- *Corresponding Author:
- Min Wan
Department of Pharmacy,
People’s Hospital of Dongxihu District,
Dongxihu,
Wuhan, Hubei 430000,
China
E-mail: 165561447@qq.com
| This article was originally published in a special issue, “Novel Therapeutic Approaches in Biomedicine and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2021:83(6) Spl Issue “12-18” |
Abstract
The purpose of this research project was to clarify the influence of Cefoperazone/Sulbactam plus Azithromycin on the alleviation of serum inflammatory factors and the improvement of pulmonary function in patients with severe lower respiratory tract infection, aiming at improving the management of patients with the disease. We first retrospectively analyzed 201 patients with severe lower respiratory tract infection from May 2019 to May 2021, of which 101 patients (research group) were treated with Cefoperazone/Sulbactam plus Azithromycin and the remaining 100 patients were treated with Cefoperazone/Sulbactam (control group). The efficacy, incidence of adverse reactions, bacterial clearance rate, recovery (hospitalization time, pulmonary infection control time and fever resolution time), inflammatory factors (interleukin-6, tumor necrosis factor-alpha, thrombocytocrit) and pulmonary function indicators (peak airway pressure, airway resistance, work of breathing, dynamic compliance) were observed and compared. The results revealed notably less time of hospitalization, pulmonary infection control and fever resolution, as well as a statistically higher bacterial clearance rate in research group vs. control group. After treatment, peak airway pressure, airway resistance and work of breathing were lower while dynamic compliance was higher in research group compared with control group; interleukin-6, hs-CRP and thrombocytocrit were lower in research group; and the total clinical effective rate was noticeably higher in research group compared with control group (93.07 % vs. 75.00 %). No evident difference was observed in total adverse reactions between the two cohorts. Therefore, this paper argues that Cefoperazone/Sulbactam plus Azithromycin is beneficial to alleviate inflammation and improve the pulmonary function in patients with severe lower respiratory tract infection, which is worth popularizing.
Keywords
Respiratory tract infection, cefoperazone/sulbactam, azithromycin, inflammatory factors, pulmonary function
Lower Respiratory Tract Infection (LRTI) is a common infectious disease and the main cause of death from infectious diseases [1]. The disease is mainly caused by gram negative/positive bacteria, Chlamydia pneumoniae and other sensitive bacteria or by microbial infection of the lower respiratory tract in patients with an infectious disease [2]. Patients will experience pricking pain in chest, which will lead to obvious symptoms of LRTI such as dyspnea and expectoration [3]. Severe LRTI has an acute onset and develops rapidly, which can be fatal in severe cases [4]. Therefore, finding suitable and effective intervention methods carries huge implications for improving the prognosis of patients with severe LRTI.
Sulbactam (SB) is a synthetic and irreversible competitive Beta (β)-lactamase inhibitor, which has enjoyed good clinical efficacy since its introduction [5]. Cefoperazone (CPZ) is the 3rd generation of cephalosporins, which has broad spectrum activity against most gramnegative/ positive bacteria and that the combination of CPZ and SB has obvious synergistic effect [6]. Studies have shown that CPZ/SB is a combination of broadspectrum β-lactam and β-lactamase inhibitors, which has a wide range of clinical applications, including the intervention of upper/lower RTI, urinary tract infection and peritonitis [7]. The increasingly serious drug resistance of gram negative bacilli has led to the need for more effective clinical treatment. Azithromycin (AZM) is a macrolide drug that is commonly used to treat a variety of infections [8]. It is used by more than 40 million patients each year due to its antibacterial activity. AZM is used clinically to intervene in bacterial infections of the upper respiratory tract and has been shown to improve Pulmonary Function (PF) in patients with lung diseases [9].
However, there are currently few studies on CPZ/SB plus AZM for patients with severe LRTI. Herewith, this study aims to provide reference for the treatment of patients with severe LRTI by observing the impacts of the combined medication on curative effect, inflammatory factors and PF.
Materials and Methods
General data:
The baseline data of 201 patients with severe LRTI from May 2019 to May 2021 were analyzed retrospectively. Among them, 101 patients (Research Group (RG)) were treated with CPZ/SB plus AZM and the remaining 100 patients were treated with CPZ/SB (Control Group (CG)).
Inclusion criteria: Patients in both groups met the diagnostic criteria of severe LRTI [10], with stable vital signs, independent thinking, complete general case data and no history of allergy to CPZ/SB and AZM. All participants provided the written informed consent, after this study was ethically approved by the internal Ethics Committee.
Exclusion criteria: Patients with abnormal liver, kidney and hematopoietic function, unconsciousness, mental illness, multiple organ dysfunction, allergy to test drugs, severe pulmonary hypertension, atelectasis, pulmonary edema and against-advice discharge, as well as dropouts or those who lost to follow up.
Treatment methods:
All patients were treated symptomatically and routinely, including sputum aspiration and oxygen inhalation. In CG, 2.0 g CPZ/SB (Pfizer Pharmaceuticals Limited, Liaoning, China, H10960113) was given intravenously twice a day. Based on the treatment in CG, AZM (Sunho Pharmaceutical Co., Ltd., Beijing, China, H20057821) was additionally given to RG, with intravenous drip of 500 mg once a day. Patients in both groups were treated with 10 d as a course of treatment for a total of 3 courses.
Endpoints:
Recovery: The time of hospitalization, Pulmonary Infection Control (PIC) and fever resolution after treatment and intervention was observed.
Bacterial clearance rate: It was divided into clearance and non-clearance. Morning sputum was collected from patients for 3 times and for those with endotracheal intubation, the sputum was obtained through a sterile sputum suction tank. If there is no growth of pathogenic bacteria in all specimens collected from the same patient on the 1st d after treatment, it is considered to be cleared. On the contrary, if the proto-pathogenic bacteria still existed in the samples taken on the 1st d after treatment, it was not cleared.
Respiratory mechanics indexes: Pre-treatment and post-treatment Peak Airway Pressure (PIP), Airway Resistance (Raw), Work Of Breathing (WOB) and Dynamic Compliance (Cdyn) were measured.
Inflammation indexes: Venous blood from elbow (5 ml) collected 24 h before and after treatment was centrifuged in a 1500 xg centrifuge at 4° for 10 min and the resulting serum was stored in a -70° refrigerator for later use. Enzyme-Linked Immunosorbent Assay (ELISA) [11] detected Interleukin-6 (IL-6), High- Sensitivity C-Reactive Protein (hs-CRP) and Calcitonin (PCT), referring to the instructions of IL-6, hs-CRP and PCT kits (Yipu Biology Co., Ltd., Wuhan, China, YXE10140, YX-E11183, YX-E10643).
Clinical efficacy: Clinical efficacy was divided into four criteria; basically cured, markedly effective, effective and ineffective. The patient was considered basically cured if the clinical symptoms and signs disappeared after treatment and the etiology and laboratory tests were normal. Markedly effective was indicated if the clinical symptoms and signs were obviously ameliorated and the etiology and laboratory examination results were improved after treatment. Effective corresponded to alleviated clinical symptoms and signs and relatively improved etiology and inflammatory examination results after treatment. And ineffective was considered if there was no amelioration in the patient’s condition. Overall response rate was calculated as (markedly effective+effective) cases/total number of cases×100 %.
Adverse reactions: A series of adverse reactions, including nausea, lethargy, vomiting and headache, were observed in two cohorts during treatment.
Statistical methods:
The statistical analysis and plotting of the data employed Statistical Package for the Social Sciences (SPSS) 21.0 statistical software (SPSS, Inc, Chicago, IL, USA) and GraphPad Prism6.0 software (GraphPad Software Inc., SanDiego, CA, USA) respectively. The normal distribution test was performed using the Shapiro-Wilk test. Categorical variables were expressed by number of cases/percentages (n/%); the Chi-square test was used for comparison between groups and continuous correction Chi-square test was applied when the theoretical frequency was less than 5. Mean±Standard Error of Mean (SEM) was used to represent continuous variables, which were compared by the independent sample T test between groups. p<0.05 was regarded as a difference with statistical significance.
Results and Discussion
RG and CG were similar in general data such as gender, age, smoking history, drinking history, hypertension history, diabetes history, disease type, intubation and mechanical ventilation, and non-invasive ventilation (p>0.05) as shown in Table 1.
| Classification | Research group (n=101) | Control group (n=100) | t/χ2 value | p value |
|---|---|---|---|---|
| Gender | 0.891 | 0.345 | ||
| Male | 56 (55.45) | 65 (62.00) | ||
| Female | 45 (44.55) | 38 (38.00) | ||
| Age (y) | 1.145 | 0.254 | ||
| 41.65±4.05 | 42.31±4.12 | |||
| BMI (kg/m2) | 1.466 | 0.144 | ||
| 22.67±2.79 | 22.09±2.82 | |||
| History of smoking | 1.819 | 0.177 | ||
| Yes | 67 (66.34) | 75 (75.00) | ||
| No | 34 (33.66) | 25 (25.00) | ||
| History of drinking | 0.398 | 0.528 | ||
| Yes | 59 (58.42) | 54 (54.00) | ||
| No | 42 (41.58) | 46 (46.00) | ||
| History of hypertension | 0.239 | 0.625 | ||
| Yes | 61 (60.40) | 57 (57.00) | ||
| No | 40 (39.60) | 43 (43.00) | ||
| History of diabetes | 0.244 | 0.621 | ||
| Yes | 49 (48.51) | 52 (52.00) | ||
| No | 52 (51.49) | 48 (48.00) | ||
| History of asthma | 0.243 | 0.622 | ||
| Yes | 35 (34.65) | 38 (38.00) | ||
| No | 66 (65.35) | 62 (62.00) | ||
| Disease type | 0.978 | 0.613 | ||
| COPD complicated with infection | 37 (36.63) | 32 (32.00) | ||
| Bronchiectasis complicated with infection | 29 (28.71) | 35 (35.00) | ||
| Acute pneumonia | 35 (34.65) | 33 (33.00) |
Table 1: Comparison of General Data Between Two Groups [N (%)] (Mean±Sd)
The time of hospitalization, PIC and fever resolution was noticeably shorter in RG compared with CG (p<0.05) as shown in Table 2.
| Group | Number of cases | Hospitalization time (d) | Lung infection control time (d) | Fever resolution time (d) |
|---|---|---|---|---|
| Research group | 101 | 10.05±1.04 | 6.79±0.72 | 5.81±0.63 |
| Control group | 100 | 16.68±1.32 | 11.93±1.25 | 7.49±0.81 |
| t | - | 39.570 | 35.770 | 16.420 |
| p | - | <0.001 | <0.001 | <0.001 |
Table 2: Comparison of Recovery Between Two Groups
The bacterial clearance rate after treatment was evidently higher in RG than in CG (83.33 % vs. 60.29 %, p<0.05) as shown in Table 3.
| Bacteria category | Research group | Control group | ||||
|---|---|---|---|---|---|---|
| Remove | Not cleared | Total strains | Remove | Not cleared | Total strains | |
| Acinetobacter | 24 | 3 | 27 | 14 | 10 | 24 |
| Pseudomonas aeruginosa | 9 | 9 | 18 | 6 | 11 | 17 |
| Staphylococcus aureus | 5 | 0 | 5 | 1 | 2 | 3 |
| Staphylococcus epidermidis | 0 | 0 | 0 | 2 | 1 | 3 |
| Klebsiella pneumoniae | 8 | 0 | 8 | 4 | 1 | 5 |
| Citrobacter | 2 | 0 | 2 | 4 | 1 | 5 |
| Serratia | 2 | 0 | 2 | 0 | 0 | 0 |
| Proteobacteria | 0 | 0 | 0 | 3 | 0 | 3 |
| Escherichia coli | 5 | 0 | 5 | 5 | 0 | 5 |
| Enterobacter cloacae | 5 | 0 | 5 | 2 | 1 | 3 |
| Total | 60 | 12 | 72 | 41 | 27 | 68 |
Table 3: Comparison of Bacterial Clearance Rate Between Two Groups
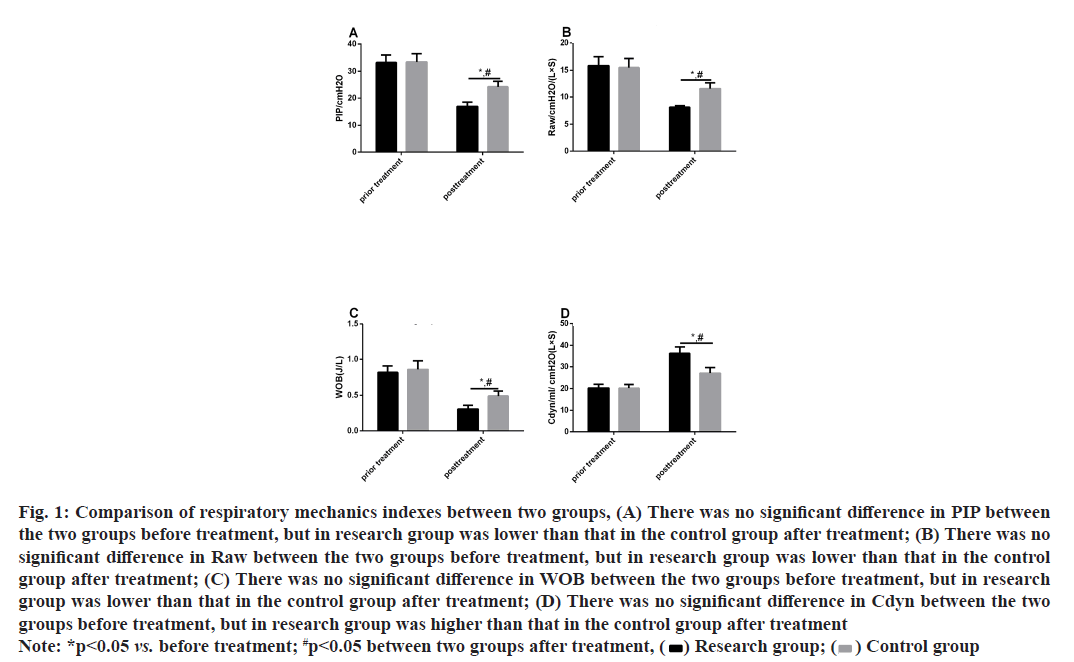
The pre-treatment respiratory mechanics indexes differed insignificantly between CG and RG (p>0.05). After treatment, PIP, Raw and WOB reduced while Cdyn increased in both cohorts (p<0.05) and the increase in PIP, Raw and WOB and the decrease in Cdyn were more evident in RG (p<0.05) as shown in fig. 1.
Fig. 1: Comparison of respiratory mechanics indexes between two groups, (A) There was no significant difference in PIP between the two groups before treatment, but in research group was lower than that in the control group after treatment; (B) There was no significant difference in Raw between the two groups before treatment, but in research group was lower than that in the control group after treatment; (C) There was no significant difference in WOB between the two groups before treatment, but in research group was lower than that in the control group after treatment; (D) There was no significant difference in Cdyn between the two groups before treatment, but in research group was higher than that in the control group after treatment
Note: *p<0.05 vs. before treatment; #p<0.05 between two groups after treatment, (  ) Research group; (
) Research group; (  ) Control group
) Control group
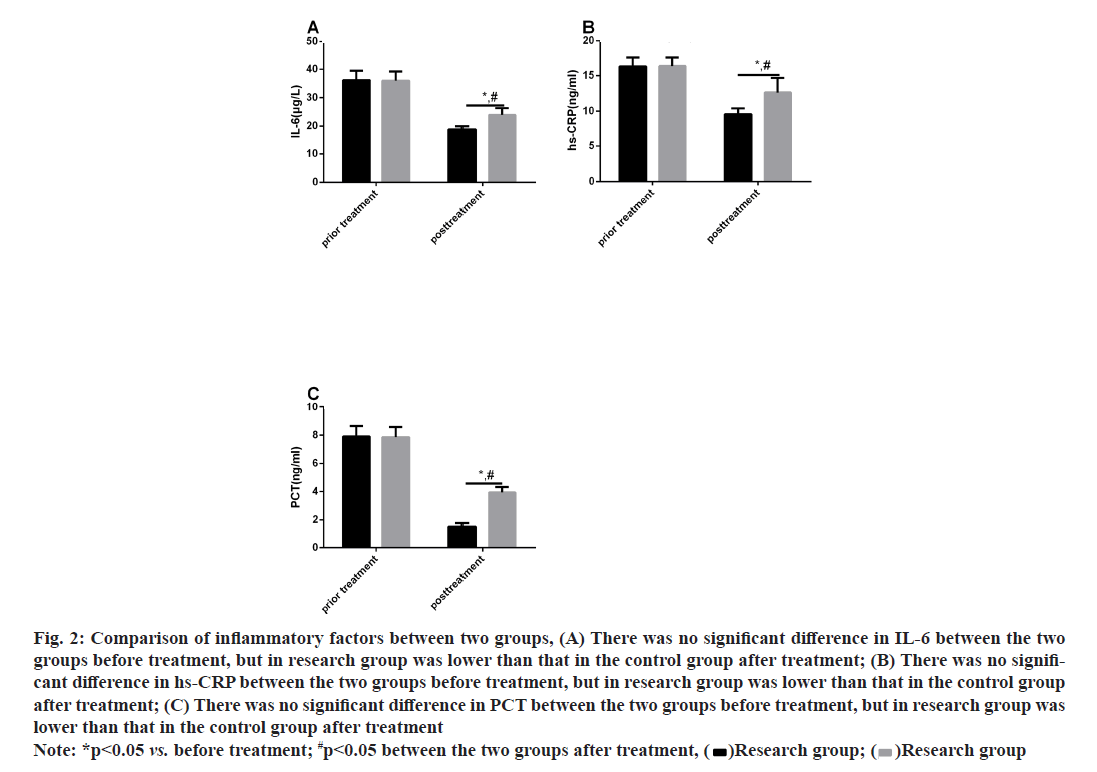
Significant difference was absent regarding the pretreatment levels of inflammatory factors between CG and RG (p>0.05). After treatment, IL-6, hs-CRP and PCT reduced in both cohorts (p<0.05) and the reductions were more distinct in RG (P<0.05) as shown in fig. 2.
Fig. 2: Comparison of inflammatory factors between two groups, (A) There was no significant difference in IL-6 between the two groups before treatment, but in research group was lower than that in the control group after treatment; (B) There was no significant difference in hs-CRP between the two groups before treatment, but in research group was lower than that in the control group after treatment; (C) There was no significant difference in PCT between the two groups before treatment, but in research group was lower than that in the control group after treatment
Note: *p<0.05 vs. before treatment; #p<0.05 between the two groups after treatment, (  )Research group; (
)Research group; (  )Research group
)Research group
After treatment, the overall response rate in RG was 93.07 %, a rate markedly higher than 75.00 % in CG (p<0.05) as shown in Table 4.
| Group | Number of cases | Basically cured | Markedly effective | Effective | Ineffective | Overall response rate |
|---|---|---|---|---|---|---|
| Research group | 101 | 27 (26.73) | 32 (31.68) | 35 (34.65) | 7 (6.93) | 94 (93.07) |
| Control group | 100 | 17 (17.00) | 28 (28.00) | 30 (30.00) | 25 (25.00) | 75 (75.00) |
| t | - | - | - | - | - | 12.261 |
| p | - | - | - | - | - | 0.001 |
Table 4: Comparison of Clinical Efficacy Between Two Groups
No significant difference was observed in the overall incidence of adverse reactions between RG and CG (5.94 % vs. 7.00 %, p>0.05) as shown in Table 5.
| Group | Number of cases | Diarrhea | Nausea | Lethargy | Vomiting | Headache | Total incidence (%) |
|---|---|---|---|---|---|---|---|
| Research group | 101 | 0 (0.00) | 1 (0.99) | 1 (0.99) | 2 (1.98) | 2 (1.98) | 6 (5.94) |
| Control group | 100 | 1 (1.00) | 2 (2.00) | 2 (2.00) | 2 (2.00) | 0 (0.00) | 7 (7.00) |
| t | - | 1.015 | 0.349 | 0.349 | 0.001 | 2.000 | 0.093 |
| p | - | 0.314 | 0.555 | 0.555 | 0.992 | 0.157 | 0.760 |
Table 5: Comparison of Total Adverse Reactions Between Two Groups
LRTI is the prime reason for global morbidity and mortality, especially among young children and the elderly [12]. With trachea, bronchus and lung as the disease site, LRTI usually shows the presentations of fever, runny nose and cough [13]; and for some, there will be varying degrees of fatigue and difficulty in breathing out, which poses a grave threat to patients’ life and work, as well as life safety [14]. Therefore, it is of utmost importance to study the disease in depth and propose scientific and standardized treatment programs to help patients relieve clinical symptoms control the progression of the disease and improve the prognosis.
At the present stage, the number of antibacterial drugs is gradually increasing, with further expanded scope of clinical application. However, more and more resistant strains appear, which complicates the patient’s condition and increases the difficulty of disease treatment [15]. In this study, we used CPZ/SB plus AZM to treat severe LRTI and found that after treatment, the inflammatory level in patients was markedly reduced and the PF was greatly improved. In Gao et al. [16] study, administration of Tanreqing injection combined with CPZ/SB to patients with chronic bronchial exacerbation was effective in shortening the duration of symptom resolution and improving the efficacy of treatment. It shows that the intervention of CPZ/ SB has an effective effect, while the intervention plus other drugs can better resolve the clinical symptoms of patients and get a good prognosis. This study compared patients’ recovery after treatment and found that the hospital stay, PIC time and fever resolution time of patients in RG were notably less, suggesting that CPZ/ SB plus AZM was effective in the treatment of severe LRTI and greatly shortened the treatment course. Severe LRTI is mostly caused by bacterial infection; However, clinical abuse of antibiotics is easy to lead to multidrug resistance symptoms, so CPZ/SB intervention is not highly sensitive to bacteria [17]. Research has shown that AZM intervention in ventilator associated pneumonia can suppress the progression of lung inflammation and improve the efficacy of intervention by reducing multidrug-resistant Acinetobacter baumannii [18]. This study revealed that the bacterial clearance rates were 83.33 % and 60.29 % in RG and CG respectively, indicating that the combined treatment intervention can better remove bacteria in patients, thus improving the clinical efficacy. Patients with severe LRTI often suffer from severe respiratory dysfunction [19]. While AZM has direct activity on airway epithelial cells, which can maintain their functions and reduce mucus secretion [20]. The results of this study identified lower PIP, Raw and WOB and higher Cdyn in RG compared with CG, demonstrating that the combined medication can not only improve the clearance rate of bacteria in vivo, but also prevent the disease from worsening and further damaging respiratory function.
The overinduction of inflammatory response is directly related to severe complications of viral pneumonia and respiratory tract infection [21]. According to Mainguy, AZM is an immunomodulatory antibiotic that can reduce pulmonary neutropenia and inflammation in asthmatic patients [22], which indicates that AZM can effectively reduce the inflammatory reaction of infessssssctious patients. In the study of Lv, giving CPZ/SB plus Tigecycline to patients with pulmonary infection can effectively reduce the inflammatory response in patients, thus obtaining a better prognosis [23]. The findings of this study revealed notably lower IL-6, hs-CRP and PCT in RG after treatment, which suggested that the combined medication can effectively reduce the inflammatory response in patients with severe LRTI, thus playing an effective role in anti-infection treatment. Dokic reported that AZM alleviated the clinical symptoms and improved the PF of asthmatic patients caused by chronic Chlamydia pneumoniae [24]. And in the research of Liu et al. [25], patients with hospital acquired pneumonia and health care-related pneumonia were given CPZ/ SB and cefepime alone; They found that there were fewer adverse events with two different treatments. It shows that AZM and CPZ/SB have a good effect in intervening patients with pulmonary infection. In this study, the overall clinical response rate was 93.07 % in RG and 75.00 % in CG, with a statistically significant difference between the two cohorts. In terms of adverse reactions, no distinct difference was observed. It shows that the combined medication can effectively improve the curative effect of patients with no obvious adverse reaction after intervention.
To sum up, CPZ/SB plus AZM is beneficial to alleviate the inflammation response and improve the PF of patients with severe LRTI, which is worth popularizing. However, there is still room for improvement in this study. For example, we can supplement the basic experiments of the therapeutic mechanism of the two treatment methods and explore the risk factors affecting the curative effect of patients from the molecular level. In the future, we will gradually improve the study from the above perspectives.
Conflicts of Interest:
The authors declared no conflicts of interest.
References
- Gale CR, Deary IJ, Batty GD. Cognitive ability and risk of death from lower respiratory tract infection: findings from UK Biobank. Sci Rep 2019;9(1):1-6.
- Sherchan JB, Humagain S. Antimicrobial Susceptibility Pattern of Gram-Negative Bacteria Causing Lower Respiratory Tract Infections in Kathmandu University Hospital. J Nepal Health Res Counc 2021;18(4):661-6.
- Hansen LS, Lykkegaard J, Thomsen JL, Hansen MP. Acute lower respiratory tract infections: Symptoms, findings and management in Danish general practice. Eur J Gen Pract 2020;26(1):14-20.
- Lhopitallier L, Kronenberg A, Meuwly JY, Locatelli I, Dubois J, Marti J, et al. Procalcitonin and lung ultrasonography point-of-care testing to decide on antibiotic prescription in patients with lower respiratory tract infection in primary care: protocol of a pragmatic cluster randomized trial. BMJ 2021;374:n2132.
- Niu T, Luo Q, Li Y, Zhou Y, Yu W, Xiao Y. Comparison of Tigecycline or Cefoperazone/Sulbactam therapy for bloodstream infection due to Carbapenem-resistant Acinetobacter baumannii. Antimicrob Resist Infect Control 2019;8(1):1-2.
- Wang Z, Li Z, Luo S, Yang Z, Xing Y, Pu C, et al. Cefoperazone and sulbactam-related eosinophilic peritonitis: a case report and literature review. J Int Med Res 2021;49(6):03000605211025367.
- Zhou L, Bao J, Ma J. Hemolytic anemia and reactive thrombocytosis associated with cefoperazone/sulbactam. Front Pharmacol 2019;10:1342.
- Echarte-Morales J, Minguito-Carazo C, del Castillo-García S, Borrego-Rodríguez J, Rodríguez-Santamarta M, Sánchez-Muñoz E, et al. Effect of hydroxychloroquine, azithromycin and lopinavir/ritonavir on the QT corrected interval in patients with COVID-19. J Electrocardiol 2021;64:30-5.
- Venditto VJ, Haydar D, Abdel-Latif A, Gensel J, Anstead MI, Pitts MG, et al. Immunomodulatory effects of azithromycin revisited: potential applications to COVID-19. Front Immunol 2021;12:285.
- Vos LM, Bruyndonckx R, Zuithoff NP, Little P, Oosterheert JJ, Broekhuizen BD, et al. Lower respiratory tract infection in the community: associations between viral aetiology and illness course. Clin Microbiol Infect 2021;27(1):96-104.
- Dvornicky-Raymond ZT, Donnelly KA, Emerson JA, Penfold LM, Citino SB. Evaluation of a visual enzyme-linked immunosorbent assay (elisa) for pregnancy detection in four ungulate species. J Zoo Wildl Med 2021;51(4):926-32.
- Malosh RE, Martin ET, Ortiz JR, Monto AS. The risk of lower respiratory tract infection following influenza virus infection: a systematic and narrative review. Vaccine 2018;36(1):141-7.
- Pan DS, Huang JH, Lee MH, Yu Y, Mark I, Chen C, et al. Knowledge, attitudes and practices towards antibiotic use in upper respiratory tract infections among patients seeking primary health care in Singapore. BMC Fam Pract 2016;17(1):1-9.
- Jannelli E, Castelli A, Calderoni EF, Annunziata S, Maccario G, Ivone A, et al. Fractures in patients with COVID-19 infection: Early prognosis and management. A case series of 20 patients in a single institution in Lombardy, Northern Italy. J Orthop Trauma 2020;34(10):e389-97.
- Guo Y, Song G, Sun M, Wang J, Wang Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front Cell Infect Microbiol 2020;10:107.
- Gao LN, Lyu J, Wang ZF, Yu DD, Sun MH. Meta-analysis of randomized controlled trials on effect of Tanreqing Injection combined with Western medicine on acute exacerbation of chronic bronchitis. Zhongguo Zhong Yao Za Zhi 2019;44(24):5313-21.
- Cheng L, Gong YL, Luo XQ, Zhang C, Liu MX, Peng YZ. Clinical characteristics of burn patients infected with Stenotrophomonas maltophilia and antibiotic resistance of the strains. Zhonghua Shao Shang Za Zhi 2018;34(2):78-82.
- Yamada K, Yanagihara K, Kaku N, Harada Y, Migiyama Y, Nagaoka K, et al. Azithromycin attenuates lung inflammation in a mouse model of ventilator-associated pneumonia by multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2013;57(8):3883-8.
- Flerlage T, Boyd DF, Meliopoulos V, Thomas PG, Schultz-Cherry S. Influenza virus and SARS-CoV-2: pathogenesis and host responses in the respiratory tract. Nat Rev Microbiol 2021:1-7.
- Cramer CL, Patterson A, Alchakaki A, Soubani AO. Immunomodulatory indications of azithromycin in respiratory disease: a concise review for the clinician. Postgrad Med 2017;129(5):493-9.
- Mahooti M, Miri SM, Abdolalipour E, Ghaemi A. The immunomodulatory effects of probiotics on respiratory viral infections: A hint for COVID-19 treatment?. Microb Pathog 2020;148:104452.
- Mainguy?Seers S, Vargas A, Labrecque O, Bédard C, Hélie P, Lavoie JP. Randomised study of the immunomodulatory effects of azithromycin in severely asthmatic horses. Vet Rec 2019;185(5):143.
- Lv Q, Deng Y, Zhu X, Ruan J, Chen L. Effectiveness of Cefoperazone-sulbactam alone and Combined with Tigecycline in the Treatment of Multi-drug Resistant Acinetobacter Baumannii Pulmonary Infection. J Coll Physicians Surg Pak 2020;30(3):332-4.
- Dokikj D, Poposki B, Karkinski D. Azithromycin in treatment of patients with asthma and C. Pneumoniae infection. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2013;34:71-7.
- Liu JW, Chen YH, Lee WS, Lin JC, Huang CT, Lin HH, et al. Randomized noninferiority trial of cefoperazone-sulbactam versus cefepime in the treatment of hospital-acquired and healthcare-associated pneumonia. Antimicrob Agents Chemother 2019;63(8):e00023-19.