- *Corresponding Author:
- H. F. Xu
Department of Traditional Chinese Medicine,
Shaoxing Hospital of Traditional Chinese Medicine,
Shaoxing 312000,
Zhejiang,
China
E-mail: xmy200311@163.com
|
This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “1-11” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Ruangan Xiaoji decoction is a traditional Chinese medicine formula used clinically to treat liver fibrosis. However, due to the complex composition of Ruangan Xiaoji decoction, its potential anti-liver fibrosis and the combination mechanism remain unclear. The present work was to further investigate the active mechanism of Ruangan Xiaoji decoction against liver fibrosis using an integrated strategy of network pharmacology. In this study, network pharmacology analysis was performed to identify the active compounds and target genes, which might be responsible for the effect of Ruangan Xiaoji decoction. Meanwhile, the combined effect of Ruangan Xiaoji decoction on carbon tetrachloride-induced liver fibrosis rats and changes of biochemical and histopathology was detected. The results of the network pharmacology analysis showed 160 compounds and 337 potential target genes related to the treatment of Ruangan Xiaoji decoction with liver fibrosis. Functional enrichment analysis indicated that the potential mechanism was mainly associated with the Hepatitis B and phosphatidylinositol 3-kinase-protein kinase B signaling pathway. In addition, the carbon tetrachlorideinduced liver fibrosis rat was used to study the mechanism of Ruangan Xiaoji decoction against liver fibrosis. Compared with the model group, Ruangan Xiaoji decoction can alleviate the pathological damage and fibrosis of rat liver tissue, significantly reduce rat serum alanine aminotransferase, aspartate aminotransferase levels and significantly affect liver superoxide dismutase and malondialdehyde levels, and can significantly inhibit tumour necrosis factor alpha, interleukin 1 beta and interleukin 11 level. Western blot analysis showed that Ruangan Xiaoji decoction can down-regulate the expression of phosphorylated-p38 mitogen-activated protein kinase, phosphorylated phosphoinositide 3-kinase and phosphorylated protein kinase B protein.
Keywords
Ruangan Xiaoji decoction, liver fibrosis, network pharmacology, carbon tetrachloride-induced liver fibrosis rats
Liver Fibrosis (LF) is not an independent disease, but a histopathological concept. It is a reversible wound repair process that causes chronic liver damage due to various reasons. The disease is characterized by excessive accumulation of collagen and other extracellular matrix components in the liver due to increased synthesis and decreased degradation. There are many causes of LF, such as viral infection, alcoholism, cholestasis, metabolic and genetic diseases, circulatory disorders, drug damage, schistosomiasis and malnutrition. Different types of liver injury have similar changes in the extracellular matrix, which are different from normal livers, which are mainly manifested by the overexpression of fibroblast collagen, glycoproteins, proteoglycans and other substances [1,2]. At the same time, liver injury can stimulate the activation of quiescent hepatic stellate cells, produce excessive proliferation of myofibroblasts, synthesize large amounts of collagen and ultimately lead to liver cirrhosis, which seriously affects the quality of life and life span of patients [3]. In short, LF is a disease with very complex etiology and pathogenesis and a dynamic process. To achieve the true reversal of LF, we still have a long way to go.
At present, the research mechanism for LF has been deep into the cell and molecular studies, but the pathogenesis has not been fully revealed [4-7]. If timely and effective treatment is not given for the cause of the disease, it directly inhibit the synthesis of liver extracellular matrix and promote degradation, it may delay and reverse LF and even early liver cirrhosis [8]. Good anti-LF drugs should be long-term tolerable, safe and effective drugs with low toxic and side effects. Due to the many factors and complex pathogenesis of LF, although modern pharmacological research on anti-LF has been carried out for many years, some progress has been made in some animal models and the research ideas and drug targets are also quite clear [9,10]. In terms of therapeutic application, the research of anti-LF drugs has not yet made major breakthroughs.
Chinese herbal medicines, especially Chinese herbal compound preparations, have been clinically verified for many years. They have good anti-LF effects and low toxic and side effects [11,12]. From the perspective of modern medicine, the pharmacology of anti-LF Chinese medicine is a breakthrough point in the treatment of LF. Most anti-LF are single-target drugs and the effectiveness is low, so anti-LF with multiple targets, high-efficiency and low toxicity still need to be developed [12].
Based on the dialectical theory of LF and the characteristics of qi deficiency and blood stasis, our self-made prescriptions Ruangan Xiaoji Decoction (RGXJD) composed of Sargassum, Jujubae fructus, Ardisiae japonicae, Carapax trionycis, Hedyotis diffusa, Benincasa exocarpium, Laminaria thallus, Areca pericarpium, Salviae miltiorrhiza, Semen coicis, Dendrobium officinale and Curcumae radix has achieved very good curative effects in clinical treatment of liver diseases [13,14]. However, the exact liver protection mechanism of RGXJD in LF was still looking forward to further elucidation. Traditional Chinese Medicine (TCM) is characterized by complex chemical composition and multiple targets. It’s very difficult to understand the mechanism of herbal medicines. Therefore, studying this overall treatment effect will provide a comprehensive understanding of the liver protective effects of RGXJD in the treatment of LF. Network pharmacology is a new discipline that could elucidate the disease pathogenesis and therapeutic mechanisms of TCM. The combined use of these two strategies has been applied in the field of TCM, including drug target discovery, efficacy evaluation and mechanism investigation [15,16].
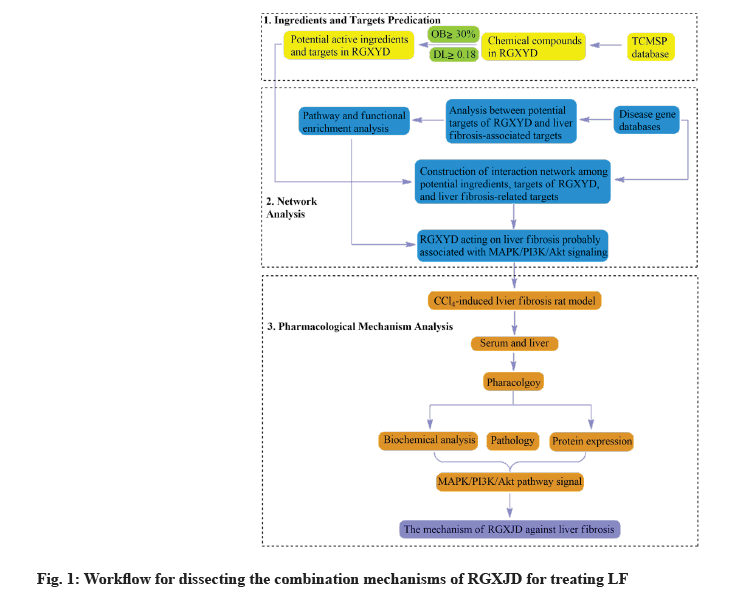
Therefore, the study of this overall therapeutic effect will give a deeper understanding RGXJD in the treatment of LF. This study is based on the Carbon Tetrachloride (CCl4 ) induced rat LF animal model and network pharmacology are effective tool to elucidate the potential combination mechanisms of TCM. A network pharmacology approach was developed to discover the combination mechanisms of RGXJD for anti-LF in terms of targets and pathways. Finally, the key metabolites and enzymes in this pathway were verified by the experiment (fig. 1).
Materials and Methods
Reagents and apparatus:
Commercial kits (Nanjing Jiancheng Institute of Biotechnology, Nanjing, China) were used for determining Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), total Superoxide Dismutase (SOD), Malondialdehyde (MDA), Tumor Necrosis Factor Alpha (TNF-α), leukocyte-mediated detection kit for Interleukin 1 Beta (IL-1β) and Interleukin-11 (IL-11). Olive oil was obtained from Aladdin Industrial Corporation (Shanghai, China). 4 % paraformaldehyde solution, Phosphate-Buffered Saline solution (PBS), heparin sodium, Mitogen- Activated Protein Kinase (MAPK) p38 antibody (Affinity), Phosphoinositide 3-kinase (PI3K) p85α antibody (Affinity), pan-Protein kinase B (AKT) 1/2/3 antibody (Affinity), Anti-Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) antibody (Abcam), antibody diluent, Bicinchoninic acid (BCA) protein quantitative reagent Box (Solarbio), Polyvinylidene Fluoride (PVDF) membrane (GE Healthcare Life).
Preparation of RGXJD:
The 12 herbs of RGXJD were supplied by the Shaoxing Hospital of TCM (Shaoxing, China). Botanical material was authenticated by Prof. Hongfeng Xu, a pharmacist of TCM at the Shaoxing Hospital of TCM. Ultimately, the extraction was concentrated to 1 g/ml using a rotary evaporator (BUCHI, Switzerland) for prospective use.
Network pharmacology analysis:
Composite compounds and target genes of RGXJD acting on LF: We used the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) database [17] and Integrative Pharmacology-Based Traditional Chinese Medicine (TCMIP) platform [18] databases to determine the compound information of RGXJD. In addition, we obtained further information regarding the compounds through a literature review. We used Oral Bioavailability (OB) and drug-likeness (Absorption, Distribution, Metabolism and Excretion (ADME) related properties) as screening criteria. Compounds with OB ≥30 %, Drug-like properties (DL) ≥0.18 and intestinal epithelial permeability (Human Intestinal Epithelial Cell Line (Caco-2)) ≥0.4 were selected for the following analysis.
The related targets of RGXJD were determined by analyzing compound target mappings obtained from the TCMSP. All retrieved targets were converted to their official symbols using the Protein Database (UniProt) Knowledgebase search function [19]. Enter "liver fibrosis" in the Gene Cards Database [20] search box, collect target genes related to LF and remove genes with a relevance score <1. The mined LF-related genes and RGXJD target genes were mapped to screen out common targets and RGXJD anti-LF targets were obtained.
Enrichment analysis and network construction: Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were further performed to probe the biological function and potential mechanisms of the detected targets. Meanwhile, the acquired target genes were submitted to the Search Tool for the Search Tool for the Retrieval of Interacting Genes/ Proteins (STRING) database [21]. Furthermore, a herb- compounds–target network of RGXJD for treating LF was constructed by utilizing the network visualization software Cytoscape [22].
Experimental design:
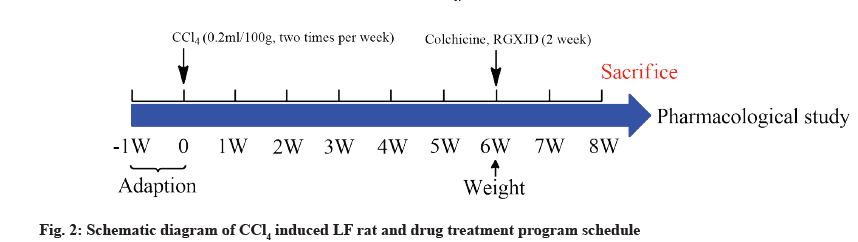
The study was approved by the Experimental Animal Center of Zhejiang Academy of TCM (Hangzhou, China; Certification number SYXK-Zhe 2014-0003). 60 Male Sprague-Dawley (SD) rats (200±20 g), were obtained from the Experimental Animal Center of Zhejiang University (Hangzhou, China). All rats were housed in a standard environment (25±2°, 50±5 % humidity, 12/12 h dark/light cycle) and free access to standard rat chow and water. After 1 w of adaptation, the rats were randomly divided into control, model, colchicine and RGXJD groups. Except control group which received olive oil, the other groups received CCl4 and olive oil which were mixed at the rate of 1:1 (v/v) (0.1 ml/100 g body weight) twice a week for 6 w to induce LF. All groups were administered once a day after 6 w modeling. The control and model group were administered with Intragastric (i.g.) distilled water, the RGXJD treated groups were given i.g. dose of 5 g/kg, 10 g/kg, 15 g/kg and the colchicine group with i.g. dose of 0.1 mg/kg (fig. 2).
At 24 h after the last drug administration, rats were sacrificed by cervical dislocation and the blood was collected from the abdominal aorta and separated by centrifugation at 4° (Eppendorf Centrifuge 5430R, Hamburg, Germany), 13 000 rpm for 10 min to obtain serum and stored at -80° for biochemical analyses. The liver was rapidly dissected to weigh and cut into small pieces. Then, a part of the liver tissue was fixed in 10 % formalin for histological investigation and the remaining tissue was stored at -80° for further analysis.
Liver weight and histopathological analysis:
Rat liver tissue samples were collected, weighed, sectioned into small pieces, fixed in 4 % paraformaldehyde, embedded in paraffin, serial sections, 5 μm thick, stained with Hematoxylin and Eosin (H&E) and Sirius red, respectively, and observed the pathological changes of liver tissue under an optical microscope.
Biochemistry analysis:
Serum ALT and AST were analyzed to evaluate the liver functions using commercial kits. The contents of SOD and MDA in liver homogenates were determined to evaluate the antioxidant capacity. The serum TNF-α, IL-1β and IL-11 contents were determined by Enzyme- Linked Immunosorbent Assay (ELISA).
Western blot assay:
Liver tissue homogenates were sonicated for complete dissolution and centrifuged at 12 000 revolutions per minute for 30 min at 4° to separate the membrane- containing fraction (pellet) from the cytosolic fraction. Proteins (100 μg) were separated by 10 % Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE). The separated proteins were blotted onto a Nitrocellulose (NC) membrane (NC membrane, Millipore, Massachusetts, USA) and the membrane was washed for 10 min with Tris-Buffered Saline (TBST) with 0.1 % Tween 20 and then immersed in a blocking buffer containing 5 % nonfat dry milk in TBST for 1 h at room temperature. Primary antibody incubation: Take the membrane out of the blocking solution, add 6 ml of TBST to the labeled incubator and wash 3 times on a shaker, 10 min each time. Remove the membrane in the order of labeling and add the corresponding diluted primary antibody. After labeling, incubate at 4°overnight. Secondary antibody incubation: Take out the membrane, add 6 ml of TBST to the labeled incubator, wash 3 times on a shaker, 10 min each time, add the secondary antibody diluted in the corresponding proportion and incubate for 1 h at room temperature on a shaker development.
Statistical analysis:
GraphPad Prism 8.0 (GraphPad Software Inc., California, USA) and Statistical Package for the Social Sciences (SPSS) 21.0 for Windows (SPSS, Chicago, USA) were used for statistical analysis. The statistical indicators were expressed as mean±standard deviation (x̅ ±s). Statistical differences between groups were evaluated by one-way Analysis of Variance (ANOVA) and rank-sum test; p value of p<0.05 was considered statistically significant.
Results and Discussion
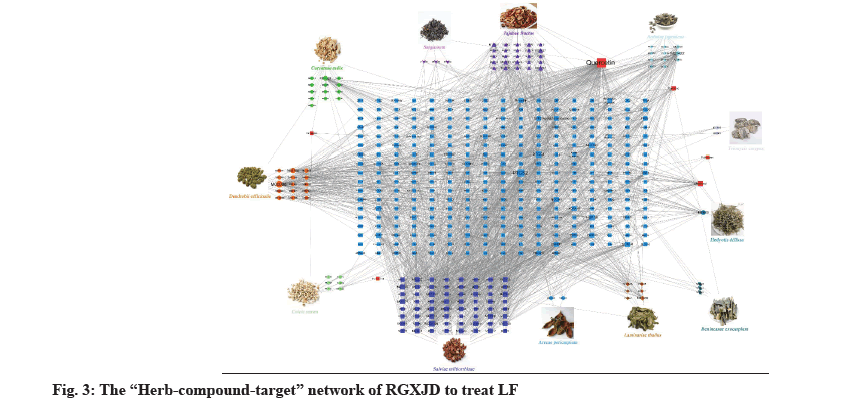
In order to better study its action mechanism of RGXJD against LF, we further use the network pharmacology technology to predict its potential targets. A total of 160 active compounds were found from the TCMSP and TCMIP databases according to the two criteria: DL ≥0.18 and OB ≥30 % and the similarity search in the SwissProt databases found an additional 337 potential targets. To further explore the combined mechanisms of RGXJD for LF, combining the targets related to active compounds of RGXJD with the targets of LF, the 160 compounds and total 337 potential targets of the herbs in RGXJD were used to construct the compound-target network. Most of these active compounds were related to multiple targets, resulting in 1892 compounds-target associations. The results showed that RGXJD handles multi-compounds and multi-target characteristics for treating LF. The topological analysis has suggested that the targets Prostaglandin-Endoperoxide Synthase 2 (PTGS2), Prostaglandin-Endoperoxide Synthase 1 (PTGS1), Nuclear Receptor Coactivator 2 (NCOA2), Heat Shock Protein 90 (HSP90), Protein Kinase CAMP- Activated Catalytic Subunit Alpha (PRKACA), Sodium Voltage-Gated Channel Alpha Subunit 5 (SCN5A), Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Gamma (PIK3CG), Cysteine- Aspartic Proteases (Caspase-3), REL-associated protein (RELA) and Adrenoceptor Beta 2 (ADRB2) and the compounds quercetin, apigenin, sitosterol, kaempferol, luteolin, isorhamnetin, tanshinone IIA, isocryptotanshinone, danshexinkum D, salviolone and naringenin are important in network, indicating that they may have synergistically exerted the therapeutic effect of RGXJD.
Interestingly, 17 of the 30 active ingredients were found to belong to 57 representative compounds in fig. 3, whereas only 13 of the 221 isolated nodes performed well in the docking analysis, which indicated that the highly connected nodes in the compound similarity network had greater opportunity to be biologically active compounds with similar structures might represent the driving force within the RGXJD, since they could work synergistically to achieve the identified therapeutic effects.
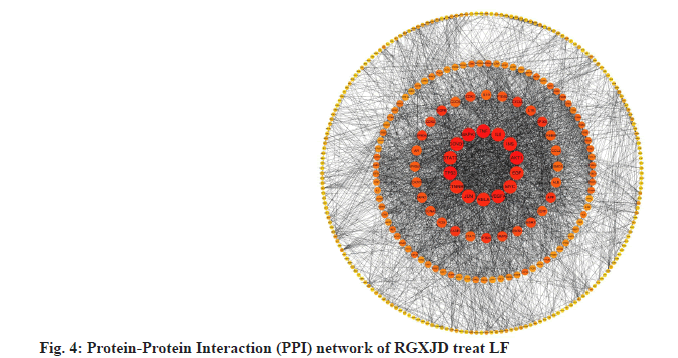
Study on the mechanism of RGXJD on LF is described below. String database was used to analyze known and predicted protein interaction databases. Targets from the network pharmacology analysis were input into String database, where human species was defined and 0.7 high confidences were selected. The nodes in the graph represent targets, while the edges represent the association between proteins. The results showed that there were 346 nodes and 3322 lines were involved in the association among these targets. Topological analysis has indicated that Tumor protein p53 (TP53) (36), AKT1 (35), TNF (33), MAPK1 (32) and Cyclin D1 (CCND1) (31) have a large degree and located at the central position, indicating that these genes interact most closely; they may regulate multiple metabolic pathways and thus play a comprehensive role in the development of LF and RGXJD treatment (fig. 4).
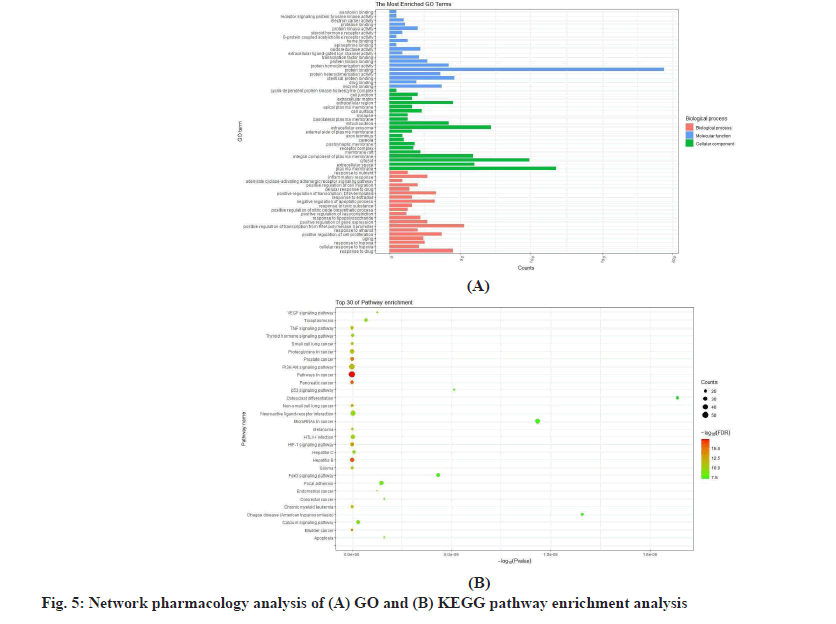
The results of the network pharmacology analysis are mentioned below. To further study the molecular mechanism of RGXJD against LF, the shared targets were utilized for GO and KEGG enrichment analysis. GO analysis was described by the 151 Biological Process (BP), 50 Cell Component (CC) and 78 Molecular Function (MF) were recognized as p<0.01.
The GO analysis was reviewed for the top 29 remarkably enriched terms in BP, CC and MF (fig. 5). The KEGG enrichment analysis showed that 113 shared genes contributed to 114 pathways (p<0.01). Among them, pathways in cancer, hepatitis B, pancreatic cancer, prostate cancer, bladder cancer, Hypoxia-Inducible Factor 1 (HIF-1) signaling pathway, Non-small cell lung cancer, chronic myeloid leukemia, Glioma and Phosphatidylinositol 3 Kinase-Protein Kinase B (PI3K- AKT) signaling pathway were closely correlated with the pathogenesis of LF.
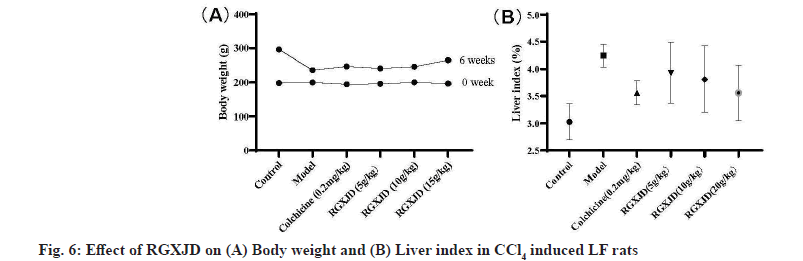
RGXJD alleviated CCl4 -induced body weight and liver histopathological changes. To evaluate the effects of RGXJD treatment on CCl4 administration in rats, body weight and liver index (liver weight/body weight×100 %) of rats were monitored. The results of the statistical analysis shown in fig. 6 demonstrated that the body weight significantly decreased, while the liver weight and liver index significantly increased in the model group compared with those in the normal group. However, RGXJD treatment significantly attenuated this decrease in body weight and increase in liver weight and liver index compared with those in the model group.
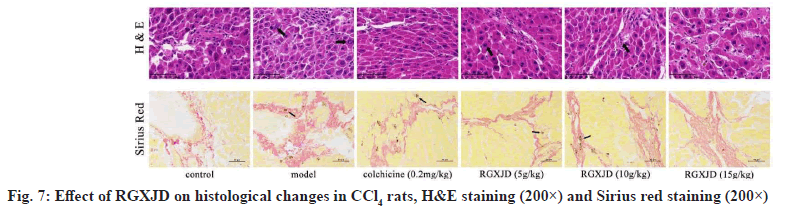
To further evaluate the potential of RGXJD to reduce LF, histological changes in the rat liver were detected by pathological examination. The liver sections were stained with H&E and Sirius Red for histological evaluation of LF. The results showed that the hepatocytes in rats receiving CCl4 exhibited increased swelling, degeneration and lymphocyte infiltration in the liver. Treatment with RGXJD and colchicine clearly improved CCl4 -induced pathological changes (fig. 7), the Sirius red staining label collagen and fibers in histologic preparations. In the model group, obvious depositions of collagen fibers were found in the central vein and portal area of liver tissue and the shape and structure of liver lobules were changed with the formation of pseudo-lobules. In RGXJD groups, only a small number of collagen fiber depositions were observed (fig. 7). These results suggest that CCl4 induces liver injury in rats and that RGXJD can improve liver injury induced by CCl4 in rats.
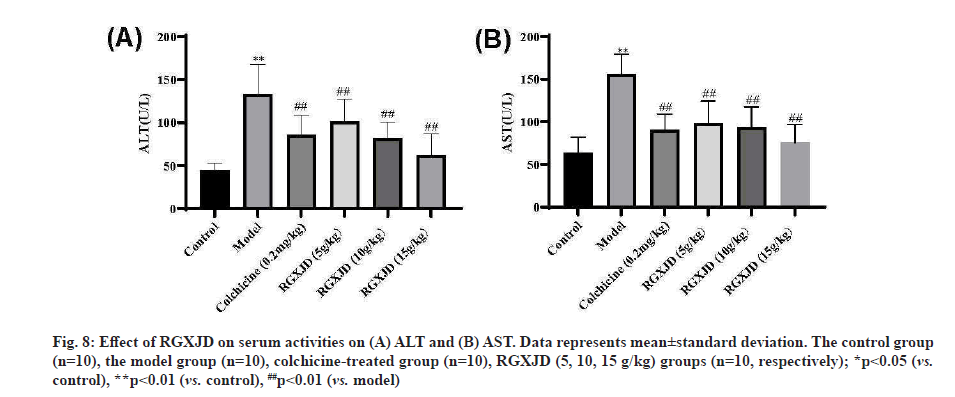
RGXJD improved serum biomarkers of liver function in rats. When liver cells are damaged, ALT and AST in the cytoplasm will be released into the blood and their contents in serum will be significantly increased. After CCl4 injection twice a week for 6 w, the rats experienced serious liver damage, as indicated by increased serum biochemical indices, including ALT and AST (fig. 8). Compared with the control group, the CCl4 model group had significantly increased serum ALT, AST levels (p<0.01) (fig. 8). Furthermore, the levels of these markers were significantly decreased in RGXJD treatment group compared with the model group; this finding indicated a restored hepatic function after the treatment with RGXJD.
Fig. 8: Effect of RGXJD on serum activities on (A) ALT and (B) AST. Data represents mean±standard deviation. The control group (n=10), the model group (n=10), colchicine-treated group (n=10), RGXJD (5, 10, 15 g/kg) groups (n=10, respectively); *p<0.05 (vs. control), **p<0.01 (vs. control), ##p<0.01 (vs. model)
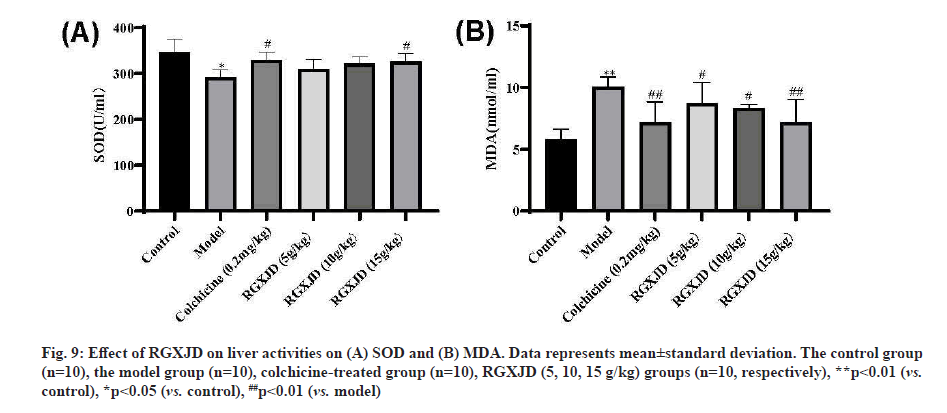
RGXJD increases antioxidant capacity in CCl4 -induced LF rat liver. To evaluate the antagonistic effects of RGXJD on oxidative stress, the activity of antioxidant enzymes (SOD) and the level of MDA (hallmarks of oxidative damage) were detected, as shown in fig. 9. Compared with model mice, SOD level decreased and MDA content significantly increased in liver homogenate of model mice after 6 w of CCl4 treatment, indicating that free radical scavenging ability decreased and lipid peroxidation was serious after CCl4 stimulation. Compared to the model group, RGXJD administration made the antioxidant enzymes more active and the accumulation of MDA was significantly lower in the high-dose group than in the low-dose group. These results may explain the antioxidant capacity of RGXJD in LF.
Fig. 9: Effect of RGXJD on liver activities on (A) SOD and (B) MDA. Data represents mean±standard deviation. The control group (n=10), the model group (n=10), colchicine-treated group (n=10), RGXJD (5, 10, 15 g/kg) groups (n=10, respectively), **p<0.01 (vs. control), *p<0.05 (vs. control), ##p<0.01 (vs. model)
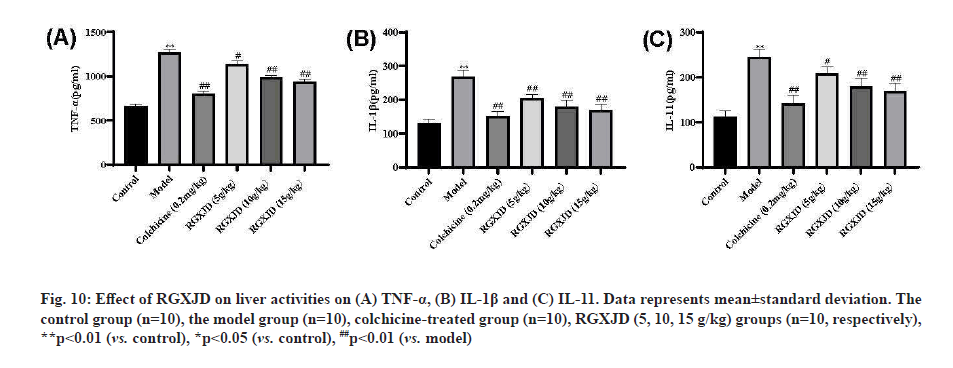
RGXJD improves inflammatory response in CCl4 - induced LF liver. IL-1β, IL-11 and TNF-α are important factors that mediate inflammatory responses. We measured the expressions of them in the liver of each group by ELISA. The promotion inflammation cytokine (TNF-α) of the model group were increased significantly compared with those of the control group (p< 0.01) and the repression inflammation cytokine (namely IL-1β, IL-11) was lower than that of the negative control group (p<0.01) after CCl4 treatment. However, compared with the model group, the treatment of different concentrations of RGXJD could callback prominently of IL-1β, TNF-α and IL-11 and the high dose of RGXJD showed the best anti-inflammatory effect. These findings provide direct evidence for the attenuation of RGXJD on CCl4 -induced inflammation and it had the similar effect as the colchicine group (fig. 10).
Fig. 10: Effect of RGXJD on liver activities on (A) TNF-a, (B) IL-1ß and (C) IL-11. Data represents mean±standard deviation. The control group (n=10), the model group (n=10), colchicine-treated group (n=10), RGXJD (5, 10, 15 g/kg) groups (n=10, respectively), **p<0.01 (vs. control), *p<0.05 (vs. control), ##p<0.01 (vs. model)
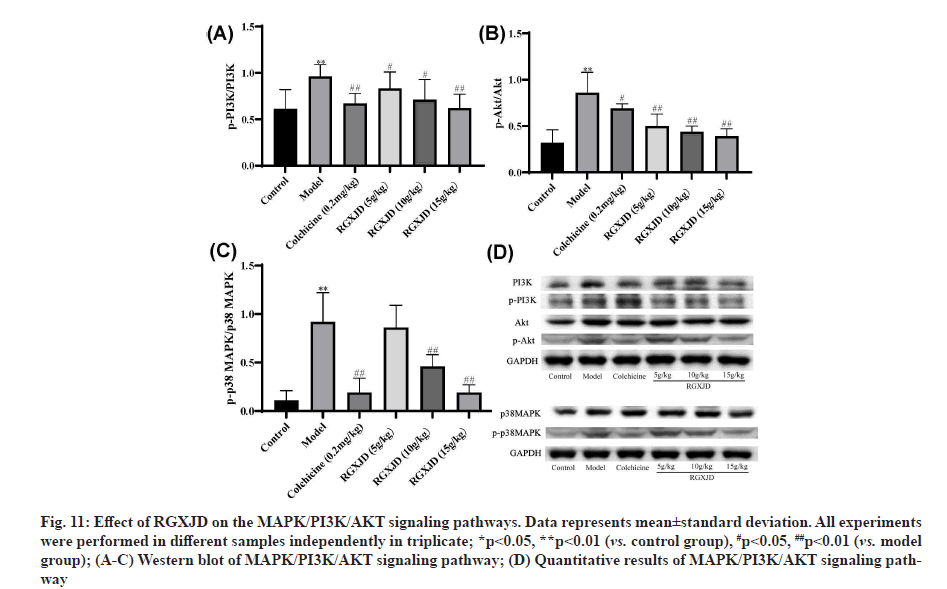
RGXJD protects against CCl4 -induced LF by targeting MAPK/PI3K/AKT pathway. Since MAPK/PI3K/ AKT pathway plays an important part in the progress of LF, we analyzed the protein expression levels of MAPK/PI3K/AKT in experimental rat liver tissue. To verify these results further, the protein levels were investigated through western blotting analyses with antibodies against the phosphorylated and total protein of p38 MAPK. Western blot analysis indicated that phosphorylated-PI3K (p-PI3K) and phosphorylated AKT (p-AKT) expression levels were significantly increased in the CCl4 -induced LF group compared with the control group. In contrast, as shown in fig. 11, the relative phosphorylation level of p38 MAPK was significantly downregulated after treatment with RGXJD as compared with model group.
Fig. 11: Effect of RGXJD on the MAPK/PI3K/AKT signaling pathways. Data represents mean±standard deviation. All experiments were performed in different samples independently in triplicate; *p<0.05, **p<0.01 (vs. control group), #p<0.05, ##p<0.01 (vs. model group); (A-C) Western blot of MAPK/PI3K/AKT signaling pathway; (D) Quantitative results of MAPK/PI3K/AKT signaling pathway
As a common disease, LF is considered as an outcome of chronic liver injury for a long period, with complex formation processes and mechanisms, many influencing factors and great individual differences. In view of the pathogenesis of LF, inhibition of Hematopoietic Stem Cell (HSC) proliferation, anti-inflammation and protection of hepatocytes are key to the treatment of LF [23]. However, there is lack of ideal drugs as the antifibrotic agents for curing hepatic fibrosis at present. Therefore, it is an urgent task to discover some effective drugs for preventing or treating LF and investigate the mechanism of action against the disease as well.
RGXJD is an herbal formula that is widely used in clinical practice involving TCM for the treatment of LF. In light of the complexity of the active ingredients in RGXJD and the diversity of potential regulatory targets in the human body, we analyzed the main components of RGXJD by network pharmacology. The holistic and systematic nature of network pharmacology coincides with the holistic view of TCM and syndrome differentiation. It can use many existing databases and pathway and network analyses to explore the molecular mechanism of TCM for the treatment of diseases [24]. Then, we constructed the RGXJD-compound-LF target network and the potential anti-LF mechanism of RGXJD was predicted. Network pharmacological analysis of RGXJD identified 12 herbs, 160 active compounds, 337 common target genes and 114 significant signal pathways regulated by target genes. For the 30 active ingredients in RGXJD may contribute to the enhancement of macrophage phagocytosis according to the analysis of network pharmacology, including ginsenosides, phytosterols, sesquiterpenes, flavonoids and alkaloids. And some compounds were reported to show the effect of anti-LF [25-27].
As observed in the results of KEGG pathway enrichment analysis, the key targets of RGXJD acting on LF were mainly related to PI3K-AKT signaling pathway, MAPK signaling pathway, neurotrophin signaling pathway, TNF signaling pathway and so on, suggesting that RGXJD has multicomponent and multitarget characteristics for the treatment of LF. The PI3K-AKT pathway participates in cell survival and death, particularly exhibiting the beneficial effects on cell survival and inhibitory effect on cell apoptosis once AKT is activated [28]. In addition, PI3K-AKT pathway is involved in the initiation of the autophagic process, which is a major intracellular machinery for degrading misfolded proteins and damaged organelles and has been reported to be involved in the pathogenesis of LF through acting on its downstream Mammalian Target of Rapamycin Complex 1 (mTOR).
MAPKs are well known in regulating HSC activation and collagen synthesis, which are the key pathological processes of LF. For example, suppression of p38 MAPK activation could effectively inhibit the collagen production of activated HSCs and Extracellular Regulated Kinase (ERK) inhibition could inhibit HSCs proliferation [29]. MAPKs are also well known in regulating oxidative stress, which is the key inducing factor of LF [30]. AKT1 is a member of the serine- threonine kinase class that plays a key role in cellular processes, including growth, proliferation, survival and angiogenesis. By auto phosphorylation, AKT1 regulates key signaling pathways such as the Janus Kinase-Signal Transducer and Activator of Transcription (JAK- STAT), apoptosis and MAPK signaling pathways, regulates apoptosis and related proteins thus affecting the process of LF [31]. Therefore, targeting MAPK/ PI3K/AKT1 signaling pathway-related proteins was a promising strategy for LF treatment.
In our study, a LF model was induced by CCl4 , which generated free radicals and promoted inflammation. Based on this classic model, our present study indicated that RGXJD could alleviate LF due to anti- inflammatory and antioxidant activities. Colchicine was frequently used in a positive control in the LF animal models. In this study, the colchicine-treatment group significantly improved the ALT level but did not show the significant effect on other fibrosis features, which that were reported before. An appropriate dose and treatment duration of colchicine would be verified to obtain consistent anti-fibrosis efficiency in further studies.
CCl4 is a toxic xenobiotics that causes hepatocyte necrosis and liver damage and is often used as a model toxin for inducting liver injury and evaluating the protective effect of drugs [32]. Under the catalysis of Cytochrome P450 2E1 (CYP2E1) in hepatocytes, CCl4 is stripped of a chlorine to form the trichloromethyl radical. This radical spontaneously loses another chlorine to form the highly oxidative metabolite carbene, which can cause hepatocyte degeneration and necrosis by inducing oxidative stress [33]. The resulting liver injury can progress to LF and sclerosis. This phenomenon indicates that CCl4 is a powerful hepatotoxic agent and may be used for the characterization of the hepatic disease treatment medicines. Elevation of liver enzymatic markers (ALT, AST) and increased lipid peroxidation are further evidence of hepatotoxicity. ALT and AST that exist in the hepatocytes can certainly leak into the peripheral blood as soon as the hepatocytes are injured [34]. Thus, serum enzymes such as ALT, AST are useful markers for detecting liver damage because activities of these enzymes are markedly elevated when hepatocytes are damaged. The liver maintains redox homeostasis in vivo by controlling the production and scavenging of Reactive Oxygen Species (ROS). Excess ROS are cleared by enzymes such as SOD. However, when the body is exposed to toxic prooxidant xenobiotics, the ROS level increases and the antioxidant system may be inadequate. ROS interact with lipids to form MDA, which forms covalent adducts with phospholipids, DNA and proteins, ultimately causing hepatocyte death [35]. The histopathological analysis showed that CCl4 caused obvious hepatic steatosis, necrosis, infiltration of inflammatory cells, massive collagen proliferation, pseudolobular formation and widely distributed fibers. Our results indicate that intragastric administration of RGXJD once per day consecutively for 5 w can significantly alleviate the symptoms in the hepatopathy rats including improving hepatic steatosis, alleviating lobular inflammatory cells infiltration and decreasing massive collagen proliferation.
Altogether, this study demonstrated that RGXJD had a protective effect on LF by exerting inhibitory activity against oxidative stress and inflammation in rats. Moreover, our findings provided scientific evidence to support its antioxidative and anti-inflammatory effects. The expression of phosphorylated-p38 MAPK, p-AKT and p-PI3K was detected and suggested MAPK/PI3K/ AKT pathways are the key signaling pathways of RGXJD effect on LF.
Author’s contributions:
Wen-Rui Zhu and Tian-Wen Zhao wrote the paper and performed the experiments. Wen-Rui Zhu revised the manuscript. Hong-Feng Xu conceived and designed the experiments.
Acknowledgements:
This work was supported by the grants of the Science and Technology Project of Shaoxing (No. 2018C30120).
Conflict of interests:
All authors have no conflict of interests.
References
- Pinzani M, Rombouts K, Colagrande S. Fibrosis in chronic liver diseases: Diagnosis and management. J Hepatol 2005;42(1):S22-36.
[Crossref] [Google Scholar] [PubMed]
- Yu L. Pathogenesis of liver injury and hepatic failure. In: Artificial Liver. Singapore: Springer; 2021. p. 105-66.
- Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med 2007;131(11):1728-34.
[Crossref] [Google Scholar] [PubMed]
- Cheng JH, She H, Han YP, Wang J, Xiong S, Asahina K, et al. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol 2008;294(1):G39-49.
[Crossref] [Google Scholar] [PubMed]
- Dobie R, Wilson-Kanamori JR, Henderson BE, Smith JR, Matchett KP, Portman JR, et al. Single-cell transcriptomics uncovers zonation of function in the mesenchyme during liver fibrosis. Cell Rep 2019;29(7):1832-47.
[Crossref] [Google Scholar] [PubMed]
- Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther 2019;10(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Mi XJ, Hou JG, Jiang S, Liu Z, Tang S, Liu XX, et al. Maltol mitigates thioacetamide-induced liver fibrosis through TGF-β1-mediated activation of PI3K/Akt signaling pathway. J Agric Food Chem 2019;67(5):1392-401.
[Crossref] [Google Scholar] [PubMed]
- Tsuchiya A, Takeuchi S, Watanabe T, Yoshida T, Nojiri S, Ogawa M, et al. Mesenchymal stem cell therapies for liver cirrhosis: MSCs as “conducting cells” for improvement of liver fibrosis and regeneration. Inflamm Regen 2019;39(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: Mechanistic concepts and therapeutic perspectives. Cells 2020;9(4):875.
[Crossref] [Google Scholar] [PubMed]
- Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol 2018;68:435-51.
[Crossref] [Google Scholar] [PubMed]
- Li H. Advances in anti-hepatic fibrotic therapy with Traditional Chinese Medicine herbal formula. J Ethnopharmacol 2020;251:112442.
- Zhang L, Schuppan D. Traditional Chinese Medicine (TCM) for fibrotic liver disease: hope and hype. J Hepatol 2014;61(1):166-8.
[Crossref] [Google Scholar] [PubMed]
- Zheng, CL. Examples of treating liver diseases with Ruangan Xiaoji Yin. J Zhejiang Coll Tradit Chin Med 1993, 17, (4), 18-18.
- He, WM. To explore the clinical curative effect of Ruangan Xiaoji Decoction in liver cirrhosis. China Pract Med 2014;(26):180-1.
- Shao LI, Zhang B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin J Nat Med 2013;11(2):110-20.
- Zhang R, Zhu X, Bai H, Ning K. Network pharmacology databases for traditional Chinese medicine: Review and assessment. Front Pharmacol 2019;10:123.
- Ru J, Li P, Wang J, Zhou W, Li B, Huang C, et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 2014;6(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Xu HY, Zhang YQ, Qing YW, Zhao HY, Wang P, Liu F. Exploration on scientific connotation of TCM syndromes and recommended prescriptions against COVID-19 based on TCMTP V2. 0. China J Chin Mat Med 2020;45(7):1488-98.
[Crossref] [Google Scholar] [PubMed]
- UniProt Consortium. Reorganizing the protein space at the Universal Protein Resource (UniProt). Nucleic Acids Res 2012;40(D1):D71-5.
[Crossref] [Google Scholar] [PubMed]
- Safran M, Dalah I, Alexander J, Rosen N, Iny Stein T, Shmoish M, et al. GeneCards Version 3: the human gene integrator. Database 2010;2010.
[Crossref] [Google Scholar] [PubMed]
- Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 2021;49(D1):D605-12.
[Crossref] [Google Scholar] [PubMed]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res 2003;13(11):2498-504.
[Crossref] [Google Scholar] [PubMed]
- Lin YL, Lu CF, Wu YH, Yang KT, Yang WY, Chen JW, et al. Protective effects of crude chalaza hydrolysates against liver fibrogenesis via antioxidation, anti-inflammation/anti-fibrogenesis, and apoptosis promotion of damaged hepatocytes. Poult Sci 2021:101175.
[Crossref] [Google Scholar] [PubMed]
- Hao DC, Xiao PG. Network Pharmacology: A rosetta stone for traditional Chinese medicine. Drug Dev Res 2014;75(5):299-312.
[Crossref] [Google Scholar] [PubMed]
- Li X, Jin Q, Yao Q, Xu B, Li Z, Tu C. Quercetin attenuates the activation of hepatic stellate cells and liver fibrosis in mice through modulation of HMGB1-TLR2/4-NF-κB signaling pathways. Toxicol Lett 2016;261:1-2.
[Crossref] [Google Scholar] [PubMed]
- Ji J, Yu Q, Dai W, Wu L, Feng J, Zheng Y, et al. Apigenin alleviates liver fibrosis by inhibiting hepatic stellate cell activation and autophagy via TGF-β1/Smad3 and p38/PPARα pathways. PPAR Res 2021;2021.
- Xu T, Huang S, Huang Q, Ming Z, Wang M, Li R, et al. Kaempferol attenuates liver fibrosis by inhibiting activin receptor-like kinase 5. J Cell Mol Med 2019;23(9):6403-10.
[Crossref] [Google Scholar] [PubMed]
- Xie D, Gore C, Zhou J, Pong RC, Zhang H, Yu L, et al. DAB2IP coordinates both PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc Natl Acad Sci 2009;106(47):19878-83.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Hu H, Yin JQ. Therapeutic strategies against TGF‐β signaling pathway in hepatic fibrosis. Liver Int 2006;26(1):8-22.
[Crossref] [Google Scholar] [PubMed]
- Li X, Wang X, Han C, Xing G, Zhou L, Li G, et al. Astragaloside IV suppresses collagen production of activated hepatic stellate cells via oxidative stress-mediated p38 MAPK pathway. Free Radic Biol Med 2013;60:168-76.
[Crossref] [Google Scholar] [PubMed]
- Himpe E, Kooijman R. Insulin‐like growth factor‐I receptor signal transduction and the Janus Kinase/Signal Transducer and Activator of Transcription (JAK‐STAT) pathway. Biofactors 2009;35(1):76-81.
[Crossref] [Google Scholar] [PubMed]
- Josan S, Billingsley K, Orduna J, Park JM, Luong R, Yu L, et al. Assessing inflammatory liver injury in an acute CCl4 model using dynamic 3D metabolic imaging of hyperpolarized [1‐13C] pyruvate. NMR Biomed 2015;28(12):1671-7.
[Crossref] [Google Scholar] [PubMed]
- Zou Y, Xiong JB, Ma K, Wang AZ, Qian KJ. Rac2 deficiency attenuates CCl4-induced liver injury through suppressing inflammation and oxidative stress. Biomed Pharmacother 2017;94:140-9.
[Crossref] [Google Scholar] [PubMed]
- Czaja MJ. Cell signaling in oxidative stress-induced liver injury. Semin Liver Dis 2007;27(4):378-89.
[Crossref] [Google Scholar] [PubMed]
- Singh R, Czaja MJ. Regulation of hepatocyte apoptosis by oxidative stress. J Gastroenterol Hepatol 2007;22:S45-8.
[Crossref] [Google Scholar] [PubMed]