- *Corresponding Author:
- H. Ke
Department of Oncology,
The Third Clinical Medical College of China Three Gorges University,
Gezhouba Central Hospital of Sinopharm,
China
E-mail: kh196651@163.com
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “57-62” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the changes in sensitivity of pancreatic cancer cells to erlotinib after specific down-regulation of Axl (Anexelekto) expression and its mechanism is the objective of the study. The expression of Axl in pancreatic cancer cells was specifically down-regulated by small interfering ribonucleic acid technology and identified by Western blotting. The survival rate of pancreatic cancer cells under the effect of erlotinib at different concentrations (0, 2.5, 5, 10 and 20 μmol/l) was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. The morphological changes of cell cycle were detected by flow cytometry and the expression of protein kinase B, phosphorylated protein kinase B, extracellular signal-regulated kinase and phosphorylated extracellular signal-regulated kinase were detected by immunoblotting. The expression of Axl was significantly inhibited at 24, 48 and 72 h after transfection with small interfering ribonucleic acid-Axl, with the most significant inhibitory effect at 48 h (p<0.05). Compared with human pancreatic cancer cell line, PANC-1/small interfering ribonucleic acid-control, erlotinib had significant proliferation inhibition and pro-apoptotic effect on human pancreatic cancer cell line, PANC-1/small interfering ribonucleic acid-Axl cells, and the difference was statistically significant (p<0.05). Compared with human pancreatic cancer cell line, PANC-1/small interfering ribonucleic acid-control, erlotinib significantly reduced the protein expression of phosphorylated extracellular signal-regulated kinase and phosphorylated protein kinase B in human pancreatic cancer cell line, PANC-1/small interfering ribonucleic acid-Axl cells (p<0.05), but total extracellular signal-regulated kinase and protein kinase B protein expression were not affected. Specific inhibition of Axl expression in pancreatic cancer cells enhances the sensitivity of pancreatic cancer cells to erlotinib by inhibiting phosphorylation of extracellular signal-regulated kinase and protein kinase B protein.
Keywords
Axl, human pancreatic cancer cell line, erlotinib, extracellular signal-regulated kinase, protein kinase B
Pancreatic cancer is a common malignancy worldwide and its mortality rate is the second highest among cancer mortality rates[1,2]. Although specialized and diversified treatment options have greatly improved the survival rate of pancreatic cancer patients, intrinsic or acquired resistance to chemotherapeutic agents have still issues that need to be addressed in the treatment of pancreatic cancer[3].
Erlotinib is a class of Tyrosine Kinase Inhibitors (TKIs) targeting the Epidermal Growth Factor Receptor (EGFR) and is widely used in the treatment of many tumors, including pancreatic cancer[4]. However, a large body of clinical data suggests that tumor cells become less sensitive and resistant to EGFR-TKIs as the treatment cycle progresses[5]. Anexelekto (Axl) is a member of the Receptor Tyrosine Kinase subfamily (TAM family), which was isolated from the Deoxyribonucleic Acid (DNA) of human Chronic Myeloid Leukemia (CML) patients in 1991 by O’bryan et al.[6]. In recent years, many studies have shown that Axl is involved as a drug resistance gene in regulating resistance to various chemotherapeutic agents such as cisplatin[7], cetuximab[8] and lapatinib[9]. However, it is not clear whether Axl is also involved in the regulation of sensitivity to erlotinib in pancreatic cancer cells. In this study, we examined the sensitivity of pancreatic cancer cells to erlotinib by specifically down-regulating the expression of Axl in pancreatic cancer cells and we also examined the possible molecular mechanisms.
Materials and Methods
Materials:
Human Pancreatic Cancer Cell Line (PANC-1) was purchased from Shanghai Cell Bank, Chinese Academy of Sciences; human small interfering Ribonucleic Acid (siRNA)-Axl was synthesized by Shanghai Kikai and control siRNA was purchased from Cell Signal, United States of America (USA); transfection reagent OligofectamineTM was purchased from Invitrogen, USA; flow-through kit was purchased from Pierce, USA; acridine orange staining reagent, Axl, phosphorylated Extracellular Signal-Regulated Kinase (p-ERK), Extracellular Signal-Regulated Kinase (ERK), phosphorylated Protein Kinase B (p-AKT) and Protein Kinase B (AKT) antibodies were purchased from Sigma, USA.
Methods:
Cell culture: PANC-1 was cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10 % Fetal Bovine Serum (FBS), 2 mmol/l glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin and the culture medium was changed every 2~3 d.
Cell transfection: Plasmid transfection was carried out according to the instructions of OligofectamineTM transfection reagent. The plasmid was diluted to 2 μg/100 μl with serum-free culture medium, 7 μl of transfection reagent was added and the cells were incubated for 30 min at room temperature.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay for cell proliferation: PANC-1, PANC-1/siRNA-control and PANC-1/siRNA-Axl cell suspensions were inoculated in 96-well culture plates, 5000~10 000 cells/well, 5 replicate wells were made for each drug concentration, 24 h post-adhesion and treated with different concentrations of erlotinib (0, 2.5, 5, 10, 20 μmol/l) diluted with FBS containing 0.5 % (2.5, 5, 10, 20 μmol/l) for 48 h. The supernatant was aspirated and 2 mg/ml of MTT solution (100 μl/well) was added to continue the incubation for 4 h. After aspirating the culture medium, Dimethyl Sulfoxide (DMSO) solution (150 μl/well) was added and the plate was shaken on a microplate shaker for 10 min to dissolve the crystals. The results were recorded and plotted.
Detection of apoptosis by flow cytometry: The above three cells were digested and counted, inoculated in 6-well plates (0.5×106 cells/well) and incubated overnight. 24 h later, the cells were treated with different concentrations of erlotinib (0, 5, 10 μmol/l) diluted with 0.5 % FBS for 48 h. The supernatant was discarded, digested with trypsin and centrifuged, and the cells were collected as 1×105/100 μl. 5 μl of Annexin V-Fluorescein Isothiocyanate (FITC) and Propidium Iodide (PI) were added, and the cells were stained for 15 min at room temperature, protected from light, and finally 400 μl of binding buffer was added, mixed well, and the apoptotic cells were detected on the machine.
Acridine orange staining: The above three cells were digested and counted, inoculated in 12-well plates (1×104 cells/well) and treated with different concentrations of erlotinib (0, 5, 10 μmol/l) for 48 h. The supernatant was discarded and washed 3 times with Phosphate Buffered Saline (PBS), and each well was stained with 100 μl of 10 % acridine orange staining reagent for 20 min and washed 3 times with PBS. The fluorescence microscope was selected to observe the excitation light at 520 nm.
Immunoblotting assay: The above three cells were treated with erlotinib and collected, rinsed 3 times in PBS, lysed in Radioimmunoprecipitation Assay (RIPA) buffer for 30 min, centrifuged at 12 000×g for 30 min, and the supernatant was collected for protein content determination by Bicinchoninic Acid (BCA). Cell lysates were diluted with 5× sampling buffer, denatured at 95° for 10 min, 10 % denaturing polyacrylamide gel electrophoresis (50 μg/well), transferred to Polyvinylidene Fluoride (PVDF) membrane, blocked with 1 % Bovine Serum Albumin (BSA) for 1 h, incubated with primary antibody (1:1000 dilution) for 3 h, incubated with alkaline phosphatase-labelled secondary antibody (1:2000 dilution) for 1 h at room temperature and visualized with 5-Bromo-4-Chloro- 3-Indolyl Phosphate (BCIP)/Nitro Blue Tetrazolium (NBT) chromogenic solution. The bands were analysed by Chemi-Genius gel analysis system for grey scale values, United Kingdom (UK).
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 22.0 software was used for data processing. Each experiment was repeated three times independently and data were expressed as x?±s. Analysis of Variance (ANOVA) was used for comparison between groups, p<0.05 was considered a statistically significant difference.
Results and Discussion
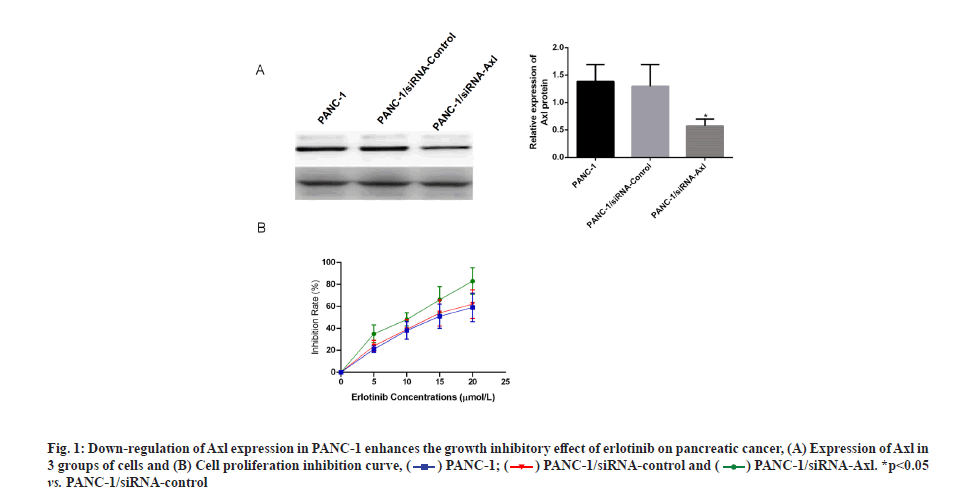
Specific down-regulation of Axl expression increases the inhibitory effect of erlotinib on pancreatic cancer. Applying siRNA technology to specifically down-regulate Axl expression in PANC-1 cells, Western blotting results showed that the protein expression of Axl was down-regulated at 24, 48 and 72 h after transfection of PANC-1 cells with siRNA-Axl, and the down-regulation effect was most obvious at 48 h (fig. 1A). The survival rate of PANC-1 cells, PANC- 1/siRNA-control and PANC-1/siRNA-Axl after 48 h treatment with different concentrations of erlotinib (0, 2.5, 5, 10 and 20 μmol/l) was then measured by MTT assay. The results showed that the survival rate of PANC-1/siRNA-Axl cells was significantly lower than that of PANC-1/siRNA-control cells with half-maximal Inhibitory Concentration (IC50) (4.99±0.83) μmol/l, which was lower than that of PANC-1/siRNA-control and PANC-1 cells with IC50 (10.92±1.36, 10.88±2.13) μmol/l by 2.2-fold and 2.1-fold, respectively (fig. 1B and Table 1). We subsequently used the close IC50 values to determine subsequent test concentrations.
| Concentration (μmol/l) | PANC-1 | PANC-1/siRNA-control | PANC-1/siRNA-Axl |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 5 | 21.74±2.33 | 24.87±1.67 | 34.20±3.19* |
| 10 | 38.76±4.18 | 39.11±3.31 | 48.28±5.24Δ |
| 15 | 51.38±2.17 | 54.89±4.98 | 67.28±7.99# |
| 20 | 59.47±10.36 | 62.30±9.40 | 84.56±10.28? |
Note: *p<0.05 vs. 5 μmol/l; #p<0.05 vs. 10 μmol/l; Δp<0.05 vs. 15 μmol/l and ?p<0.05 vs. 20 μmol/l
Table 1: Effect of Erlotinib on Cell Proliferation Inhibition Rate (%)
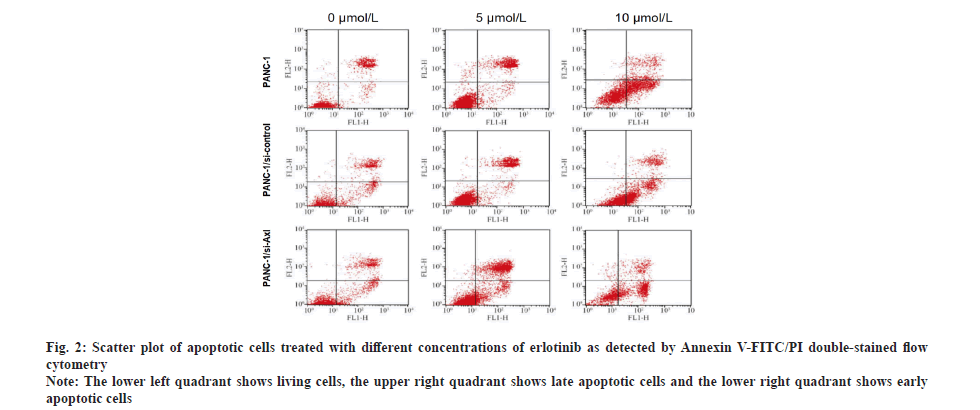
Specific down-regulation of Axl expression promoted the apoptosis-inducing effect of erlotinib on pancreatic cancer cells. To investigate the effect of erlotinib on apoptosis of pancreatic cancer cells, the PANC-1, PANC-1/siRNA-control and PANC-1/siRNA-Axl cells were treated with different concentrations of erlotinib (0, 5 and 10 μmol/l) for 48 h, respectively. Annexin V-FITC/PI double-staining assay was used to detect apoptosis. Compared with PANC-1, PANC-1/siRNA- control, PANC-1/siRNA-Axl cells showed significant apoptosis with the increase of erlotinib concentration (Table 2 and fig. 2) and the difference was statistically significant (p<0.05).
| Erlotinib concentration (μmol/l) | PANC-1 | PANC-1/siRNA-control | PANC-1/siRNA-Axl |
|---|---|---|---|
| 0 | 13.75±0.74 | 14.40±0.82 | 15.88±0.66 |
| 5 | 26.77±0.42 | 26.07±0.72 | 46.91±0.58* |
| 10 | 48.10±0.48 | 49.35±0.61 | 65.28±0.78# |
Note: *p<0.05 vs. 5 μmol/l and #p<0.05 vs. 10 μmol/l
Table 2: Effect of Erlotinib on the Rate of Apoptosis of Pancreatic Cancer Cells (%)
Fig. 2: Scatter plot of apoptotic cells treated with different concentrations of erlotinib as detected by Annexin V-FITC/PI double-stained flow cytometry
Note: The lower left quadrant shows living cells, the upper right quadrant shows late apoptotic cells and the lower right quadrant shows early apoptotic cells
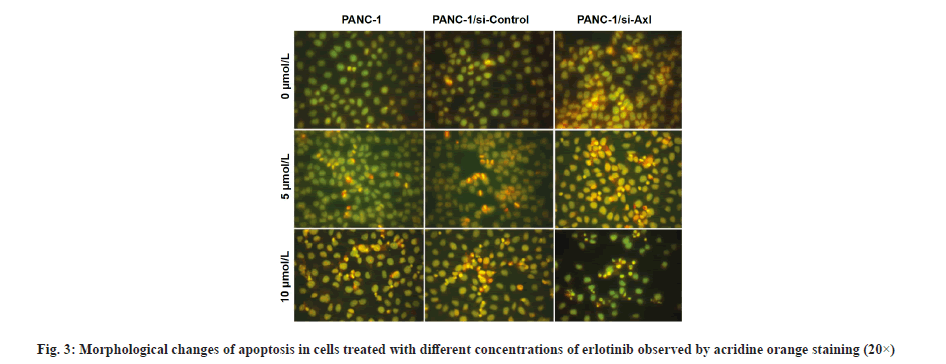
Morphological observation of erlotinib-induced cell death was described here. As shown in fig. 3, PANC- 1, PANC-1/siRNA-control and PANC-1/siRNA-Axl cells without erlotinib treatment showed uniform green chromatin and regular nuclear morphology, after treatment with 5 μmol/l erlotinib, compared with PANC-1/siRNA-control cells, PANC-1/siRNA-Axl cells showed obvious nuclear consolidation, chromatin agglutination and orange-red color. After treatment with 5 μmol/l erlotinib, the PANC-1/siRNA-Axl cells showed obvious nuclear condensation, chromatin agglutination and orange-red coloration compared with PANC-1/siRNA-control, and nuclear fragmentation was seen when erlotinib was 10 μmol/l.
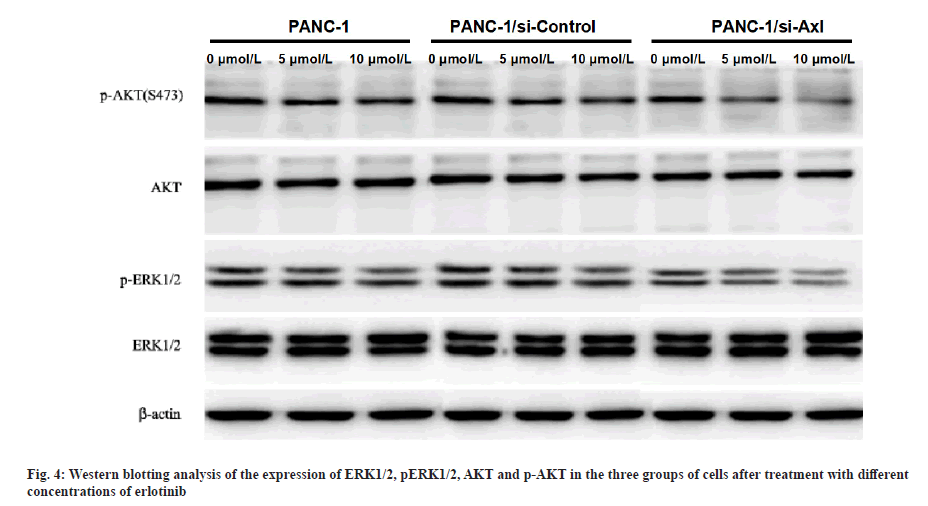
Effect of erlotinib on ERK1/2 and AKT protein and phosphorylated protein expression in pancreatic cancer cells after specific down-regulation of Axl expression was explained here. PANC-1, PANC-1/siRNA-control and PANC-1/siRNA-Axl cells were treated with different concentrations of erlotinib (0, 5, 10 μmol/l) for 48 h and then subjected to Western blotting assay. The results showed that the protein expression levels of p-ERK1/2 and p-AKT in PANC-1/siRNA-Axl cells decreased significantly with increasing drug concentrations compared with PANC-1/siRNA-control (p<0.05), while the expression levels of total ERK and AKT did not change (fig. 4).
EGFR is a member of the ErbB receptor family[10] and is highly expressed in a variety of tumour cells including pancreatic cancer[11]. In pancreatic cancer cells, when EGFR binds to the related ligands, it phosphorylates tyrosine residues in the cells, which in turn activates two important downstream signalling pathways, Mitogen-Activated Protein Kinase (MAPK) and Phosphatidylinositol 3-Kinase (PI3K)/AKT, thus playing a role in regulating cell proliferation, survival and migration[12-14]. ERK1/2 and AKT are key enzymatic proteins in these two signalling pathways. Erlotinib is an anti-tumor drug targeting EGFR. Its mechanism of action is mainly through competition with Adenosine Triphosphate (ATP) on the ATP binding site of the tyrosine kinase domain on the EGFR receptor, preventing the phosphorylation of tyrosine residues, thus blocking a series of enzymatic cascades downstream of EGFR, thereby inhibiting tumor growth[15]. Erlotinib is currently being used as an EGFR- TKIs drug in the study and treatment of pancreatic cancer[16]. In phase II clinical trials, erlotinib alone was shown to control the progression of advanced pancreatic cancer[17]. However, the frequent resistance and reduced sensitivity of these drugs over longer treatment cycles is the greatest limitation to their oncological treatment.
In recent years, many studies have shown that Axl is closely related to tumor development[18,19]. It is now known that Axl is highly expressed in lung cancer cells and is positively correlated with the malignancy of lung cancer cells[20]. In addition, Axl as a drug resistance gene is not only involved in resistance to many chemotherapeutic agents, but also has a regulatory role in the activation of PI3K/AKT and Mitogen-Activated Protein Kinase Kinase (MEK)/ERK pathways, thus promoting tumor proliferation and malignancy[21,22]. This suggests that Axl may also have a regulatory role in the sensitivity of EGFR-TKIs.
In order to investigate the effect of Axl on the sensitivity of erlotinib, the expression of Axl in the pancreatic cancer cell line PANC-1 was down-regulated by applying siRNA specificity to observe the changes in the sensitivity of tumor cells to erlotinib. The inhibition effect of erlotinib on PANC-1/siRNA-Axl cells was significantly increased compared with PANC-1/siRNA- control. Morphological changes of apoptosis were observed by acridine orange staining and significant nuclear condensation, chromatin agglutination to orange-red colour to form apoptotic vesicles, and nuclear fragmentation were observed in PANC-1/ siRNA-Axl cells at 5 and 10 μmol/l erlotinib compared with the control group. These findings suggest that specific downregulation of Axl expression increases the growth inhibitory and pro-apoptotic effects of erlotinib on pancreatic cancer.
To further investigate the molecular mechanism of Axl affecting erlotinib sensitivity, the protein expression of ERK1/2 and AKT, key enzymes in PI3K/AKT and MEK/ERK pathways, was examined by Western blotting. The results showed that the protein expression levels of p-ERK and p-AKT in PANC-1/siRNA-Axl cells were significantly lower than those in PANC-1/ siRNA-control cells after treatment with different concentrations of erlotinib (0, 5 and 10 μmol/l) for 48 h, but there was no difference in the total ERK and AKT expression.
In conclusion, the results of this study suggest that specific down-regulation of Axl expression increases the sensitivity of pancreatic cancer cells to erlotinib by inhibiting phosphorylation of ERK and AKT proteins, and Axl may be an important molecular target affecting the sensitivity of pancreatic cancer to erlotinib.
Conflict of interests:
The authors declared no conflict of interest.
References
- Lee RC, Kanhere H, Trochsler M, Broadbridge V, Maddern G, Price TJ. Pancreatic, periampullary and biliary cancer with liver metastases: Should we consider resection in selected cases? World J Gastrointest Oncol 2018;10(8):211-20.
[Crossref] [Google scholar] [PubMed]
- Lau SC, Cheung WY. Evolving treatment landscape for early and advanced pancreatic cancer. World J Gastrointest Oncol 2017;9(7):281-92.
[Crossref] [Google scholar] [PubMed]
- Koutsounas I, Giaginis C, Patsouris E, Theocharis S. Current evidence for histone deacetylase inhibitors in pancreatic cancer. World J Gastroenterol 2013;19(6):813-28.
[Crossref] [Google scholar] [PubMed]
- Hao J, Yang X, Ding XL, Guo LM, Zhu CH, Ji W, et al. Paeoniflorin potentiates the inhibitory effects of erlotinib in pancreatic cancer cell lines by reducing ErbB3 phosphorylation. Sci Rep 2016;6(1):1-9.
[Crossref] [Google scholar] [PubMed]
- Busse D, Yakes FM, Lenferink AE, Arteaga CL. Tyrosine kinase inhibitors: Rationale, mechanisms of action, and implications for drug resistance. Semin Oncol 2001;28(16):47-55.
[Crossref] [Google scholar] [PubMed]
- O'Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, et al. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol 1991;11(10):5016-31.
[Crossref] [Google scholar] [PubMed]
- Hong J, Peng D, Chen Z, Sehdev V, Belkhiri A. ABL regulation by AXL promotes cisplatin resistance in esophageal cancer. Cancer Res 2013;73(1):331-40.
[Crossref] [Google scholar] [PubMed]
- McDaniel NK, Iida M, Nickel KP, Longhurst CA, Fischbach SR, Rodems TS, et al. AXL mediates cetuximab and radiation resistance through tyrosine 821 and the c-ABL kinase pathway in head and neck cancer. Clin Cancer Res 2020;26(16):4349-59.
[Crossref] [Google scholar] [PubMed]
- Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R, et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: Activation of AXL. Cancer Res 2009;69(17):6871-8.
[Crossref] [Google scholar] [PubMed]
- Tfayli A, Mohty R. EGFR tyrosine kinase inhibitors in non-small cell lung cancer: Treatment paradigm, current evidence, and challenges. Tumori 2021;107(5):376-84.
[Crossref] [Google scholar] [PubMed]
- Meyers N, Gérard C, Lemaigre FP, Jacquemin P. Differential impact of the ERBB receptors EGFR and ERBB2 on the initiation of precursor lesions of pancreatic ductal adenocarcinoma. Sci Rep 2020;10(1):1-3.
[Crossref] [Google scholar] [PubMed]
- Huang J, Liu J, Qiu L. Transient receptor potential vanilloid 1 promotes EGFR ubiquitination and modulates EGFR/MAPK signalling in pancreatic cancer cells. Cell Biochem Funct 2020;38(4):401-8.
[Crossref] [Google scholar] [PubMed]
- Tao H, Chen X, Du Z, Ding K. Corn silk crude polysaccharide exerts anti-pancreatic cancer activity by blocking the EGFR/PI3K/AKT/CREB signaling pathway. Food Funct 2020;11(8):6961-70.
[Crossref] [Google scholar] [PubMed]
- McCubrey JA, Meher AK, Akula SM, Abrams SL, Steelman LS, et al. Wild type and gain of function mutant TP53 can regulate the sensitivity of pancreatic cancer cells to chemotherapeutic drugs, EGFR/Ras/Raf/MEK and PI3K/mTORC1/GSK-3 pathway inhibitors, nutraceuticals and alter metabolic properties. Aging 2022;14(8):3365-86.
[Crossref] [Google scholar] [PubMed]
- Dong Z, Wang Y, Ding V, Yan X, Lv Y, Zhong M, et al. GLI1 activation is a key mechanism of erlotinib resistance in human non?small cell lung cancer. Oncol Lett 2020;20(4):1-10.
[Crossref] [Google scholar] [PubMed]
- Liu C, Yu H, Hou YH, Gao ZL, Zhang YJ. Clinical efficacy evaluation of erlotinib combined with concurrent chemoradiotherapy in the treatment of locally advanced pancreatic cancer. Pak J Med Sci 2022;38(1):118-22.
[Crossref] [Google scholar] [PubMed]
- Lim SH, Yun J, Lee MY, Kim HJ, Kim KH, Kim SH, et al. Gemcitabine and erlotinib with or without oxaliplatin in previously untreated advanced pancreatic cancer: A randomized phase II trial. Yonsei Med J 2021;62(8):671-8.
[Crossref] [Google scholar] [PubMed]
- Tian Y, Zhang Z, Miao L, Yang Z, Yang J, Wang Y, et al. Anexelekto (AXL) increases resistance to EGFR-TKI and activation of AKT and ERK1/2 in non-small cell lung cancer cells. Oncol Res 2016;24(5):295-303.
[Crossref] [Google scholar] [PubMed]
- Kim SH, Choi S, Lee WS. Bevacizumab and anexelekto inhibitor, TP-0903 inhibits TGF-β1-induced epithelial-mesenchymal transition of colon cancer cells. Anticancer Drugs 2022;33(1):e453-61.
[Crossref] [Google scholar] [PubMed]
- Hwang JA, Hur JY, Kim Y, Im JH, Jin SH, Ryu SH, et al. Efficacy of newly discovered DNA aptamers targeting AXL in a lung cancer cell with acquired resistance to erlotinib. Transl Cancer Res 2021;10(2):1025-33.
[Crossref] [Google scholar] [PubMed]
- Abdel-Rahman WM, Al-Khayyal NA, Nair VA, Aravind SR, Saber-Ayad M. Role of AXL in invasion and drug resistance of colon and breast cancer cells and its association with p53 alterations. World J Gastroenterol 2017;23(19):3440-8.
[Crossref] [Google scholar] [PubMed]
- Wang F, Liu X, Bartholdy BA, Cheng H, Halmos B. Blockade of AXL activation overcomes acquired resistance to EGFR tyrosine kinase inhibition in non-small cell lung cancer. Transl Cancer Res 2019;8(6):2425-38.
[Crossref] [Google scholar] [PubMed]
 vs. PANC-1/siRNA-control
vs. PANC-1/siRNA-control