. TSH is a third-generation selective postsynaptic α1A-adrenergic receptor antagonist used to treat benign prostatic hyperplasia. In this study, TSH sustain-release capsules was released by a combination of drug-containing coating, enteric-coated coating and sustained-release coating, which could overcome the defect of single control of drug dissolution and achieve sustained-release in a wide range of pH biological environment. TSH sustain-release capsules can obtain a stable plasma concentration curve and a long time of action, reduce the incidence of cardiovascular side effects and greatly improve the safety, effectiveness and compliance of patients with medication. In terms of safety, related substance in TSH sustain-release capsules is very important. Present study aimed to study the extraction of TSH and its related substances in TSH sustained-release capsules. An LC-MS/MS method based on the extraction was applied to identify the main possible related substances in TSH sustainedrelease capsules and an appropriate HPLC method specific enough to avoid the interfering of high level excipients in the analysis was also studied and validated. The established method could be used to evaluate the quality of TSH sustained-release capsule and provide fundamental information for the analysis of similar preparations with dense core-shell structure pellets.
In present study, a NaOH-MeOH extraction and gradient HPLC method was developed and validated for the determination of related substances in TSH sustainedrelease capsules. The extraction and chromatographic conditions were optimized to avoid the interference of excipients and its degradation products and to determinate the related substances accurately. Five main degradation products, RS1 to RS5, were also identified with LC-MS/MS method. The method was also validated and proved to be selective, precision, accurate and sensitive, and could be used for the determination of related substances in TSH sustained-release capsules and for its routine quality control. Results of different batches showed related substances in TSH sustainedrelease capsules ranging from <0.1 % to 0.28 %, with RS4 as main related substance. The extraction could also be applied to other core-shell pellets contained capsules.
Authors’ contributions:
Jian Li and Li Deng have contributed equally to this work.
Acknowledgements:
This work was financially supported by the Fundamental Research Funds for the Central Universities (No. 2632017ZD07).
Conflict of interests:
The authors declared no conflict of interests.
References
- Huang Y, Chen H, Zhou X, Wu X, Hu E, Jiang Z. Inhibition effects of chlorogenic acid on benign prostatic hyperplasia in mice. Eur J Pharmacol 2017;809:191-5.
[Crossref] [Google Scholar] [PubMed]
- Lowe FC. Summary of clinical experiences with tamsulosin for the treatment of benign prostatic hyperplasia. Rev Urol 2005;7(4):S13-21.
[Google Scholar] [PubMed]
- Sudha T, Dhomane J. A validated RPHPLC method for the determination of impurities in Tamsulosin HCl. Int J Chem Res 2011;2(4):29-33.
- Rao RN, Talluri MK, Raju AN, Shinde DD, Ramanjaneyulu GS. Development of a validated RP-LC/ESI-MS–MS method for separation, identification and determination of related substances of tamsulosin in bulk drugs and formulations. J Pharm Biomed Anal 2008;46(1):94-103.
[Crossref] [Google Scholar] [PubMed]
- Basniwal PK, Panda S, Jain S, Jain D. Stability-indicating HPLC assay method and degradation profile of tamsulosin. Am Eurasian J Sci Res 2012;7(5):193-8.
- Namdev D, Borkar RM, Raju B, Kalariya PD, Rahangdale VT, Gananadhamu S, et al. Identification of forced degradation products of tamsulosin using liquid chromatography/electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal 2014;88:245-55.
[Crossref] [Google Scholar] [PubMed]
- Chandorkar JG, Kotwal VB, Dhande NS, Gurav SG, Pande VV, Yadav PV. A sensitive HPLC method for simultaneous estimation of tamsulosin hydrochloride and its impurity. Pak J Pharm Sci 2008;21(3):307-10.
[Google Scholar] [PubMed]
- Kasawar GB, Farooqui MN. Stability indicating HPLC method for the determination of chiral purity of r-(-)-5-[2-aminopropyl]-2-methoxybenzene sulfonamide. Indian J Pharm Sci 2009;71(5):533-7.
[Crossref] [Google Scholar] [PubMed]
- Nithiyananthan TS, Shankarananth V, Rajasekhar KK, Ravikiran P, Vikram Kumar E, Jayanth Kumar Reddy G. RP-HPLC method for the estimation of tamsulosin hydrochloride in bulk and tablet dosage form. Drug Invention Today 2009;1(2):154-6.
- Jadhav JN, Chandorkar JG. Simultaneous estimation of tamsulosin hydrochloride and dutasteride in combined dosage form by UV spectroscopy method. J Pharm Res 2009;2(3):507-9.
- Patel DB, Patel NJ. Validated stability indicating HPTLC method for the determination of tamsulosin hydrochloride in pharmaceutical dosage forms. Int J Chem Tech Res 2010;2(1):646-52.
- Choudhari VP, Nikalje AP. Stability-indicating HPTLC method for the determination of tamsulosin in pharmaceutical dosage forms. Chromatographia 2009;69(11):1463-7.