- *Corresponding Author:
- Yukun Zhang
Department of Traditional Chinese Medicine, Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang Province 150040, China
E-mail: zhang520-888@163.com
| This article was originally published in a special issue, “Clinical Advancements in Life Sciences and Pharmaceutical Research” |
| Indian J Pharm Sci 2024:86(5) Spl Issue “151-158” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aims to explore the potential mechanisms of Astragalus membranaceus in the treatment of Parkinson's disease with a particular focus on its role in neurotransmitter regulation. Firstly, using network pharmacology methods, we screened potential active ingredients from Astragalus membranaceus based on Traditional Chinese Medicine Systems Pharmacology Database criteria, with oral bioavailability≥40 % and drug-likeness≥0.1. Subsequently, potential protein targets of these active ingredients were predicted using databases such as SwissTargetPrediction and Search Tool for Interacting Chemicals. Targets related to Parkinson's disease and dopamine metabolism were obtained from GeneCards and Online Mendelian Inheritance in Man databases. We then constructed networks of active ingredients and targets, further filtering out targets associated with Parkinson's disease. Lastly, a protein-protein interaction network was constructed using the Search Tool for the Retrieval of Interacting Genes/Proteins database and key genes in the network were quantified using the MCODE application. Functional enrichment analysis was performed using Gene Ontology/Kyoto Encyclopedia of Genes and Genomes. Finally, molecular docking was employed to validate target genes. Our findings identified the muscarinic cholinergic receptor 2 gene as one of the potential targets of Astragalus membranaceus in treating Parkinson's disease. Further bioinformatics analysis revealed the modulatory effect of Astragalus membranaceus on the acetylcholine receptor signaling pathway, providing new theoretical insights into its neuroprotective effect. This study, employing a comprehensive approach of network pharmacology and bioinformatics analysis, elucidated the potential mechanisms of Astragalus membranaceus in Parkinson's disease treatment, emphasizing the significant role of neurotransmitter regulation.
Keywords
Astragalus membranaceus, Parkinson’s disease, network pharmacology, bioinformatics, neurotransmitter regulation
Parkinson’s Disease (PD) is a chronic and progressive neurodegenerative disorder predominantly affecting elderly individuals[1]. A key pathological characteristic of PD is the substantial degeneration of dopaminergic neurons in the Substantia Nigra pars compacta (SNpc) of the midbrain[2-4]. Normally, these neurons release dopamine to regulate motor functions in the striatum. When these neurons are lost, it leads to a marked reduction in dopamine levels in the striatum[5]. Another pathological characteristic of PD is the formation of Lewy bodies, which are mainly composed of aggregated alpha-synuclein and are present in the remaining dopaminergic neurons[6]. PD is also associated with significant neuroinflammation, characterized by the activation of microglia and the increased release of pro-inflammatory cytokines[7-9].
Dopamine is a crucial neurotransmitter that regulates motor, emotional, and cognitive functions[10]. In PD patients, the loss of dopaminergic neurons results in a significant reduction in central nervous system dopamine levels, leading to primary symptoms such as tremors, rigidity, bradykinesia, and postural instability[11]. Additionally, PD patients may experience non-motor symptoms, including depression, anxiety, sleep disturbances, and autonomic dysfunction[12].
Scutellaria baicalensis (S. baicalensis), commonly known as Astragalus, is a traditional Chinese medicinal herb widely used for treating inflammation, infections, and neurological disorders[13]. Its primary active components include baicalin, baicalein, wogonoside, and wogonin[14,15]. Modern research has demonstrated that S. baicalensis and its main constituents have significant neuroprotective effects[16]. Baicalin and baicalein possess potent anti-inflammatory properties, capable of inhibiting microglial activation and reducing the release of pro-inflammatory cytokines, thereby alleviating neuroinflammation[17,18]. These compounds can also scavenge free radicals and reduce oxidative stress, thus protecting dopaminergic neurons from damage[19]. Furthermore, baicalin regulates the expression of apoptosis-related genes, inhibiting neuronal apoptosis and slowing the progression of neurodegenerative diseases. Additionally, the components of S. baicalensis can promote the expression of nerve growth factors and synaptic formation, aiding in the recovery of neurological functions.
This study aims to systematically identify and validate the mechanisms by which S. baicalensis and its major components regulate dopamine neurotransmitter metabolism in PD. Network pharmacology methods will be used to predict the potential targets of the main components of S. baicalensis, especially those related to dopamine metabolism. Pathway enrichment analysis will be conducted on the predicted targets to identify the key pathways involved in dopamine metabolism.
Materials and Methods
Network pharmacology analysis:
The chemical structures of the major active components of S. baicalensis (baicalin, baicalein, wogonoside, and wogonin) were sourced from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (http://tcmspw.com/tcmsp.php).
Target prediction:
Utilize databases such as SwissTarget (http://www.SwisstargetPrediction.ch/) Prediction and Stitch to predict potential protein targets of the identified active compounds.
PD-related target screening:
Utilize general databases from Online Mendelian Inheritance in Man (OMIM) (https://omim.org/), and GeneCards (https://www.genecards.org/) to search for genes and proteins linked to PD.
Protein-Protein Interaction (PPI):
Common targets were entered into the Search Tool for the Retrieval of Interacting Genes (STRING) database (https://string-db.org/) for analysis. The protein type was set to Homo sapiens, and the minimum interaction threshold was set to 0.4.
Network construction:
Construct a compound-target network using Cytoscape version 3.8.2 to visualize the interactions between the active compounds and their predicted targets.
Pathway enrichment analysis:
Perform pathway enrichment analysis on the identified targets using tools such as DAVID or the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database to identify key pathways related to dopamine metabolism. Focus on pathways known to be involved in the pathophysiology of PD.
Molecular docking:
Obtaining the SDF format files of core drug’s major active ingredients from the PubChem database, collecting critical target protein structures from the Protein Data Bank (PDB) database, optimizing the targets using Pymol software by removing water molecules and small molecule ligands, and performing hydrogenation and charge processing using AutoDock Tools, then saving them as pdbqt format.
Results and Discussion
To further explore the molecular mechanisms of Astragalus in combating PD, we conducted a network pharmacology and molecular docking study. We identified potential active compounds in Astragalus based on screening criteria of Oral Bioavailability (OB) ≥40 % and Drug-Likeness (DL) ≥0.1. Ultimately, we identified 27 potential active compounds from Astragalus (Table 1).
| ID | Molecule | OB (%) | DL |
|---|---|---|---|
| MOL000228 | (2R)-7-hydroxy-5-methoxy-2-phenylchroman-4-one | 55.23 | 0.2 |
| MOL002573 | β-patchoulene | 50.69 | 0.11 |
| MOL002910 | Carthamidin | 41.15 | 0.24 |
| MOL002911 | 2,6,2',4'-tetrahydroxy-6'-methoxychaleone | 69.04 | 0.22 |
| MOL002913 | Dihydrobaicalin_qt | 40.04 | 0.21 |
| MOL002914 | Eriodyctiol (flavanone) | 41.35 | 0.24 |
| MOL002915 | Salvigenin | 49.07 | 0.33 |
| MOL002917 | 5,2',6'-Trihydroxy-7,8-dimethoxyflavone | 45.05 | 0.33 |
| MOL002927 | Skullcapflavone II | 69.51 | 0.44 |
| MOL002928 | Oroxylina | 41.37 | 0.23 |
| MOL002932 | Panicolin | 76.26 | 0.29 |
| MOL002934 | Neobaicalein | 104.34 | 0.44 |
| MOL002937 | Dihydrooroxylin | 66.06 | 0.23 |
| MOL000612 | (-)-Alpha-cedrene | 55.56 | 0.1 |
| MOL000073 | Ent-epicatechin | 48.96 | 0.24 |
| MOL000131 | Electron-ion collider | 41.9 | 0.14 |
| MOL000449 | Stigmasterol | 43.83 | 0.76 |
| MOL000676 | Dibutyl phthalate | 64.54 | 0.13 |
| MOL001490 | Bis((2S)-2-ethylhexyl) benzene-1,2-dicarboxylate | 43.59 | 0.35 |
| MOL001889 | Methyl linolelaidate | 41.93 | 0.17 |
| MOL002879 | 2,3-O-isopropylidene-2,3-dihydroxy-1,4-bis(diphenylphosphino)butane | 43.59 | 0.39 |
| MOL002897 | Epiberberine | 43.09 | 0.78 |
| MOL003475 | 9-Cedranone | 67.6 | 0.12 |
| MOL003568 | Patchoulene | 49.06 | 0.11 |
| MOL008206 | Moslosooflavone | 44.09 | 0.25 |
| MOL011081 | Linolenic acid methyl ester | 46.15 | 0.17 |
| MOL012246 | 5,7,4'-trihydroxy-8-methoxyflavanone | 74.24 | 0.26 |
Table 1: Information on Active Ingredients of A. membranaceus
We utilized databases such as SwissTargetPrediction and Stitch to predict potential protein targets of active ingredients in Astragalus membranaceus (A. membranaceus). Subsequent analysis yielded a substantial number of potential targets. Simultaneously, through searches in GeneCards and OMIM databases using keywords PD and dopamine metabolism, we obtained a set of targets relevant to these diseases.
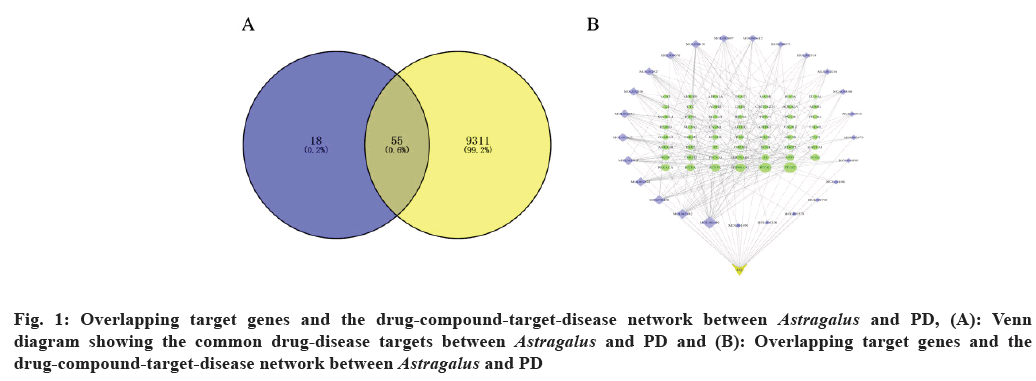
Using the mapping tool of Venny 2.1 online software, we compared the predicted targets with disease-associated targets (fig. 1A), thereby selecting specific targets closely related to PD and dopamine metabolism.
We illustrated the relationships between active ingredients of A. membranaceus and their targets through compound-target networks and target-disease networks (fig. 1B). Furthermore, we delineated the positions and roles of these targets in PD-related pathways.
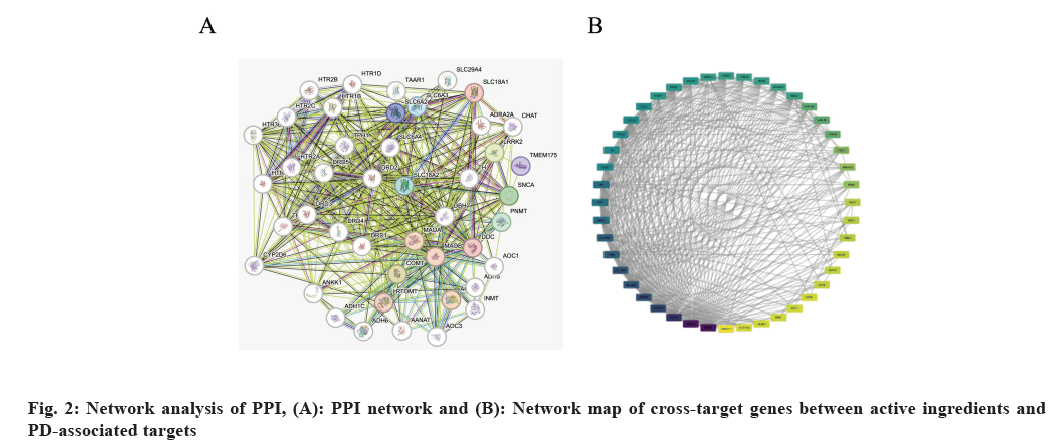
We used the STRING database to construct a PPI network to predict protein interactions. Subsequently, the significance of these genes in the network was quantified using the MCODE application in Cytoscape (fig. 2A and fig. 2B). The data indicated that SCN5A, ADRB2, CHRM3, CHRM1, CHRM2, GABRA2, and GABRA1 are the most relevant proteins (Table 2). This includes only CHRM2’s neurotransmitter-related information about PD.
| Gene | Description | Associated diseases | Neurotransmitter |
|---|---|---|---|
| SCN5A | Sodium voltage-gated channel alpha subunit 5A | Long QT syndrome, Brugada syndrome and cardiomyopathy | No |
| ADRB2 | Adrenoceptor beta 2 | Asthma and Chronic Obstructive Pulmonary Disease (COPD) | No |
| CHRM3 | Muscarinic acetylcholine receptor M3 | Asthma and Overactive bladder | Acetylcholine |
| CHRM1 | Muscarinic acetylcholine receptor M1 | Alzheimer's disease and PD | Acetylcholine |
| CHRM2 | Muscarinic acetylcholine receptor M2 | Alzheimer's disease and schizophrenia | Acetylcholine |
| GABRA2 | Gamma-aminobutyric acid receptor subunit alpha-2 | Alcoholism and epilepsy | GABA |
| GABRA1 | Gamma-aminobutyric acid receptor subunit alpha-1 | Epilepsy, anxiety and insomnia | GABA |
Table 2: Overview of Genes, Neurotransmitters and Associated Diseases
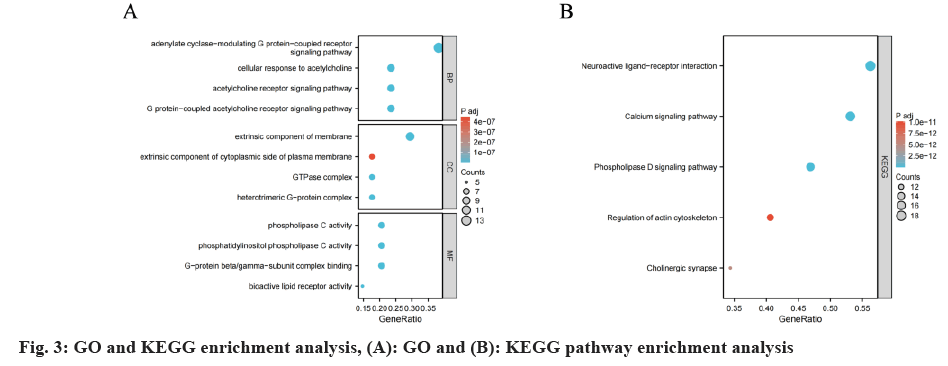
As shown in fig. 3A, biological process annotations suggest that A. membranaceus potential therapeutic mechanism in PD is primarily associated with adenylate cyclase-modulating G protein-coupled receptor signaling pathway, cellular response to acetylcholine, acetylcholine receptor signaling pathway, G protein acetylcholine coupled receptor signaling pathway. Cellular compartment annotations indicate that the action of A. membranaceus in PD is mainly related to compartment extrinsic component of membrane. Furthermore, molecular function annotations suggest that molecule phospholipase C activity, G-protein beta/gamma-subunit complex binding may be involved in A. membranaceus therapeutic effect on PD. Additionally, KEGG pathway enrichment analysis reveals that the potential therapeutic mechanism of A. membranaceus against PD mainly involves neuroactive ligand- receptor interaction, calcium signaling pathway, phospholipase D signaling pathway (fig. 3B and Table 3).
| Ontology | Description | Padjust | Gene ID |
|---|---|---|---|
| BP | G protein-coupled acetylcholine receptor signaling pathway | 2.8236E-15 | GNA15/GNAQ/CHRM2/GRK2/CHRM3/CHRM1/PLCB1/CHRM5 |
| BP | Adenylate cyclase-modulating G protein-coupled receptor signaling pathway | 5.27948E-14 | GNA15/GNA12/GNAQ/CHRM2/GRK5/GNAS/CHRM3/CHRM1/LPAR3/LPAR1/GNA14/CHRM5/LPAR2 |
| BP | Acetylcholine receptor signaling pathway | 5.27948E-14 | GNA15/GNAQ/CHRM2/GRK2/CHRM3/CHRM1/PLCB1/CHRM5 |
| BP | Cellular response to acetylcholine | 7.25964E-14 | GNA15/GNAQ/CHRM2/GRK2/CHRM3/CHRM1/PLCB1/CHRM5 |
| BP | Response to acetylcholine | 7.73408E-14 | GNA15/GNAQ/CHRM2/GRK2/CHRM3/CHRM1/PLCB1/CHRM5 |
| CC | Heterotrimeric G-protein complex | 1.68788E-09 | GNA15/GNA12/GNAQ/GNAS/GNG12/GNA14 |
| CC | GTPase complex | 1.68788E-09 | GNA15/GNA12/GNAQ/GNAS/GNG12/GNA14 |
| CC | Extrinsic component of membrane | 3.16186E-09 | GNA15/GNA12/GNAQ/GNAS/KALRN/ARHGEF25/GNG12/GNA14/PIK3R6/PIK3R5 |
| CC | Extrinsic component of cytoplasmic side of plasma membrane | 4.49376E-07 | GNA15/GNA12/GNAQ/GNAS/GNG12/GNA14 |
| CC | Postsynaptic membrane | 6.43986E-06 | F2R/CHRM2/CHRM3/CHRM1/CHRM5/CHRNA5/GABRA2 |
| MF | G-protein beta/gamma-subunit complex binding | 6.08189E-13 | GNA15/GNA12/GNAQ/GNAS/PLCB2/GNA14/PIK3R5 |
| MF | Phosphatidylinositol phospholipase C activity | 1.1669E-12 | CHRM3/PLCB2/CHRM1/PLCB1/CHRM5/PLCB3/BDKRB2 |
| MF | Phospholipase C activity | 1.39668E-12 | CHRM3/PLCB2/CHRM1/PLCB1/CHRM5/PLCB3/BDKRB2 |
| MF | Bioactive lipid receptor activity | 1.63234E-09 | LPAR3/LPAR1/LPAR6/LPAR4/LPAR2 |
| MF | Phosphoric diester hydrolase activity | 4.15933E-09 | CHRM3/PLCB2/CHRM1/PLCB1/CHRM5/PLCB3/BDKRB2 |
| KEGG | Phospholipase D signaling pathway | 9.24555E-17 | GNA12/F2R/AGTR1/GNAS/PLCB2/PLCB1/LPAR3/LPAR1/LPAR6/LPAR5/LPAR4/LPAR2/PLCB3/PIK3R6/PIK3R5 |
| KEGG | Calcium signaling pathway | 9.24555E-17 | GNA15/CYSLTR2/GNAQ/F2R/CHRM2/AGTR1/GNAS/BDKRB1/CHRM3/PLCB2/CHRM1/PLCB1/CYSLTR1/GNA14/CHRM5/PLCB3/BDKRB2 |
| KEGG | Neuroactive ligand-receptor interaction | 2.40215E-15 | CYSLTR2/F2R/CHRM2/EDN2/AGTR1/BDKRB1/CHRM3/CHRM1/LPAR3/CYSLTR1/LPAR1/LPAR6/CHRM5/LPAR4/LPAR2/BDKRB2/CHRNA5/GABRA2 |
| KEGG | Cholinergic synapse | 5.12238E-12 | GNAQ/CHRM2/CHRM3/PLCB2/CHRM1/PLCB1/GNG12/CHRM5/PLCB3/PIK3R6/PIK3R5 |
| KEGG | Regulation of actin cytoskeleton | 1.01446E-11 | GNA12/F2R/CHRM2/BDKRB1/CHRM3/CHRM1/GNG12/LPAR1/CHRM5/LPAR5/LPAR4/LPAR2/BDKRB2 |
Table 3: Go Enrichment Analysis of the Top 20 Results and KEGG Pathway Enrichment Analysis of the Top 20 Results
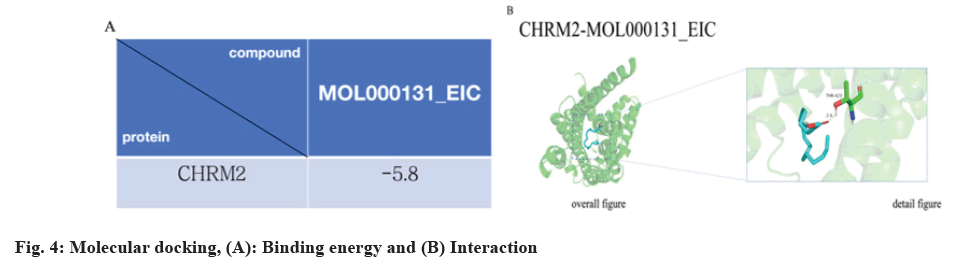
Based on degree centrality, target clustering analysis, and KEGG analysis, we hypothesized that CHRM2 may play a crucial role in the therapeutic effect of Astragalus on PD. We conducted molecular docking analysis to validate the binding of the main compounds of Astragalus with CHRM2. The binding energies between compounds and targets are shown in (fig. 4A and fig. 4B).
A. membranaceus is believed to potentially possess neuroprotective effects against PD[20]. The active compounds within Astragalus are considered to exhibit anti-inflammatory and neuroprotective properties, which may contribute to alleviating neuroinflammation, safeguarding neurons from damage, and potentially playing a role in treating PD[21,22].
27 potential active compounds were identified in Astragalus. Using databases such as SwissTargetPrediction and Stitch, potential protein targets of active ingredients in Astragalus were predicted, and specific targets relevant to PD and dopamine metabolism were selected. A PPI network was constructed using the STRING database, and key proteins related to PD, including SCN5A, ADRB2, CHRM3, CHRM1, CHRM2, GABRA2, and GABRA1 were identified. Only the neurotransmitter-related information of CHRM2 was included regarding PD.
In the context of PD, CHRM2, encoding the muscarinic acetylcholine receptor M2, emerges as a significant gene of interest[23]. Acetylcholine, a neurotransmitter, is intricately involved in modulating various aspects of neuronal function, including motor control, cognition, and memory. The muscarinic acetylcholine receptors, particularly M2 subtype, play a crucial role in mediating cholinergic neurotransmission within the central nervous system. Research suggests that alterations in cholinergic signaling, including dysregulation of muscarinic receptors, could contribute to the pathophysiology of PD[24]. Specifically, CHRM2 dysfunction or alterations in its expression levels might impact cholinergic neurotransmission and subsequently influence motor and cognitive functions implicated in PD[25]. Moreover, studies have implicated muscarinic acetylcholine receptors, including M2, in the regulation of dopaminergic signaling pathways, which are central to the pathogenesis of PD[26-28]. Dysfunctional interactions between cholinergic and dopaminergic systems may exacerbate neurodegenerative processes and contribute to PD symptomatology[29,30].
The results of GO enrichment analysis indicate that Astragalus may exert its potential effects in the treatment of PD by modulating various biological processes, cellular components, and molecular functions. Specifically, Astragalus may influence biological processes relevant to PD pathology, including neurotransmitter signaling, apoptosis regulation, and inflammation modulation. In terms of cellular components, Astragalus effects may primarily involve the regulation of extracellular membrane structures, potentially related to cell signaling and intercellular interactions. Additionally, Astragalus may impact various molecular functions such as phosphatase activity, G protein-coupled receptor binding, and cytokine activity, which may contribute to its therapeutic effects on PD.
In terms of potential therapeutic pathways, KEGG pathway enrichment analysis identified several pathways enriched in PD, including neuroactive ligand-receptor interaction, calcium signaling pathway, and phospholipase D signaling pathway. These pathways are known to be involved in dopamine neurotransmission and neuronal function regulation, suggesting that Astragalus may mediate its neuroprotective effects on PD by modulating the activity of these pathways.
Furthermore, the active components of Astragalus may also possess anti-inflammatory and antioxidant properties, which could help alleviate neuroinflammation and oxidative stress responses, thereby protecting neurons from damage[31]. Additionally, Astragalus may promote the expression of neurotrophic factors and synaptic formation, facilitating neuronal survival and functional recovery.
In comparison to previous studies, our research has further deepened the understanding of the mechanism of Astragalus in the treatment of PD. Despite being widely used in traditional medicine for the treatment of various diseases, the mechanism of Astragalus in PD remains relatively understudied. Through the comprehensive application of network pharmacology methods and bioinformatics analysis, our study has delved into the potential mechanisms of Astragalus in PD, providing a new perspective for understanding its therapeutic effects.
However, our study also has some limitations. Firstly, although we utilized advanced computational tools and databases for prediction and analysis, further experimental validation is still needed to confirm the results. Secondly, our research only explored the potential effects of Astragalus in a preliminary manner, and further clinical studies are required to confirm its specific therapeutic effects and dosage effects.
Therefore, while our study provides a new theoretical basis for the role of Astragalus in PD treatment, further research is needed to validate our findings and further elucidate its potential value in clinical practice.
This study employed a comprehensive approach integrating network pharmacology methods and bioinformatics analysis to investigate the potential mechanisms of A. membranaceus in treating PD. The results revealed that we specifically screened neurotransmitter-related genes associated with PD, including the CHRM2 gene. Our findings suggest that the CHRM2 gene might be one of the potential targets for A. membranaceus in treating PD. By modulating neurotransmitter signaling pathways, particularly the acetylcholine receptor signaling pathway, A. membranaceus may regulate the release and signal transduction of neurotransmitters such as dopamine, thereby exerting its neuroprotective effects.
Funding:
The work was supported by research program for philosophy and social sciences in Heilongjiang province (No: 23TQD182).
Conflict of interests:
The authors declared no conflict of interests.
References
- Bej E, Cesare P, Volpe AR, d’Angelo M, Castelli V. Oxidative stress and neurodegeneration: Insights and therapeutic strategies for Parkinson’s disease. Neurol Int 2024;16(3):502-17.
[Crossref] [Google Scholar] [PubMed]
- Kelty-Stephen DG, Kiyono K, Stergiou N, Mangalam M. Spatial variability and directional shifts in postural control in Parkinson’s disease. Clin Parkinsonism Relat Disord 2024;10:100249.
[Crossref] [Google Scholar] [PubMed]
- Yan S, Lu J, Zhu H, Tian T, Qin Y, Li Y, et al. The influence of accelerated brain aging on coactivation pattern dynamics in Parkinson's disease. J Neurosci Res 2024;102(5):e25357.
[Crossref] [Google Scholar] [PubMed]
- Meyer M, Montel S, Colnat-Coulbois S, Frismand S, Llorca PM, Vidailhet P, et al. Parkinson’s disease: Coping strategies, cognitive restructuring and deep brain stimulation. J Geriatr Psychiatry Neurol 2024:08919887241248831.
[Crossref] [Google Scholar] [PubMed]
- James D, Smith J, Lane E, Thomas R, Brown S, Seage H. Adherence to Parkinson's disease medication: A case study to illustrate reasons for non-adherence, implications for practice and engaging under-represented participants in research. Explor Res Clin Soc Pharm 2024;14:100450.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, Tu Y, Yan G, Ji X, Chen S, Niu C, et al. Integrated bioinformatics analysis for revealing CBL is a potential diagnosing biomarker and related immune infiltration in Parkinson’s disease. Int J Gen Med 2024;17:2371-86.
[Crossref] [Google Scholar] [PubMed]
- Zou M, Wu Y, Lan Y, Xie H, Sun H, Liu W, et al. Identification and optimization of nitrophenolic analogues as dopamine metabolic enzyme inhibitors for the treatment of Parkinson’s disease. Bioorgan Chem 2024;148:107488.
- Zhang N, Zhang S, Dong X. Plant-derived bioactive compounds and their novel role in central nervous system disorder treatment via ATF4 targeting: A systematic literature review. Biomed Pharmacother 2024;176:116811.
[Crossref] [Google Scholar] [PubMed]
- Danz K, Fleddermann J, Koch M, Fecioru E, Maahs L, Kinsinger N, et al. Evaluation of the transport and binding of dopamine-loaded PLGA nanoparticles for the treatment of Parkinson’s disease using in vitro model systems. Pharmaceutics 2024;16(5):571.
[Crossref] [Google Scholar] [PubMed]
- de Moraes Santos Corrêa É, Christofoletti G, de Souza AS. Effects of intracerebral aminophylline dosing on catalepsy and gait in an animal model of Parkinson’s disease. Int J Mol Sci 2024;25(10):5191.
[Crossref] [Google Scholar] [PubMed]
- Gonzalez-Ramos A, Puigsasllosas-Pastor C, Arcas-Marquez A, Tornero D. Updated toolbox for assessing neuronal network reconstruction after cell therapy. Bioengineering 2024;11(5):487.
[Crossref] [Google Scholar] [PubMed]
- Hussain MS, Moglad E, Afzal M, Sharma S, Gupta G, Sivaprasad GV, et al. Autophagy-associated non-coding RNAs: Unraveling their impact on Parkinson's disease pathogenesis. CNS Neurosci Ther 2024;30(5):e14763.
[Crossref] [Google Scholar] [PubMed]
- Lyu S, Zhang CS, Mao Z, Guo X, Li Z, Luo X, et al. Real-world Chinese herbal medicine for Parkinson's disease: A hospital-based retrospective analysis of electronic medical records. Front Aging Neurosci 2024;16:1362948.
[Crossref] [Google Scholar] [PubMed]
- Xu Z, Yang D, Huang X, Huang H. Astragaloside IV protects 6-hydroxydopamine-induced SH-SY5Y cell model of Parkinson’s disease via activating the JAK2/STAT3 pathway. Front Neurosci 2021;15:631501.
[Crossref] [Google Scholar] [PubMed]
- Tan Y, Yin L, Sun Z, Shao S, Chen W, Man X, et al. Astragalus polysaccharide exerts anti-Parkinson via activating the PI3K/AKT/mTOR pathway to increase cellular autophagy level in vitro. Int J Biol Macromol 2020;153:349-56.
[Crossref] [Google Scholar] [PubMed]
- Stępnik K, Kukula-Koch W, Plazinski W, Gawel K, Gaweł-Bęben K, Khurelbat D, et al. Significance of astragaloside IV from the roots of Astragalus mongholicus as an acetylcholinesterase inhibitor-From the computational and biomimetic analyses to the in vitro and in vivo studies of safety. Int J Mol Sci 2023;24(11):9152.
[Crossref] [Google Scholar] [PubMed]
- Liu R, Zhang Y, Li S, Liu C, Zhuang S, Zhou X, et al. Extraction and preparation of 5-lipoxygenase and acetylcholinesterase inhibitors from Astragalus membranaceus stems and leaves. J Separation Sci 2023;46(4):2200812.
[Crossref] [Google Scholar] [PubMed]
- Du Y, Li C, Xu S, Yang J, Wan H, He Y. LC-MS/MS combined with blood-brain dual channel microdialysis for simultaneous determination of active components of astragali radix-safflower combination and neurotransmitters in rats with cerebral ischemia reperfusion injury: Application in pharmacokinetic and pharmacodynamic study. Phytomedicine 2022;106:154432.
[Crossref] [Google Scholar] [PubMed]
- Abd Elkader HT, Essawy AE, Al-Shami AS. Astragalus species: Phytochemistry, biological actions and molecular mechanisms underlying their potential neuroprotective effects on neurological diseases. Phytochemistry 2022;202:113293.
[Crossref] [Google Scholar] [PubMed]
- Lee YA, Kim YJ, Lee JS, Lee S, Goto Y. Imbalance between dopamine and serotonin caused by neonatal habenula lesion. Behav Brain Res 2021;409:113316.
[Crossref] [Google Scholar] [PubMed]
- Guo LY, Shi FL, Li M, Sun JH, Li CG, Liu ZX. Astragalus protects PC12 cells from 6-hydroxydopamine-induced neuronal damage: A serum pharmacological study. J Physiol Inv 2021;64(1):24-31.
[Crossref] [Google Scholar] [PubMed]
- Zhao L, Sun Y, Yu C, Chen J, Xu X, Zhang X, et al. Astragaloside protects rat brain from microwave-induced functional injuries via restoring acetylcholine and normalizing electroencephalogram. Environ Sci Pollut Res 2020;27(32):40787-94.
[Crossref] [Google Scholar] [PubMed]
- Chorlian DB, Meyers JL, Manz N, Zhang J, Kamarajan C, Pandey A, et al. Genetic influences vary by age and sex: Trajectories of the association of cholinergic system variants and theta band event related oscillations. BioRxiv 2023.
[Crossref] [Google Scholar] [PubMed]
- He Y, Su H, Li N, Zhang Y, Zhang P, Zhang Y, et al. In utero hypoxia attenuated acetylcholine-mediated vasodilatation via CHRM3/p-NOS3 in fetal sheep MCA: Role of ROS/ERK1/2. Hypertension Res 2022;45(7):1168-82.
- Liu W, Li J, Yang M, Ke X, Dai Y, Lin H, et al. Chemical genetic activation of the cholinergic basal forebrain hippocampal circuit rescues memory loss in Alzheimer’s disease. Alzheimer's Res Ther 2022;14(1):53.
[Crossref] [Google Scholar] [PubMed]
- Refisch A, Komatsuzaki S, Ungelenk M, Chung HY, Schumann A, Schilling SS, et al. Associations of common genetic risk variants of the muscarinic acetylcholine receptor M2 with cardiac autonomic dysfunction in patients with schizophrenia. World J Biol Psychiatry 2023;24(1):1.
[Crossref] [Google Scholar] [PubMed]
- Katayama K, Suzuki K, Suno R, Kise R, Tsujimoto H, Iwata S, et al. Vibrational spectroscopy analysis of ligand efficacy in human M2 muscarinic acetylcholine receptor (M2R). Commun Biol 2021;4(1):1321.
[Crossref] [Google Scholar] [PubMed]
- Heinrich M, Sieg M, Kruppa J, Nürnberg P, Schreier PH, Heilmann-Heimbach S, et al. Association between genetic variants of the cholinergic system and postoperative delirium and cognitive dysfunction in elderly patients. BMC Med Genom 2021;14:248.
[Crossref] [Google Scholar] [PubMed]
- Wan YJ, Sheng L. Regulation of bile acid receptor activity. Liver Res 2018;2(4):180-5.
[Crossref] [Google Scholar] [PubMed]
- Więckowska A, Gajewska-Woźniak O, Głowacka A, Ji B, Grycz K, Czarkowska-Bauch J, et al. Spinalization and locomotor training differentially affect muscarinic acetylcholine receptor type 2 abutting on α-motoneurons innervating the ankle extensor and flexor muscles. J Neurochem 2018;147(3):361-79.
[Crossref] [Google Scholar] [PubMed]
- Sakata K, Overacre AE. Promoter IV-BDNF deficiency disturbs cholinergic gene expression of CHRNA5, CHRM2, and CHRM5: Effects of drug and environmental treatments. J Neurochem 2017;143(1):49-64.