- *Corresponding Author:
- J. Lin
Department of Rheumatology and Immunology
Taizhou People’s Hospital, No. 366 Taihu Road
Taizhou, Jiangsu 225300, China
E-mail: m16531755539_1@163.com
| This article was originally published in a special issue, “Biomedical Research in Healthcare Setting” |
| Indian J Pharm Sci 2020:82(3)Spl issue5;119-124 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of the present study was to explore the role and potential mechanism of miR-5571-3p in rheumatoid arthritis. In this study, miR-5571-3p level in rheumatoid arthritis tissues and fibroblast-like synoviocytes was detected using qRT-PCR analysis. Cell Counting Kit-8 assay was performed to determine proliferation of fibroblast-like synoviocyte. Transwell assay was conducted to examine the invasion of fibroblast-like synoviocytes, and western blot analysis was used to examine the ornithine decarboxylase antizyme 3 protein expression. Results showed that miR-5571-3p expression was elevated in vivo and in vitro in rheumatoid arthritis. Inhibition of miR-5571-3p suppressed rheumatoid arthritis fibroblastlike synoviocyte proliferation and invasion. ornithine decarboxylase antizyme 3 siRNA could partially abolish the suppressor effect of miR-5571-3p inhibitor in cell proliferation and invasion in fibroblastlike synoviocytes. In conclusion, miR-5571-3p and ornithine decarboxylase antizyme 3 played important regulatory roles in proliferation and invasion of rheumatoid arthritis fibroblast-like synoviocytes, miR5571-3p/ornithine decarboxylase antizyme 3 axis could serve as a new target for therapy of rheumatoid arthritis.
Keywords
Rheumatoid arthritis, miR-5571-3p, Fibroblast-like synoviocytes, Ornithine decarboxylase antizyme 3, Proliferation, Invasion
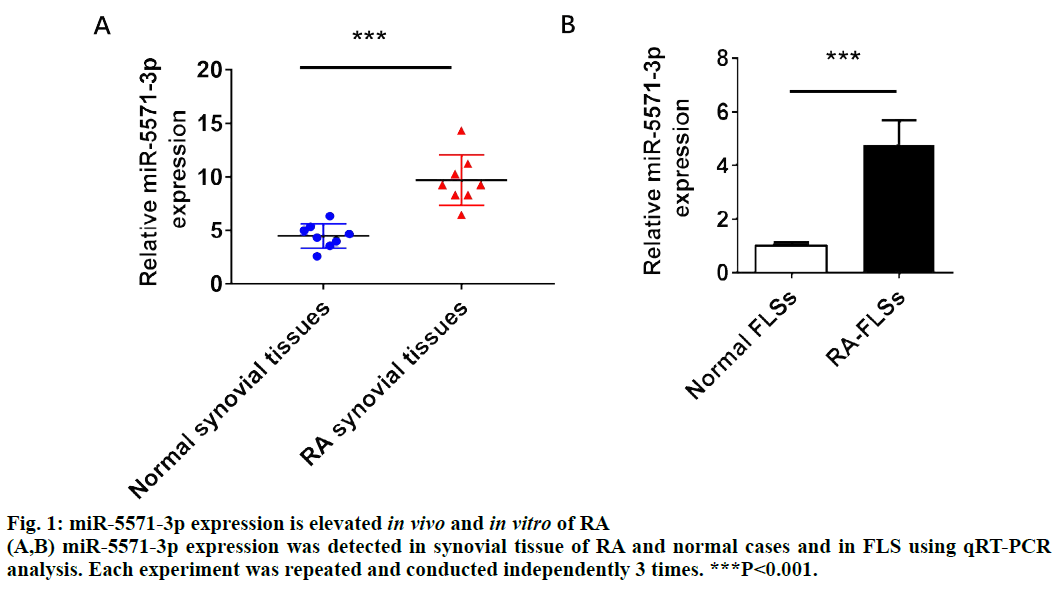
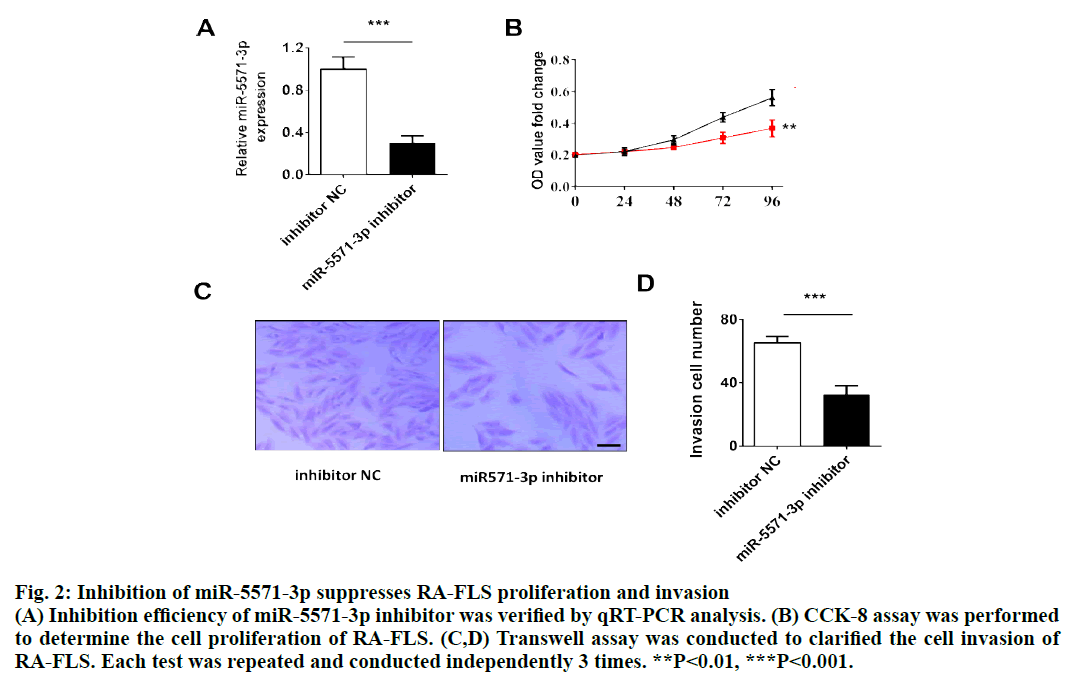
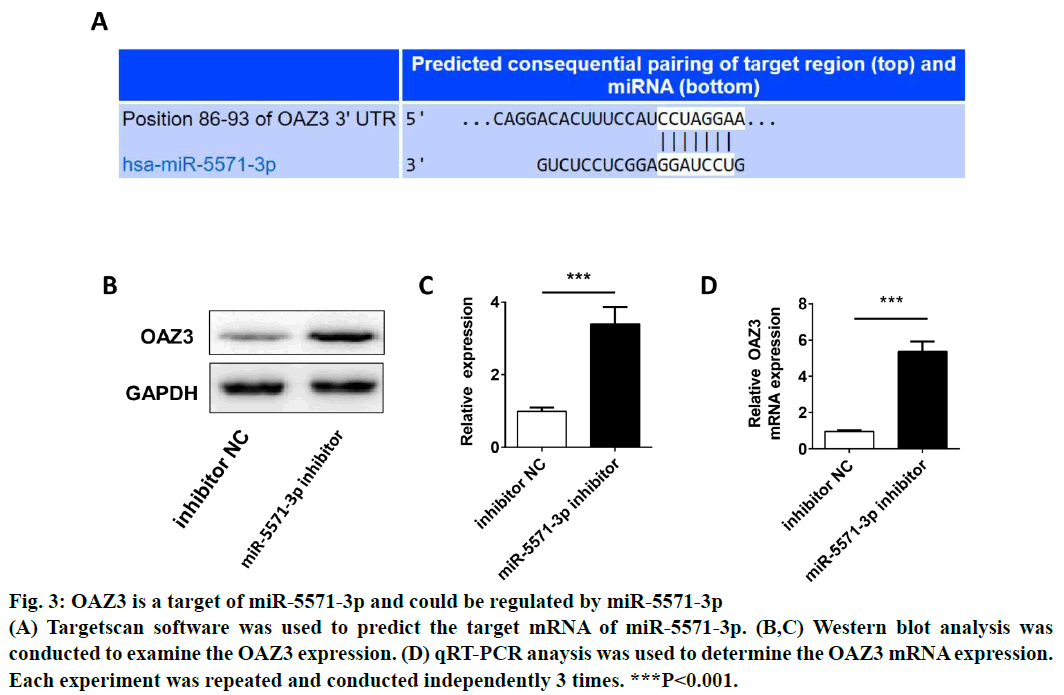
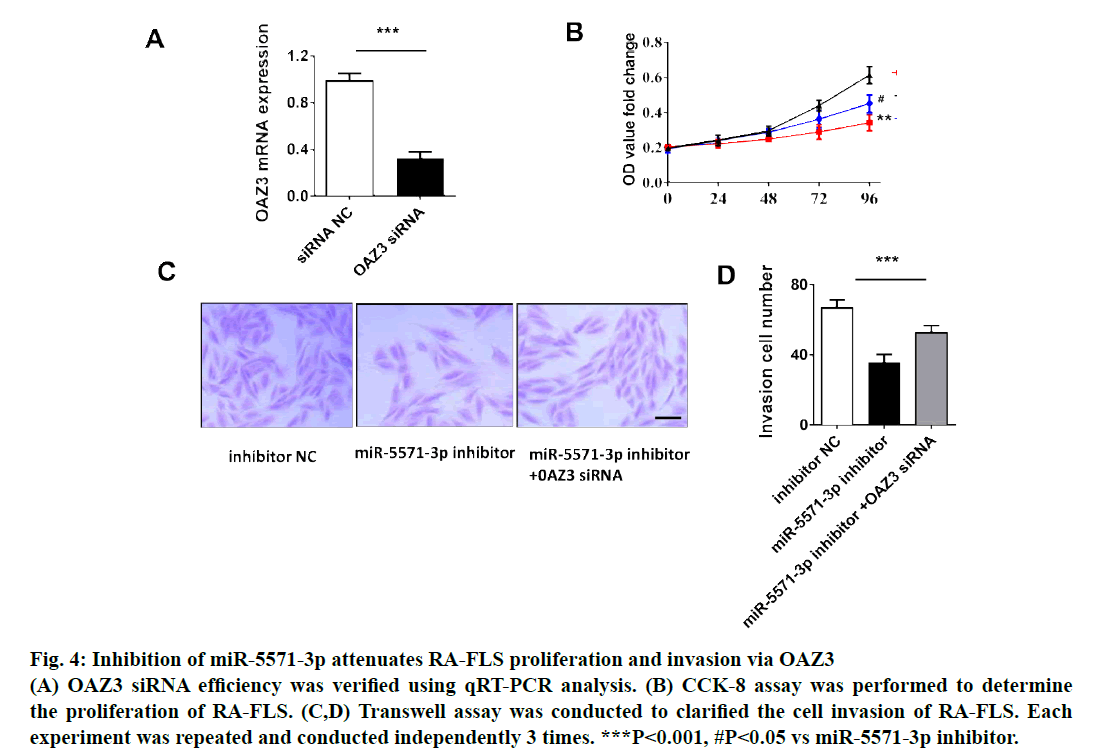
Rheumatoid arthritis (RA), characterized by erosive joint destruction and chronic synovitis, is also one of the most common autoimmune diseases in rheumatology and immunology[1]. Epidemiological studies showed that RA can occur at any age, especially in the young and middle-aged. Among them, 20 to 45-y old patients account for more than 80 % and the ratio of male to female is about 1:3[2,3]. In the past 2 decades, the survey of RA in China showed that the incidence rate of RA has changed little and has remained at 0.3-0.4%[4]. Although RA is not a fatal disease, it still poses a great threat to human life, health and quality of life due to its incurable and high disability rate. Traditionally, T-and B-cell-dependent immune responses are the main pathogenesis of RA. In this process, fibroblast-like synoviocytes (FLS) is only passive victims or bystanders[5,6]. However, numerous studies have revealed that FLS play an extremely important role in the occurrence and development of RA, which can promote the formation of new blood vessels, pannus and cartilage degeneration[6,7]. It is an important pathophysiological process that the proliferation and invasion of synovial cells become more and more serious. Therefore, a systematic study of the pathophysiology of FLS hyperproliferation, reduction of apoptosis and the search for molecular targets that can regulate FLS may lead to a new breakthrough in the mechanism of RA. MicroRNA (miRNA) is a kind of non-coding RNA (ncRNA) which has been studied widely in various diseases[8]. With the regulatory effect of miRNAs on target functional genes, miRNA has rapidly become a hot topic in the field of life science research[9]. In RA, there are also multiple miRNAs that were shown to be dysregulated compared to normal cases[10]. In addition, previous studies have confirmed that miRNAs play a regulatory role in the development of RA. For instance, miR-145-5p affected the biological behavior of RA by regulating Wnt1/β-catenin pathway[11]. However, the role and mechanism of miRNAs in RA need to be further explored. In the present study, it was revealed that the expression of miR-5571-3p was upregulated in RA tissues and RA FLS. The effect of miR-5571-3p on cell proliferation and invasion in RA-FLS was further explored and confirmed that the ornithine decarboxylase antizyme 3 (OAZ3) could mediate the role of miR-5571-3p on cell proliferation and invasion in RA-FLS. Synovium samples of RA cases (n=8) and non-RA patients (n=8) were collected from Taizhou People’s Hospital. All selected RA patients were diagnosed clearly and other immune diseases were excluded. Present study was approved by the ethics committee of the Taizhou People’s Hospital and all selected subjects signed the formal informed consent. The human RA-FLS (MH7A) were obtained from the Shanghai Honsun Biological Technology Co., Ltd. (Shanghai, China). The cells were incubated in RPMI 1640 medium (Gibco, USA) with 10% fetal bovine serum (Gibco, USA) and penicillin/ streptomycin (1:100, Sigma, St. Louis, Mo) at 37° in 5% CO2. When grown at 50% density, the cells were transfected with miR-5571-3p inhibitor or inhibitor NC (Genekey, Shanghai, China) for 36 h and then the cells were collected for corresponding experiments. OAZ3 siRNA was obtained from NanjinhgGenscript Co. Ltd (Nanjing, China). The concentration of OAZ3 siRNA transfected cells was 50 nM. RA-FLS proliferation treated with inhibitor NC or miR-5571-3p inhibitor was determined using a Cell Counting Kit-8 (CCK-8, Abcam, USA) experiments. Not only cell viability, activities of dehydrogenases in cells is also identified by CCK-8. The specific operation refers to the previous study[12]. Briefly, the RA-FLS were cultured on 96-well plates, and 12 μlsolution was added to each well for 24 h, cultured for 2 h, and then detected. Cell invasion of RA-FLS were determined by Transwell assay as previously described[13]. Briefly, RA-FLS (4×105 each well) were seeded in the top compartment with serumfree DMEM, and DMEM with 10 % FBS was added into the bottom compartment for 24 h culture, then fixed with 4 % paraformaldehyde and stained with crystal violet. Finally, randomly select 10 fields of vision from each hole to take photos and count the number of RA-FLS. The proteins of RA-FLS transfected with inhibitor NC or miR-5571-3p inhibitor were extracted. After electrophoresis and transmembrane separation, the proteins was transferred to polyvinylidene fluoride (PVDF, Thermo Fisher, USA) membrane. The PVDF membrane was incubated with primary antibody of OAZ3 and GAPDH (Abcam, CA, USA) at 4° overnight. Membranes were then incubated with second antibody for 2 h and determined using supersensitive Ecl-chemiluminescence (Thermo Fisher, USA). Trizol was used to isolated RNA of RA and normal synovial tissues, and MH7A cells as reported previously[14]. RNA concentration was detected by NanoDrop ND-1000 spectrophotometer (Thermo Scientific, USA) and was reverse transcribed using a PrimeScript RT Kit (Takara, Japan). The detection was normalized using GAPDH and U6 and analyzed by 2−ΔΔCT method. The primers of miR-5571-3p were Forward primer, 3’-TAGGGTTGAAAACC-5’; reverse primer, 3’-CTTCGGCCCGGCACG-5’; U6, forward primer, 3’-GGGCTTACGCCTAGG-5’; reverse primer, 3’-GCCCACATTCACTTG-5’; OAZ3,forward primer, 3’-AGGGTTGCCCTCGT-5’; reverse primer: 3’-TCGTAACCCCTAGG-5’ and GAPDH,forward, 3’-CGAGTTCTGGGCTCACA-5’; reverse, 3’-TCCCGGTTTACGTTACC-5’. The data were presented as means±SD using GraphPad 7.0. Values were analyzed using the Student’s t test between 2 groups, and one-way analysis of variance (ANOVA) was performed for 3 groups. P value less than or equal to 0.05 was considered as statistically significant. To verify the expression of miR-5571-3p in vivo and in vitro of RA, first the expression of miR-5571-3p in 8 RA cases and 8 normal synovium tissues were compared using RT-PCR analysis. The results indicated that the miR-5571-3p level in RA group was markedly upregulated than that in normal synovium tissues (fig. 1A). Similarly, the expression of miR-5571-3p in RA-FLSs was also significantly elevated than that in normal cells (fig. 1B). Inhibition of miR-5571-3p suppressed RA-FLS proliferation and invasion. To confirm the effect of miR-5571-3p in proliferation and invasion of RA-FLS, the expression of miR-5571-3p was knocked down using miR-5571-3p inhibitor in RA-FLS, the inhibition efficiency of miR-5571-3p inhibitor was shown in (fig 2A).Suppression of miR- 5571-3p significantly inhibited proliferation of RAFLS, which was detected by CCK-8 assay (fig 2B).In addition, Transwell assay demonstrated that miR-5571- 3p inhibitor attenuated invasion of RA-FLS (fig 2C and 2D). OAZ3 is a target of miR-5571-3p and could be regulated by miR-5571-3p. To further study the potential mechanism of miR-5571-3p, Targetscan software was used to predict the target gene of miR- 5571-3p, and the results showed that miR-5571-3p has binding sites with OAZ3 (fig 3A). By transfecting miR- 5571-3p inhibitor into RA-FLS, it was found that OAZ3 expression was significantly upregulated at protein and mRNA levels (fig 3B-3D). Inhibition of miR-5571-3p attenuated RA-FLS proliferation and invasion via OAZ3. Given the OAZ3 was a target and regulated by miR-5571-3p, it was next investigated whether OAZ3 siRNA could reverse the inhibitory effect of miR-5571- 3p inhibitor in RA-FLS. First OAZ3 siRNA was constructed to knockdown the OAZ3 expression (fig 4A). Then the miR-5571-3p inhibitor was used to transfect RA-FLS with or without OAZ3 siRNA for 36 h and proliferation and invasion of RA-FLS was detected. The CCK-8 and transwell assay results showed that OAZ3 siRNA partially abolished the inhibitory role of miR-5571-3p in cell proliferation (fig 4B), and invasion (fig 4C,4D). FLS were not only the effector cells of RA joint injury, but also mediate joint damage, which plays an important role in the disease process of RA[15,16]. The morphology and biological behavior of RA related FLS showed tumor like changes, which are in a state of stable activation[17,18]. Numerous abnormal proliferation and invasion of FLS could aggravate the formation of pannus, erode and destroy articular cartilage and bone, and cause adverse effects on the prognosis of the disease[19,20]. There are only 1-3 layers of cells in the inner layer of synovium of normal joint, but the inner layer cells of RA patients can be expanded to 10 layers or more[21]. Although there is inflammatory infiltration in the synovium lining of RA patients, the main cause of synovium hyperplasia is the excessive proliferation of FLS[22]. In this study, it was observed that the expression of miR-5571-3p in RA tissues and FLS increased significantly. By inhibiting the expression of miR-5571-3p in FLS, it was confirmed that miR-5571-3p could significantly inhibit proliferation of FLS. Moreover, the increased ability to of RA cells to invade could further aggravate the destruction of articular cartilage and bone. Similarly, it could be demonstrated that inhibition of miR-5571- 3p could markedly reduce the invasive cells of RA. These clues suggested that miR-5571-3p might be a critical ncRNA with important regulatory functions in the development of RA. MiRNA regulates biological function of diseases via binding and negatively regulating downstream target mRNAs, which is one of the main mechanisms[23]. OAZ3 was predicted to be a potential target binding gene of miR-5571-3p by biology analysis and confirmed that miR-5571-3p could negatively regulate the expression of OAZ3 using qRTPCR and Western blot analysis. In view of the fact that OAZ3 also played a regulatory role in the proliferation of disease[24,25], it is of great significance to explore whether OAZ3 mediates miR-5571-3p in the proliferation and invasion of RA cells. Through rescue experiments, it was confirmed that OAZ3 could reverse the effect of miR-5571-3p on the proliferation and invasion of FLS. In conclusion, the present study indicated that miR-5571-3p attenuated proliferation and invasion of RA-FLS by overexpressing OAZ3. Further studies are necessary to provide a theoretical basis and new vision for RA therapy.
Figure 2: Inhibition of miR-5571-3p suppresses RA-FLS proliferation and invasion
(A) Inhibition efficiency of miR-5571-3p inhibitor was verified by qRT-PCR analysis. (B) CCK-8 assay was performed
to determine the cell proliferation of RA-FLS. (C,D) Transwell assay was conducted to clarified the cell invasion of
RA-FLS. Each test was repeated and conducted independently 3 times. **P<0.01, ***P<0.001.
Figure 3: OAZ3 is a target of miR-5571-3p and could be regulated by miR-5571-3p
(A) Targetscan software was used to predict the target mRNA of miR-5571-3p. (B,C) Western blot analysis was
conducted to examine the OAZ3 expression. (D) qRT-PCR anaysis was used to determine the OAZ3 mRNA expression.
Each experiment was repeated and conducted independently 3 times. ***P<0.001.
Figure 4: Inhibition of miR-5571-3p attenuates RA-FLS proliferation and invasion via OAZ3
(A) OAZ3 siRNA efficiency was verified using qRT-PCR analysis. (B) CCK-8 assay was performed to determine
the proliferation of RA-FLS. (C,D) Transwell assay was conducted to clarified the cell invasion of RA-FLS. Each
experiment was repeated and conducted independently 3 times. ***P<0.001, #P<0.05 vs miR-5571-3p inhibitor.
Acknowledgments
This work was supported by Taizhou People’s Hospital.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Batko B, Stajszczyk M, Swierkot J, Urbanski K, Raciborski F, Jedrzejewski M, et al. Prevalence and clinical characteristics of rheumatoid arthritis in poland: A nationwide study. Arch Med Sci 2019;15:134-40.
- Viecceli D, Garcia MP, Schneider L, Alegretti AP, Silva CK, Ribeiro AL, et al. Correlation between cellular expression of complement regulatory proteins with depletion and repopulation of b-lymphocytes in peripheral blood of patients with rheumatoid arthritis treated with rituximab. Rev Bras Reumatol 2017;57:385-91
- Gomes RKS, Pires FA, Nobre MRC, Marchi MFS, Rickli JCK. Impact of rheumatoid arthritis in the public health system in santa catarina, brazil: A descriptive and temporal trend analysis from 1996 to 2009. Rev Bras Reumatol 2017;57:204-9.
- Huang RY, Pan HD, Wu JQ, Zhou H, Li ZG, Qiu P, et al. Comparison of combination therapy with methotrexate and sinomenine or leflunomide for active rheumatoid arthritis: A randomized controlled clinical trial. Phytomedicine 2019;57:403-10.
- Hammaker D, Nygaard G, Kuhs A, Ai R, Boyle DL, Wang W, et al. Joint location-specific jak-stat signaling in rheumatoid arthritis fibroblast-like synoviocytes. ACR open rheumatology 2019;1:640-8.
- Zhang Q, Liu J, Zhang M, Wei S, Li R, Gao Y, et al. Apoptosis induction of fibroblast-like synoviocytes is an important molecular-mechanism for herbal medicine along with its active components in treating rheumatoid arthritis. Biomolecules 2019;9(12):795.
- Bi X, Guo XH, Mo BY, Wang ML, Luo XQ, Chen YX, et al. Lncrna picsar promotes cell proliferation, migration and invasion of fibroblast-like synoviocytes by sponging mirna-4701-5p in rheumatoid arthritis. EBioMedicine 2019;50:408-20.
- Allmer J, Yousef M. Computational mirnomics. J Integr Bioinform 2016;13:1-2
- Wang Y, Zeng J, Pan J, Geng X, Liu Y, Wu J, et al. Microrna-200c is involved in proliferation of gastric cancer by directly repressing p27(kip1). Biochem Biophys Rep 2016;8:227-33.
- Wang Y, Feng T, Duan S, Shi Y, Li S, Zhang X, et al. Mir-155 promotes fibroblast-like synoviocyte proliferation and inflammatory cytokine secretion in rheumatoid arthritis by targeting foxo3a. Exp Ther Med 2020;19:1288-96.
- Dinesh P, Kalaiselvan S, Sujitha S, Rasool M. Mir-145-5p mitigates dysregulated wnt1/beta-catenin signaling pathway in rheumatoid arthritis. Int Immunopharmacol 2020;82:106328.
- Li B, Zhou C, Yi L, Xu L, Xu M. Effect and molecular mechanism of mtor inhibitor rapamycin on temozolomide-induced autophagic death of u251 glioma cells. Oncol Lett 2018;15:2477-2484
- Yu M, Gong ZP, Ma ZL, Yao ZF, Liu C, Wang J, et al. Crkl promotes proliferation, migration, invasion of laryngeal squamous cell carcinoma. Eur Rev Med Pharmacol Sci 2020;24:5473-80.
- Palit P, Ghosh R, Tolani P, Tarafdar A, Chitikineni A, Bajaj P, et al. Molecular and physiological alterations under elevated co2 concentrations in chickpea. Plant Cell Physiol. 2020;61(8):1449-63.
- Li Y, Wang LM, Xu JZ, Tian K, Gu CX, Li ZF. Gastrodia elata attenuates inflammatory response by inhibiting the nf-kappab pathway in rheumatoid arthritis fibroblast-like synoviocytes. Biomed Pharmacother2017;85:177-81
- Lebre MC, Vieira PL, Tang MW, Aarrass S, Helder B, Newsom-Davis T, et al. Synovial il-21/tnf-producing cd4(+) t cells induce joint destruction in rheumatoid arthritis by inducing matrix metalloproteinase production by fibroblast-like synoviocytes. J leukocyte biol 2017;101:775-83.
- Guo Q, Zhang S, Huang J, Liu K. Alogliptin inhibits il-1beta-induced inflammatory response in fibroblast-like synoviocytes. Int immunopharmacol 2020;83:106372.
- Wei Q, Lv F, Zhang H, Wang X, Geng Q, Zhang X, et al. Microrna-101-3p inhibits fibroblast-like synoviocyte proliferation and inflammation in rheumatoid arthritis by targeting ptgs2. Biosci Rep 2020;40(1):BSR20191136.
- Cai Y, Jiang C, Zhu J, Xu K, Ren X, Xu L, et al. Mir-449a inhibits cell proliferation, migration, and inflammation by regulating high-mobility group box protein 1 and forms a mutual inhibition loop with yin yang 1 in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Res Ther 2019;21:134.
- Wang J, Zhao Q. Betulinic acid inhibits cell proliferation, migration, and inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes. J Cell Biochem 2018.
- Ma JD, Jing J, Wang JW, Yan T, Li QH, Mo YQ, et al. A novel function of artesunate on inhibiting migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Res Ther 2019;21:153.
- Bustamante MF, Oliveira PG, Garcia-Carbonell R, Croft AP, Smith JM, Serrano RL, et al. Hexokinase 2 as a novel selective metabolic target for rheumatoid arthritis. Ann Rheum Dis 2018;77:1636-43.
- Huang Z, Xing S, Liu M, Deng W, Wang Y, Huang Z, et al. Mir-26a-5p enhances cells proliferation, invasion, and apoptosis resistance of fibroblast-like synoviocytes in rheumatoid arthritis by regulating pten/pi3k/akt pathway. Biosci Rep 2019;39(7):BSR20182192.
- Lenis YY, Wang X, Tang W, Wu G, Bazer FW. Effects of agmatine on secretion of interferon tau and catecholamines and expression of genes related to production of polyamines by ovine trophectoderm cells. Amino Acids 2016;48:2389-99.
- Zhao QG, Lu BS, Huang PT. Functions of fancl in primordial germ cell formation and fanconi anemia. Yi Chuan Xue Bao2005;32:993-1000.