- *Corresponding Author:
- J. Puralae Channabsavaiah
Department of Pharmacognosy, P. A. College of Pharmacy, Kairangala, Mangalore, Karnataka 574153, India
E-mail: jagadish.pc@manipal.edu
| Date of Received | 07 December 2020 |
| Date of Revision | 24 August 2021 |
| Date of Acceptance | 11 June 2022 |
| Indian J Pharm Sci 2022;84(3):730-739 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of the study was to develop and validate a simple, robust and accurate method for detection and simultaneous estimation of lupeol and beta-sitosterol as dual bioactive markers in ethanol and n-hexane extracts of Cissus quadrangularis Linn. and its marketed formulation using reverse phase highperformance liquid chromatography for standardization by integrated quality by design approach. A reverse phase high-performance liquid chromatography method with photodiode array detection was developed for simultaneous estimation and standardization of two major phytosterols compounds, betasitosterol and lupeol, in marketed formulation and extracts of Cissus quadrangularis Linn. by integrated quality by design approach. The design of experiments was generated by Box-Behnken three level 3 factorial design to optimize the pH of the mobile phase, percentage of the buffer and organic modifier for better separation between lupeol and beta-sitosterol. The optimized chromatographic separation was performed on a Merck Hibar LiChrospher® reverse phase C18 (250 mm×4.6 mm, 5 μm) column as a stationary phase with isocratic elution program using a mixture of mobile phase A: Methanol and acetonitrile in the ratio of 35:15 v/v and mobile phase B: 20 mM ammonium acetate 40 % (pH 4.5) with the flow rate of 1.0 ml/ min at 210 nm. The experimental results of the predicted method by the design of experiment were similar to the suggested responses and all fall in acceptance criteria with the desirability of 0.961. The developed method was validated as per International Conference on Harmonisation guidelines Q2(R1). The betasitosterol and lupeol showed good regression (R2>0.9995) within test ranges and the percent recovery was found to be 1.77 % w/w and 3.007 % w/w in marketed formulation. The recovery in ethanol extract was found to be 0.926 % and 3.63 %, and 0.065 % and 0.19 %, respectively, in n-hexane extract. The method was found to be highly specific without the interference of impurities. Thus, the method could be extended for the marker-based standardization and routine quality control of Cissus quadrangularis Linn. extracts and their formulation.
Keywords
Box-Behnken experimental design, Cissus quadrangularis Linn., quality by design, reverse phase high performance liquid chromatography, validation parameters
Cissus quadrangularis Linn. (C. quadrangularis L.) is a well-known bone healing plant known in Sanskrit as Astisamdhani, which means bone healer. It belongs to the Vitaceae family of food plants[1]. It is also used in gout, syphilis, venereal illness, piles, leucorrhea and as an aphrodisiac in Ayurveda for its therapeutic applications as a general tonic and painkiller, with particular bone fracture mending qualities[2]. Numerous researches have been published establishing that a variety of phytochemicals present in the aerial portions of the plant, stem bark, fruits and roots of C. quadrangularis L. notably sterols and triterpenoids, are commonly considered as important physiologically active components[3,4].
Beta (β)-sitosterol, one of the most important steroidal substances, has been isolated and described as having estrogenic characteristics[5]. β-sitosterol is one of the most abundant sterols, estimated at 5.31 % in plants and has shown profound biological effects in the reduction of carcinogen-induced colon cancer and inflammation as well[6]. Lupeol, a pentacyclic lupane-type triterpene, is another significant phytoconstituent found in a variety of flowering plant groups at around 2.3 %[7]. Lupeol has been proven in experimental animals to have potent biological effects on inflammation, rheumatism and cancer[6]. Inflammation and its triggers, such as certain kinases, play a key role in the pathophysiology of osteoporosis. Anti-osteoporosis activity has been demonstrated for agents that inhibit such kinases. Both of these chemicals are found in C. quadrangularis L. and they have been identified as bioactive markers in the plant[7].
Medicinal plants continue to play an essential role in supplying lead compounds for further pharmaceutical research as well as alternate sources of effective treatment, despite improvements in new drug development[8]. Because of the complex nature of herbs, it is critical to monitor quality control and standardize active components responsible for the product’s pharmacological activity because product quality and standardization have a direct influence on therapeutic efficacy[9,10]. Given the wide range of therapeutic advantages, β-sitosterol and lupeol have shown promise in the clinical treatment and management of a variety of diseases. Therefore, quantification of the major phytosterols of C. quadrangularis L., such as β-sitosterol and lupeol as shown in fig. 1, is not only important but also the appropriate way to standardize the plant extracts[11]. Thus, there is a need to develop a satisfactory method for controlling the quality of C. quadrangularis L. active constituent’s β-sitosterol and lupeol.
A review of the literature indicates that a number of analytical techniques utilizing High-Performance Thin Layer Chromatography (HPTLC) and High- Performance Liquid Chromatography (HPLC) have been described for the quantification of phytosterols, β-sitosterol, lupeol, stigmasterol and oleanolic acid from various sources and matrices. Shailajan et al. reported on the simultaneous measurement of lupeol, stigmasterol and β-sitosterol in the fruit and root of the Carissa carandas species using HPLC[12]. Previously reported HPTLC methods are not sensitive in terms of quantification of β-sitosterol and lupeol[13]. Shah et al. estimated stigmasterol and β-sitosterol from C. quadrangularis crude powder, but HPLC detection wavelength is very low, which leads to a decrease in specificity due to interference[14].
Chromatographic technology has been accepted and authorized by the World Health Organization (WHO) as a technique for identifying and standardizing herbal medicines. This approach entails the creation of techniques by optimizing one element at a time[15]. As of now, no papers utilizing intergraded experimental (Design of Experiments (DOE)) methodology to investigate lupeol and β-sitosterol as dual bioactive indicators have been published. Analytical Quality by Design (AQbD) is a systematic development methodology that was recently used in the development of analytical methods for pharmaceutical bulk medicines[16]. AQbD plays an important role in developing a robust method as an early risk assessment and helps to identify the critical analytical parameters and to focus on these factors in method development for a robust method[17]. Due to the complexity of herbs, quality control and standardization of the active ingredients responsible for the product’s pharmacological activity should be closely monitored since quality management and product standardization have a direct effect on treatment effectiveness[18]. Thus, the contemporary QbD technique is utilized to standardize herbal medications in order to assure product quality and follow the drug’s full life cycle.
The current article takes a novel and contemporary approach to the development and validation of an RPHPLC system for the standardization of β-sitosterol and lupeol from C. quadrangularis L. and marketed formulations, while adhering to established Food and Drug Administration (FDA) specifications and quality control criteria for drugs.
Materials and Methods
Plant material collection and authentication:
The entire plant of C. quadrangularis L. was collected from areas of Pavagada, Karnataka in November 2017. Dr. G. K. Bhat, Professor of Botany, Poorna Prajna College, Udupi, Karnataka, confirmed the botanical identity of the crude drug and a voucher specimen of the drug (No. 514) has been deposited in the Department of Pharmacognosy, Manipal College of Pharmaceutical Sciences, Manipal.
Chemicals and reagents:
The reference standards of β-sitosterol (99.99 %) and lupeol (99.99 %) were procured from Sigma-Aldrich. HPLC solvents like acetonitrile and methanol were purchased from Finar Ltd. (Ahmedabad, Gujarat, India), and other chemicals used were of analytical grade. Bonton capsule is manufactured and marketed by Vasu Healthcare Pvt. Ltd., Gujarat, India.
QbD approach for method development and optimization:
DOE: A literature survey revealed that lupeol and β-sitosterol are aqueous soluble compounds and more polar, so for mobile phase preparation, methanol may be used as the solvent. Lupeol is a strong acidic molecule having a hexamethyl group (pKa=~18.49), whereas β-sitosterol is a phenanthrene-7-ol based acidic molecule (pKa=18.2). Therefore, the QbD approach was used to set a few mandatory features for the determination of the Analytical Target Profile (ATP). The interaction effects of Critical Method Attributes (CMA) of the method of Critical Quality Attributes (CQA) were evaluated by DOE and statistical analysis of data was performed by Design-Expert® software (version 12.0.1) using the Box-Behnken Design (BBD). The independent variables selected were the mobile phase pH (X1), aqueous percentage (%) in the mobile phase (X2), acetonitrile ratio in the mobile phase (X3) and Retention Time (RT) (R1, R2), were considered as covariates or dependent variables. A 3-factor, 3-level BBD constructed 17 experimental runs. The significance of the design was determined by the evaluation of statistical parameters by the Analysis of Variance (ANOVA) method. A 10 μg/ml concentration of both analytes was selected for the experiments. The responses obtained for experiments were assessed for the RT of both analytes (Y) as the response variable. The obtained data were fitted to an appropriate mathematical model by using Design- Expert® software. The chromatographic conditions were optimized by numerical and graphical modes within the robust design space.
Optimized chromatographic conditions: The chromatographic method was developed using the Shimadzu prominence HPLC system with LC solution software and a configuration of LC-20AD quaternary pumps along with the DGU-20A5 degasser unit, SPD-M 10A photo diode array detector, SIL 20AC HT autosampler and CTO-10AS column oven. The chromatographic separation was achieved using Merck Hibar LiChrospher® Reverse Phase (RP) C18 (250 mm×4.6 mm, 5 μm) column as a stationary phase with a mobile phase comprising of a mixture of mobile phase A: Methanol and acetonitrile in the ratio of 35:15 v/v and mobile phase B: 20 mM ammonium acetate 40 % (pH 4.5) with 1.0 ml/min flow rate at 210 nm. The column was maintained at a temperature of 25°. The injection volume was 40 μl with a run time of 30 min.
Solution preparations:
Preparation of standard solutions: Accurately weighed and transferred 1 mg standards of markers β-sitosterol and lupeol to 10 ml volumetric flasks. Standards were dissolved in methanol and sonicated for 10 min. The final volume was made up with the methanol to get stock solutions containing 0.1 mg/ml (100 μg/ml) of β-sitosterol and lupeol.
Preparation of ethanolic extracts of C. quadrangularis L.: The weighed quantity (3 kg) of the whole powdered plant of C. quadrangularis L. was exhaustively extracted with 95 % ethanol using the Soxhlet apparatus at an elevated temperature (60° for 16 h). A rotary evaporator was used to concentrate the ethanolic extract under reduced pressure at 40°. 10 mg of the dried ethanol extract was transferred to a 10 ml volumetric flask and dissolved in methanol and the volume was made up to the mark to obtain sample stock solution. Aliquot 500 μl from the sample stock solution was transferred to a 1 ml Eppendorf tube and made up to mark with the mobile phase.
Preparation of hexane extracts of C. quadrangularis L.: The weighed quantity (3 kg) of the whole powdered plant of C. quadrangularis L. was exhaustively extracted with hexane using the Soxhlet apparatus at an elevated temperature of 50° for 16 h. A rotary evaporator was used to concentrate hexane extract under reduced pressure at 40°. 10 mg of the hexane extract was transferred to a 10 ml volumetric flask and dissolved in methanol and the volume was made up with a mobile phase to obtain a sample stock solution. Aliquot 500 μl of the sample stock solution was transferred to a 1 ml Eppendorf tube and made up to mark with the mobile phase.
Preparation of formulation for extraction: 20 capsules of Bonton were weighed and the contents were removed carefully from the capsule. An equivalent quantity of 15.493 g drug powder was weighed accurately and dissolved in a 100 ml volumetric flask in methanol. The mixture was sonicated for 15 min at 40° and the final volume was made up of methanol. The solution was then filtered using Whatman filter paper 0.45 μ. The above stock solution was injected into the HPLC system and analyzed.
Method validation:
The developed analytical method was validated concerning International Conference of Harmonization (ICH) Q2(R1) guidelines over the parameters of specificity, linearity, Limit of Detection (LOD), Limit of Quantification (LOQ), accuracy, precision and robustness[19].
Results and Discussion
A factorial design using BBD was applied for observing the effect of three independent variables, mobile phase pH (X1), aqueous percent (%) in the mobile phase (X2), acetonitrile ratio in the mobile phase (X3), on two responses-RT of β-sitosterol (R1) and RT of lupeol as parameters for optimization of the proposed method. The chromatographic conditions and ranges fixed for selected variables are given in Table 1. A total of 17 runs were obtained for the fixed variables to test the predictive validity of the model. Each combination of independent factors suggested by BBD was finally run on the system and observed for the responses such as RT for both lupeol and β-sitosterol. Details of RT and resolution between two markers for combination of independent factors were given in supplementary material. Among the various models, the quadratic model was suggested by design with the maximum least square regression coefficients for all two responses R1 and R2 as compared to other models. High values of R2 and significant differences (p<0.05) were observed during the assessment of the model. Among the numerous trials, run no. 04 demonstrated the greatest resolution between lupeol and β-sitosterol and also followed the design-suggested paths.
| Factor | Name | Units | Type | Minimum | Maximum | Coded low | Coded high |
|---|---|---|---|---|---|---|---|
| A | pH | 1 | Numeric | 3 | 6 | -1↔3.00 | +1↔6.00 |
| B | % aqueous content | % | Numeric | 40 | 60 | -1↔40.00 | +1↔60.00 |
| C | % ACN | % | Numeric | 15 | 30 | -1↔15.00 | +1↔30.00 |
Table 1: Selection of Independent Variables and Their Levels
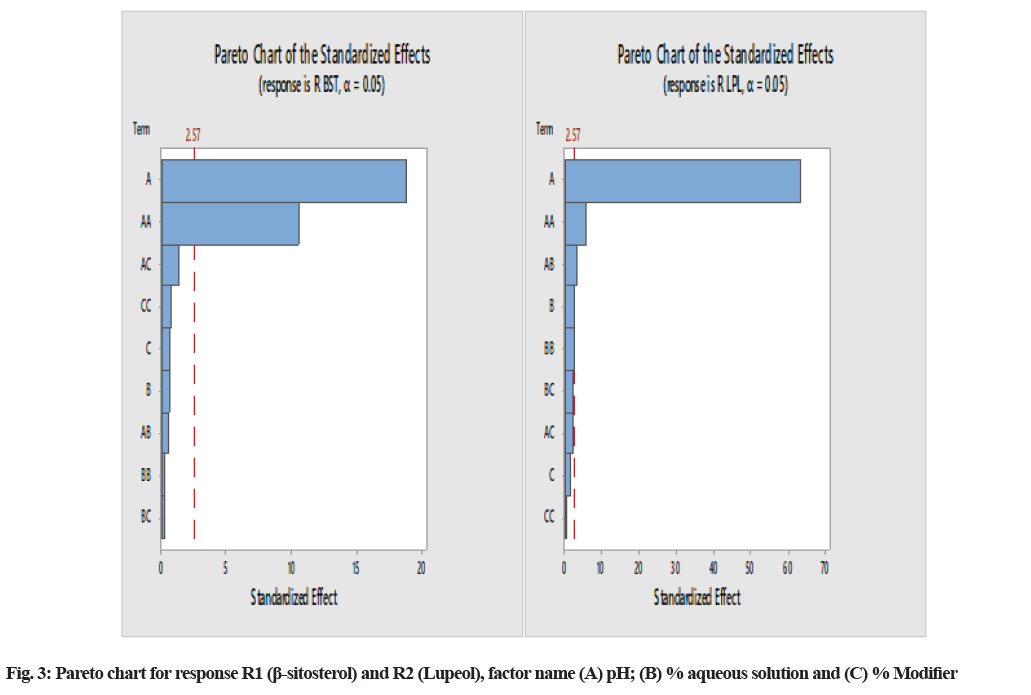
The normal plot of standardized effect and Pareto chart were analyzed to identify the riskiest CMA of the model. Fig. 2 presents that factor A: pH has a more significant influence on the retention of β-sitosterol and both factor pH and aqueous % of the mobile phase are the influential factors on the RT of lupeol. Specifically we have also reviewed the Pareto chart (fig. 3), which graphically presents the terms on the y-axis and the relative standard effects for the response on the x-axis. In this chart, the most influential term appears with the longest length on the top of horizontal bars and the vertical straight red line represents the limit of the predefined confidence interval.
The model was validated by the application of ANOVA, which showed that the model was significant. The results of ANOVA for responses R1 and R2 showed that the model F-value of 73.21 and 5.38, respectively, implies the models are significant. The p-values for the model terms showed that both the variables R1 and R2 are significant (p<0.05) (Table 2) in all cases. Factor B and C, p-values indicated that these factors are not influential factors for retention of lupeol and β-sitosterol (p>0.05), but factor A, i.e., pH of mobile phase, has a significant effect on response. From Table 2, the predicted R-squared for all responses R1 (0.8361) and R2 (0.7873) are in reasonable agreement with the adjusted R-squared values of 0.976 and 0.7112, respectively, i.e., the difference was less than 0.2 in each case. The ratio of 22.34 and 6.90 indicate an adequate signal (ratio>4.0). The desirability of the model was found to be 0.961 (Table 3).
| Response | R1 (RT of β-sitosterol) | R2 (RT of lupeol) |
|---|---|---|
| Standard deviation | 1.34 | 5.19 |
| Mean | 23.25 | 15.15 |
| % Coefficient of Variation (CV) | 5.74 | 34.27 |
| p-value | <0.0001 | 0.0187 |
| F-value | 73.21 | 5.38 |
| R2 | 0.9895 | 0.8736 |
| Adjusted R2 | 0.976 | 0.7112 |
| Predicted R2 | 0.8361 | 0.7873 |
| Adequate precision | 22.3482 | 6.9057 |
Table 2: Anova Results for Response
| Factor | Predicted level | Actual level | Desirability |
|---|---|---|---|
| pH | 4.66 | 4.5 | 0.961 |
| % aqueous | 40 | 40 | |
| % ACN | 15 | 15 | |
| R1 | 26.042 | 26.042 | |
| R2 | 15.6245 | 16.936 |
Table 3: The Optimized Method According to DOE
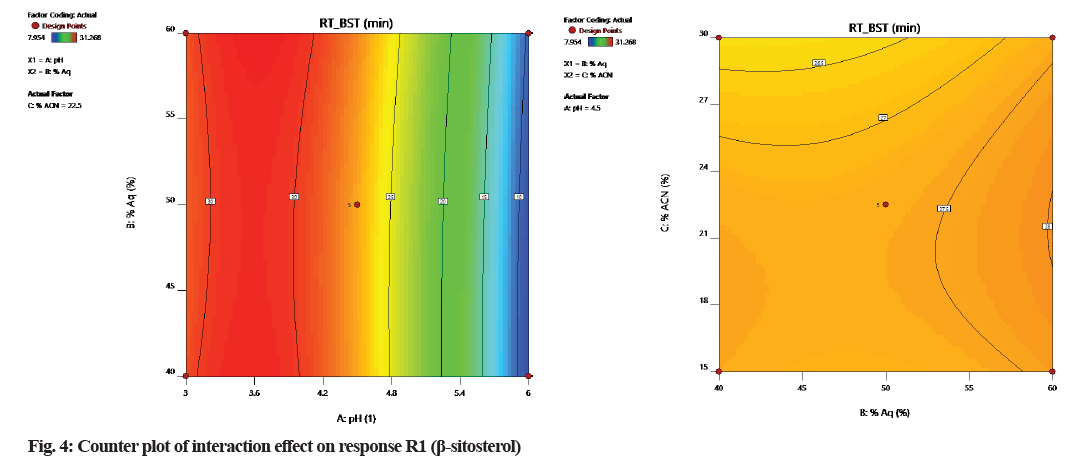
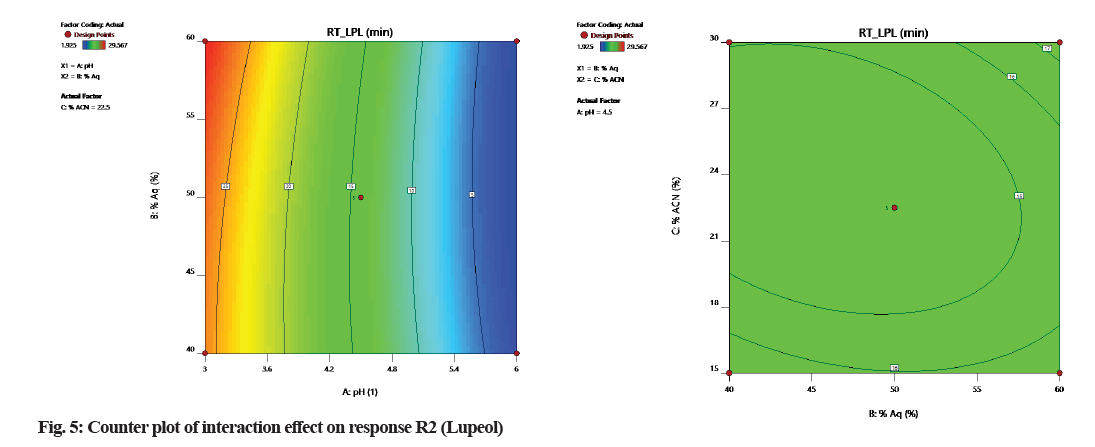
According to counterplots of interactions (fig. 4 and fig. 5), it was observed that variable A (pH of the mobile phase) is having a positive interaction with response to R1 and R2. Factor B and C has a negative effect on response R1 and R2 because it shows that the relationship between factors and response is not linear.
The optimized experimental conditions were obtained based on statistical method verification. The experimental results of the predicted method were similar to the suggested responses and all fall in acceptance criteria as shown in Table 4. The optimized chromatographic separation was achieved using Merck Hibar LiChrospher® RP C18 (250 mm×4.6 mm, 5 μm) column as a stationary phase with a mobile phase comprising of a mixture of mobile phase A: Methanol and acetonitrile in the ratio of 35:15 v/v and mobile phase B: 20 mM ammonium acetate 40 % (pH 4.5) with 1.0 ml/min flow rate at 210 nm.
| Recovery level | Amount added (µg) | Average % recovery | |||||
|---|---|---|---|---|---|---|---|
| Lupeol | β-sitosterol | ||||||
| Hexane extract | Ethanol extract | Formulation | Hexane extract | Ethanol extract | Formulation | ||
| 80 % | 24 | 101.17 | 101.37 | 101.36 | 101.17 | 101.37 | 101.36 |
| 100 % | 30 | 102.02 | 101.81 | 101.87 | 102.02 | 101.81 | 101.87 |
| 120 % | 36 | 100.95 | 101.66 | 101.87 | 100.95 | 101.66 | 101.87 |
Table 4: Accuracy for Lupeol and β-Sitosterol
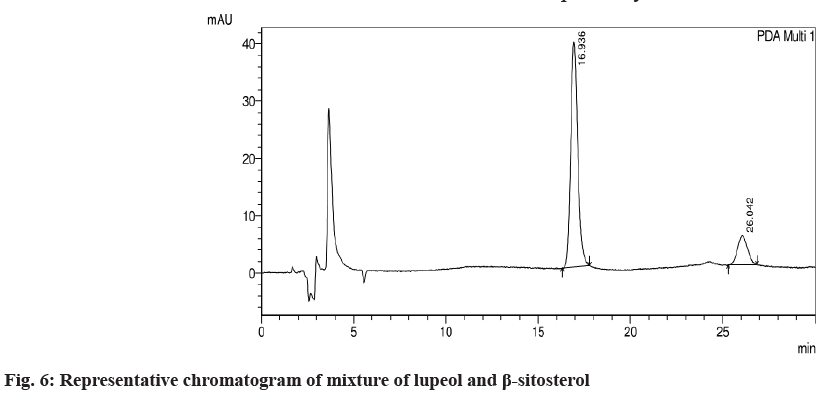
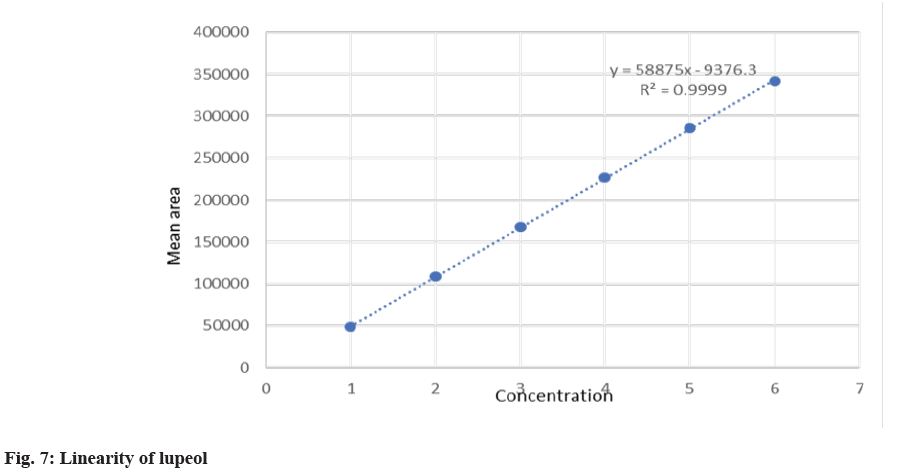
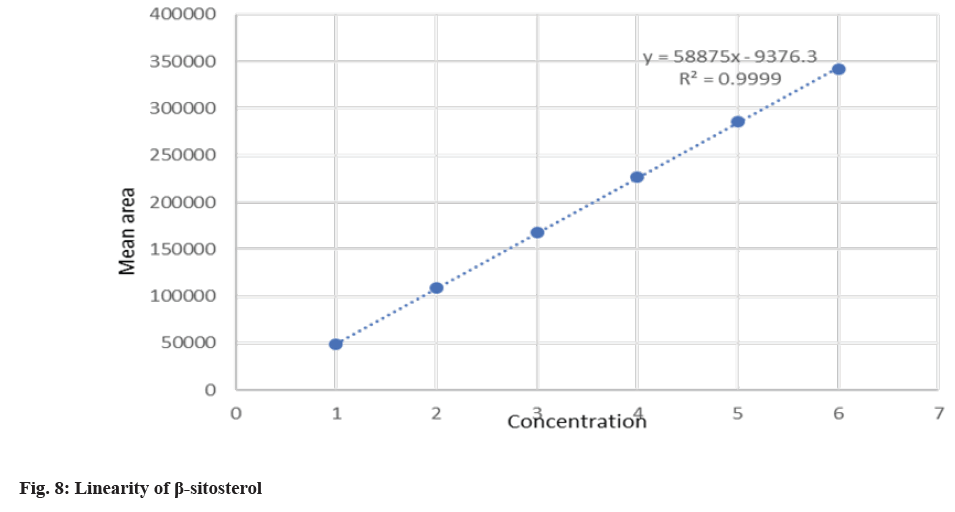
The system suitability was performed to check the reproducibility of the chromatographic method. The percentage Relative Standard Deviation (% RSD) was found to be 0.77 % and 0.25 for β-sitosterol and lupeol respectively. The present method was found to be highly specific as there was no interference at the RT of lupeol and β-sitosterol as shown in fig. 6. The peak purity was found to be greater than the peak purity threshold, indicating that lupeol and β-sitosterol was pure and there was no interference of impurities or degradants. The linearity for β-sitosterol and lupeol was established, and the concentration versus peak area calibration curve was plotted as shown in fig. 7 and fig. 8. The method was found to be linear for β-sitosterol and lupeol over the concentration range of 10 μg/ml- 60 μg/ml. The correlation coefficient (r2) was found to be 0.99947 and 0.9999 for lupeol and β-sitosterol, respectively. The LOD for lupeol and β-sitosterol was found to be 0.617 μg/ml and 0.581μg/ml, respectively, and the LOQ for lupeol and β-sitosterol was found to be 1.871 μg/ml and 1.781 μg/ml. The mean % recovery for lupeol and β-sitosterol was found to be within the limit of 98 %-102 %. The recovery data for lupeol and β-sitosterol extracts and formulations are shown in Table 4. The precision of the given method was determined by intermediate precision and repeatability. The reporting of results was done in terms of % RSD as shown in Table 5. The method was found to be robust on the change of mobile phase, wavelength, column temperature, flow rate. The RSD was found to be less than 2 %.
| Determinations | d 1 | d 2 | ||
|---|---|---|---|---|
| Lupeol | b-sitosterol | Lupeol | b-sitosterol | |
| 1 | 58.5993 | 5.8907 | 58.3574 | 5.8897 |
| 2 | 58.3799 | 5.8596 | 58.2839 | 5.8963 |
| 3 | 58.6350 | 5.8797 | 58.6688 | 5.8763 |
| 4 | 58.4083 | 5.8567 | 58.4164 | 5.9076 |
| 5 | 58.3793 | 5.8906 | 58.435 | 5.8928 |
| 6 | 58.5993 | 5.8476 | 58.600 | 5.8468 |
| Mean | 58.5002 | 5.8708 | 58.4605 | 5.8849 |
| Standard | 0.1227 | 0.0186 | 0.1465 | 0.0212 |
| % RSD | 0.2098 | 0.3169 | 0.2507 | 0.3610 |
Table 5: Intermediate Precision for Lupeol and β-Sitosterol
A single chromatogram of lupeol and β-sitosterol at RT 16.936 and 26.052 min was observed in the chromatogram of drug samples extracted. The % recovery for lupeol and β-sitosterol in the marketed formulation was found to be 1.77 % w/w and 3.007 % w/w, respectively. The % recovery for lupeol and β-sitosterol in ethanol extract was found to be 0.926 % and 3.63 %, respectively and 0.065 % and 0.19 % respectively in n-hexane extract, as shown in Table 6.
| Extracts | Formulation | Hexane extract | Ethanol extract |
|---|---|---|---|
| Lupeol | 1.77 % w/w | 0.065 % w/w | 0.926 % w/w |
| b-sitosterol | 3.007 % w/w | 0.19 % w/w | 3.63 % w/w |
Table 6: Assay of Lupeol and β-Sitosterol
C. quadrangularis L. is an Ayurvedic herb and employed as raw material for the herbal formulation (primarily churns, tablets and Bhasma) manufacture in various areas of India for therapy. The qualitative variety of herbal raw materials is comparatively substantial compared with chemical remedies. Medicinal plants from various areas exhibit enormous variations and even herbs picked in the same place but at a different time may have active chemicals of significantly varying amounts. The usage of QbD paradigms has been penetrating the industrial, academic and government sectors at an alarming rate with the goal to obtain a comprehensive process and product knowledge. As a result, randomized order integrated QbD experiments for optimization was carried out to reduce the impact of uncontrolled factors that might bias the response. The pH of the mobile phase and the percent of aqueous phase were found as risk factors for retention of β-sitosterol and lupeol with the aid of DOE. The improved experimental model was statistically significant, as evidenced by the histogram plot, ANOVA and residual contour plots. In terms of accuracy and precision, the created optimized technique was shown to be more accurate and sensitive. An analytical method has successfully developed and validated by QbD technique for RP-HPLC method development for detection and simultaneous quantification of lupeol and β-sitosterol in C. quadrangularis L. and its commercial product in compliance with ICH Q2(R1). The QbD methodology was implemented, resulting in a more robust system that can provide consistent, dependable and high-quality data throughout the process which also saving time and money. As a result, this technique may be expanded to include marker-based standardization of other herbal products, including β-sitosterol and lupeol, as well as routine quality monitoring of herbal raw materials. In a nutshell, the investigations demonstrate the technique created by QbD design has exceptional usefulness for chromatographic fingerprinting and quality control of herbs in accordance with industrial standards.
Acknowledgements:
The authors are grateful to Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, for providing the facilities to carry out the research work.
Conflict of interests:
The authors declared no conflict of interest.
References
- Udupa KN, Chaturvedi GN, Tripathi SN. Advances in research in Indian medicine. Varanasi: Banaras Hindu University; 1970.
- Kirtikar KR, Basu BD. Indian medicinal plants. In: Basu LM, editors. Delhi: CSIR; 2000. p. 841-3.
- Prasad GC, Udupa KN. Effect of Cissus quadrangularis on the healing of cortisone treated fractures. Indian J Med Res 1963;51:667-76.
[Google Scholar] [PubMed]
- Udupa KN, Prasad GC. Further studies on the effect of Cissus quadrangularis in accelerating fracture healing. Indian J Med Res 1964;52:26-35.
[Google Scholar] [PubMed]
- Prasad GC, Udupa KN. Pathways and site of action of a phytogenic steroid from Cissus quadrangularis. J Res Indian Med 1972;4:132.
- Maridass M, Ramesh U. Investigation of phytochemical constituents from Eulophia epidendraea. Int J Biol Tech 2010;1(1):1-23.
- Gallo MB, Sarachine MJ. Biological activities of lupeol. Int J Biomed Pharm Sci 2009;3(1):46-66.
- Colombo R, de L. Batista AN, Teles HL, Silva GH, Bomfim GC, Burgos RC, et al. Validated HPLC method for the standardization of Phyllanthus niruri (herb and commercial extracts) using Corilagin as a phytochemical marker. Biomed Chromatogr 2009;23(6):573-80.
[Crossref] [Google Scholar] [PubMed]
- Yoganarasimhan SN. Medicinal Plants of India. 2nd ed. Tamilnadu: Cyber Media; 2000.
- Mate GS, Naikwade NS, Magdum CS, Chowki AA, Patil SB. Evaluation of anti-nociceptive activity of Cissus quadrangularis on albino mice. Int J Green Pharm 2008;2(2):118-21.
- Shailajan S, Menon S, Sayed N, Tiwari B. Simultaneous estimation of three triterpenoids from Carissa carandas using validated high performance liquid chromatography. Int J Green Pharm 2012;6(3):241-7.
- Manjula K, Rajendran K, Eevera T, Kumaran S. Quantitative estimation of lupeol and stigmasterol in Costus igneus by high-performance thin-layer chromatography. J Liq Chromatogr Relat Technol 2013;36(2):197-212.
- Preet R, Gupta RC, Pradhan SK. Chromatographic determination of β-sitosterol, lupeol and oleanolic acid in Leptadenia pyrotechnica (Forsk.) decne.-A botanical source of the Ayurvedic drug Jivanti. J Planar Chromatogr Mod TLC 2018;31(2):150-4.
- Shah UM, Patel SM, Patel PH, Hingorani L, Jadhav RB. Development and validation of a simple isocratic HPLC method for simultaneous estimation of phytosterols in Cissus quadrangularis. Indian J Pharm Sci 2010;72(6):753-8.
[Crossref] [Google Scholar] [PubMed]
- Calixto JB. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz J Med Biol Res 2000;33(2):179-89.
[Crossref] [Google Scholar] [PubMed]
- Fukuda IM, Pinto CF, Moreira CD, Saviano AM, Lourenço FR. Design of experiments (DoE) applied to pharmaceutical and analytical quality by design (QbD). Braz J Pharm Sci 2018;54:1-16.
- Sultana S, Kumar U, Hossain MS, Lira DN, Rouf AS. QbD approach for the development and validation of RP-UHPLC method for quantitation of vildagliptin. Dhaka Univ J Pharm Sci 2017;16(1):107-17.
- Tang D, Yang D, Tang A, Gao Y, Jiang X, Mou J, et al. Simultaneous chemical fingerprint and quantitative analysis of Ginkgo biloba extract by HPLC-DAD. Anal Bioanal Chem 2010;396(8):3087-95.
[Crossref] [Google Scholar] [PubMed]
- ICH harmonised tripartite guideline. Validation of analytical procedures: Text and methodology Q2(R1). Geneva, Switzerland: International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use; 2005.
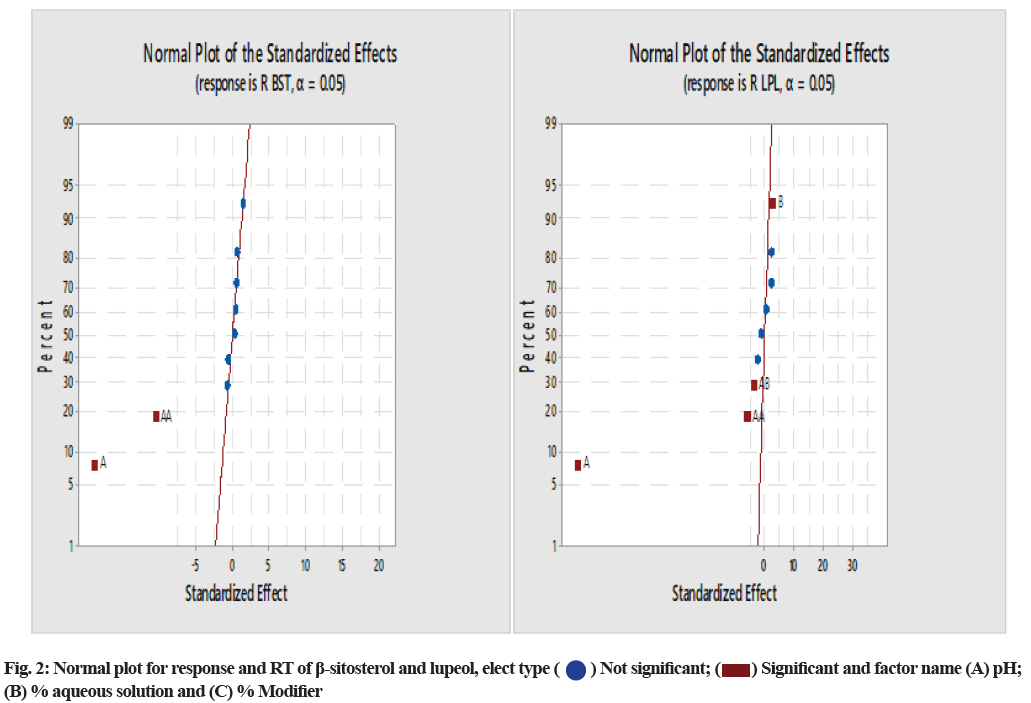
 ) Not significant; (
) Not significant; ( ) Significant and factor name (A) pH; (B) % aqueous solution and (C) % Modifier
) Significant and factor name (A) pH; (B) % aqueous solution and (C) % Modifier