- *Corresponding Author:
- Q. X. Li
Department of Pharmacy, Wuhan City College, Wuhan, Hubei 430083, China
E-mail: xiongqili54321@163.com
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “154-161” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Male infertility is influenced by psychological, biological and environmental factors. Nowadays, the morphological change of genes and biological molecules are the focus of attention. Therefore, this study was conducted with the aim of investigating the relationship between tumor necrosis factor alpha-308 and interleukin-1beta C+3953T polymorphisms with male fertility in Erbil province. This study was conducted between 2020 and 2021 on 270 men who referred to the infertility center in Erbil, Iraq, with infertility problems. The sampling was purposeful and the samples were divided into three groups of 90 people with the characteristics of the first group without fertility problems, the second group with primary infertility and the third group with secondary infertility. Blood samples were taken from each sample and the samples were sent to the laboratory for deoxyribonucleic acid extraction and allele analysis. A polymerase chain reaction machine was used for this purpose. Statistical package for the social sciences version 23 software and descriptive statistical tests, chi-square, odds ratio and conditional regression were used to analyze the obtained data. The results of tumor necrosis factor alpha-308 genotype showed the frequency of alleles in three groups of men, it can be concluded that there is a significant difference between the three groups of men in terms of mutation frequency and fertility (p≤0.001). The results of interleukin-1beta C+3953T genotype have shown that there is a significant difference in mutation frequency and finally fertility in three groups of men (p≤0.001). The tumor necrosis factor alpha-308 genotype GA and GG alleles have a significant relationship with male fertility. In genotype interleukin-1beta C+3953T, CT and CC alleles have a significant relationship with male fertility. The results of this study showed that tumor necrosis factor alpha-308 genotype and interleukin-1beta C+3953T genotype were identified as important risk factors in male infertility.
Keywords
Polyethylene glycol-hyaluronic acid-melphalan, transmission electron microscope, dynamic light scattering, cytotoxicity, tumor cell
Cancer is a complicated disease representing one of the leading causes of mortality in the world. The greatest challenge in developing anti-cancer drugs is delivering them to the target tumor tissues and reducing systemic toxicity. Due to the constant progress accomplished in the fields of medicinal chemistry, nanotechnology and in the understanding of the biological mechanisms of cancer diseases, some drug delivery approaches have been developed to enhance target delivery and to improve efficacy of existing anti-tumor drugs[1]. The “Prodrug” strategy was one of those strategies, designed many years ago to help the drugs to cross physiological barriers. In this approach, the prodrug consists of an active drug, linked to a carrier that will help it to reach the pharmacological target and then it is ensured that the carrier can be removed later and the biologically active compound was regenerated at the target site[2].
Melphalan (MEL) is an alkylating agent, which inhibits Deoxyribonucleic Acid (DNA) synthesis. It has been proven effective against ovarian, breast and colon cancers[3]. However, it has some obvious drawbacks such as short plasma half-life, non-target selectivity and serious adverse reactions. Because of these shortcomings, its clinical application is limited.
Hyaluronic Acid (HA) is a naturally occurring linear polysaccharide. HA receptors such as homing cell adhesion molecule (Cluster of Differentiation 44 (CD44)) and HA-mediated cell migration receptor (Receptor for Hyaluronan-Mediated Motility (RHAMM)) are abundantly presented in tumor cells[4]. Tumor cells show enhanced binding and internalization of HA. Furthermore, it has been shown that an active drug linked to HA specifically binds with tumor cells after entering the body, followed by intracellular release of active drugs, thus restoring their original cytotoxicity[5-7].
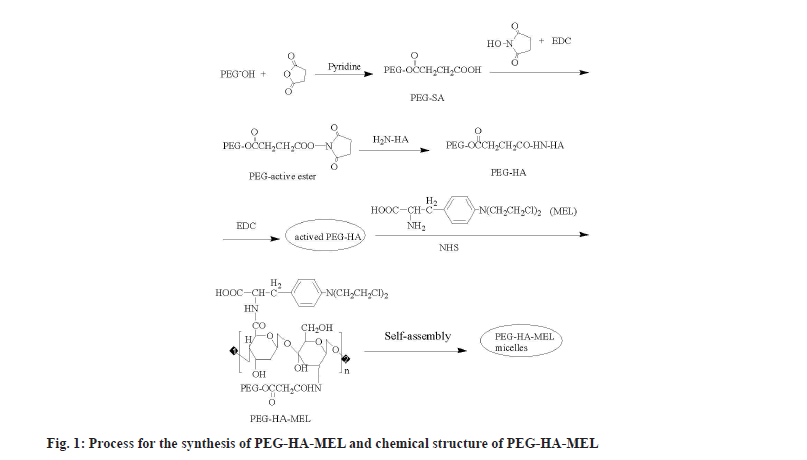
Polyethylene Glycol (PEG) is a small nontoxic linear molecule, which has been extensively investigated for the development of tumor-targetable prodrugs, due to its capability of keeping the prodrug in circulation for a long duration (extend plasma half-life of MEL), followed by their passive accumulation in the tumor tissue[8]. Particularly, the PEG surface can enable prodrugs to escape from the reticuloendothelial system, thus minimizing their removal from the liver. PEG has been used to modify biomedical materials such as proteins, polypeptides, enzymes and biochemical drugs. The activated PEG was chosen to couple with HA at a high-speed in mild conditions, in which reaction the carboxyl end of activated PEG reacts with the amino acid end of acetylated HA to get binary conjugate content of an ester chain. Therefore, the amino tail of MEL reacts with the carboxyl tail of HA to form PEG-HA-MEL (fig. 1).
Materials and Methods
N-Hydroxy Succinimide (NHS) and 1-Ethyl-3-(3-dimethyl-aminopropyl) carbodimide (EDC) were purchased from J and K chemicals. MEL and HA (Molecular weight (Mw)=1050 kDa) were obtained from Wuhan Hezhong Biochemical Manufacturing Co. Ltd. Succinic Anhydride (SA) was purchased from Tianjin Kaitong Chemical Reagent Co. Ltd. PEG-4-Dimethylaminopyridine (DMAP) was obtained from Wuhan Chemical Reagent Co. Ltd. The Fourier-Transform Infrared (FTIR) spectra were recorded on pressed Potassium bromide (KBr) pellets using a Nexus FTIR spectrometer (Therma Nicolet spectrometer, United States of America (USA)) at room temperature in the range between 4000 and 500 cm-1. Bruker Avance-400 (Brucker, Switzerland) was used to acquire Proton Nuclear Magnetic Resonance (1H NMR) spectra and a JEM-2010HT Transmission Electron Microscope (TEM) (Japan Electronics and Oxford Company, Japan) was employed to get the sample morphology, dispersion degree and particle size information.
General procedure for the preparation of PEG-active ester:
With pyridine (2 ml) as a catalyst, a solution of PEG-2000 (10 g) and SA (2.5 g) in chloroform (100 ml) was heated for 6 h then evaporated until a viscous liquid was obtained. After cooling in the ice bath, anhydrous diethyl ether was added to the solution. The produced (PEG-SA) precipitate was filtered and recrystallized in chloroform and anhydrous diethyl ether[8].
The mixture of PEG-SA, NHS and EDC were dissolved in appropriate amount of N, N-Dimethylformamide (DMF) and reacted overnight by constant magnetic stirring. After that, anhydrous diethyl ether was added to the solution in drops, stirred in ice bath for a few minutes, until no more precipitates were generated. The precipitate was filtered and then dissolved in chloroform and recrystallized in anhydrous diethyl ether.
General procedure for preparation of PEGylated HA:
HA (0.5 g) was dissolved in Sodium hydroxide (NaOH) (20 %, 15 ml) solution, after magnetic stirring until the colloidal material gradually turned into uniform solution. Then the PEG-active ester dissolved in water was added, reacted for 6 h at room temperature[9,10]. Flocs were obtained by adding ethanol (95 %) to the solution, then filtered and washed with ethanol 3 times. A pale yellow solid was obtained, namely PEGylated-HA (PEG-HA)[11,12].
General procedure for the preparation of PEG-HA-MEL micelles:
PEG-HA (300 mg), EDC (260 mg) and DMAP (0.05 mmol) were dissolved in anhydrous Dimethyl Sulfoxide (DMSO) (15 ml) while avoiding light and stirred at room temperature till PEG-HA was dissolved completely[13]. MEL (10 mg) and NHS (0.05 mmol) were added, the mixture was allowed to react without light for 24 h. Then the solution obtained was dialyzed to form micelle solution. After dialysis and freeze-drying, the product was named as PEG-HA-MEL (prodrug).
Cell culture and cytotoxicity assay:
The human ovarian cancer cell line (SK-OV-3) tumor cells (China type culture collection, Wuhan, China) were maintained at 37° in a humidified incubator with 5 % Carbon dioxide (CO2)/95 % air and propagated in Dulbecco’s Modified Eagle Medium (DMEM) containing 100 mg/dl D-glucose, 10 % fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mmol/l supplemental glutamine. Cell lines were passaged once per week after treatment with trypsin-ethylenediaminetetraacetic acid. Cells were used for experiments from passages 5-15. Assay was carried out by seeding 3000 SK-OV-3 tumor cells in each well of 96-well culture plates. During this final incubation, SK-OV-3 tumor cells were treated with one of the following: Control; MEL (10−8, 10−7, 10−6 mol/l) and PEG-HA-MEL (10−8, 10−7, 10−6 mol/l) for 0 h, 24 h and 48 h. Changes of tumor cell morphology were observed by an inverted microscope at 0 h, 24 h and 48 h after treatment. Cell viability was determined using 3-(4, 5-Dimethyltiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide (MTT). The absorbance was measured using a VarioskanTM microplate reader (Thermo Fisher Scientific Inc., Waltham, Massachusetts (MA), USA) at 440 nm with 690 nm as a reference wavelength. Cell viability was calculated as follows: Inhibition rate (%)=(Absorbance of control wells-Absorbance of treated wells)/Absorbance of treated wells×100. The experiments were repeated for a minimum of 3 times.
Chemistry:
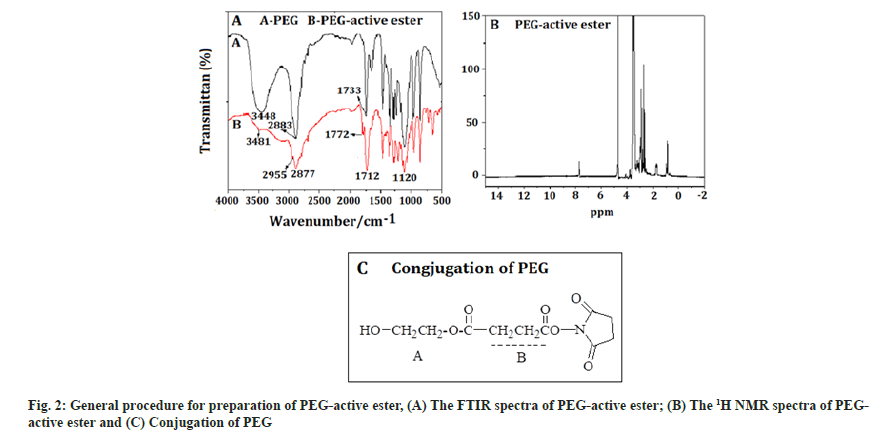
PEG-active ester:Fig. 2A showed the FTIR spectra of PEG-active ester, which is a white solid, with 70 % yield. The absorption band at 3481 cm-1 was the characteristic peak of a hydroxyl, the band at 2955 cm-1 was attributed to the hydrocarbon telescopic vibration of a methyl-CH2, which belonged to a PEG characteristic peak. The band at 2877 cm-1 was telescopic vibration of –N, which was the characteristic peak of NHS. The band at 1712 cm-1 was carbonyl telescopic vibration of an ester, the peak at 1120 cm-1 was telescopic vibration of C-O. The result showed that the compound contained the ester bond, which is PEG active ester, was synthesized successfully. The 1H NMR spectra of PEG-active ester was shown in fig. 2B. The signals at A (1.50 ppm) was attributed to the methyl protons in SA. Successful conjugation of PEG was confirmed by the peak appearing at B (3.6 ppm [-CH2-CH2-O-]) (fig. 2C).
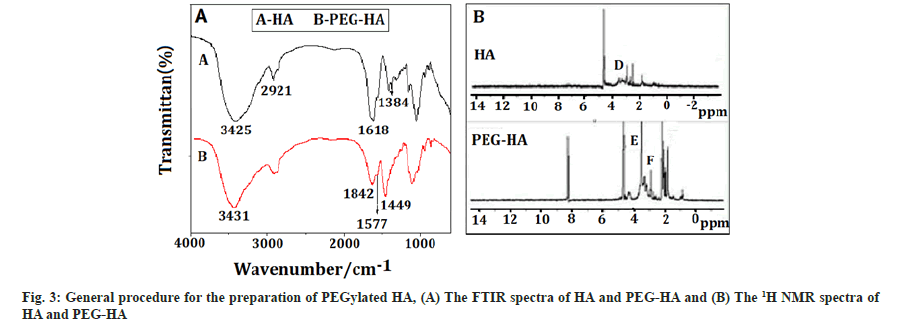
PEGylated HA: Fig. 3A showed the FTIR spectra of HA and PEG-HA, which is a light yellow solid, with 78 % yield. The bands at 3425 cm-1 and 3431 cm-1 were characteristic peaks of hydroxyl, the peak at 2921 cm-1 was the hydrocarbon telescopic vibration of -CH2-. The bands at 1626 cm-1 and 1572 cm-1 were characteristic peaks of amide. Likewise, the peaks at 1577 cm-1 and 1618 cm-1 attributed to amide characteristic peaks of PEG-HA, while the band at 1449 cm-1 was bending vibration of modified PEG part. Finally, the band at 1384 cm-1 was bending vibration of methyl hydrocarbon of HA amide. The result showed that, HA was successfully connected to the PEG, because there are characteristic absorption peaks of modified PEG and amide bond in PEG-HA.
The 1H-NMR spectra of HA and PEG-HA were shown in fig. 3B. The signals at C (2.00 pm) and D (3.75-3.20 ppm) were attributed to the methyl protons in acetamide groups and the protons in the sugar ring of HA, respectively. The signals at F (3.2-2.20 ppm) were attributed to the methylene protons of PEG. The characteristic peaks at E (3.60 ppm) were corresponded to the methylene in the alpha (α) position of the ester groups.
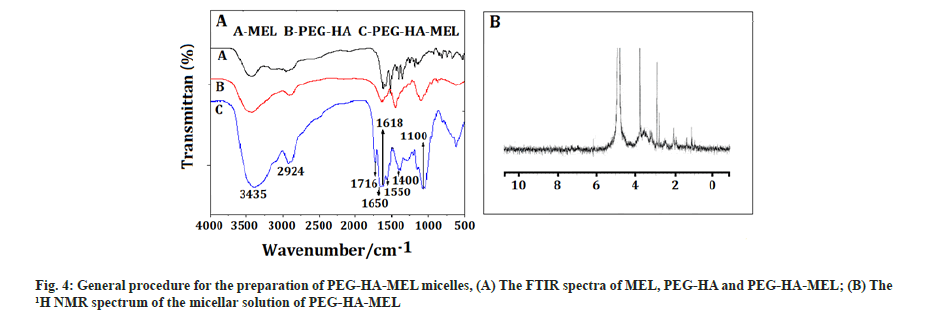
PEG-HA-MEL micelles: Fig. 4A showed the FTIR spectra of MEL, PEG-HA and PEG-HA-MEL, which is light yellow solid, with 56 % yield. As shown, the peaks between 3500 cm-1 to 3400 cm-1 may be the characteristic peaks of hydroxyl, amino or hydrogen of benzene rings. The bands at 1600 cm-1 and 1599 cm-1 were characteristic peaks of an amide and a carbonyl, while the peak at 1100 cm-1 was stretching vibration of C-O. Absorption peaks of benzene were at around 1600 cm-1 to 1400 cm-1.
The product of PEG-HA-MEL was placed into an analysis bag, dialyzed with 1.5 l deionized water until all small molecules passed through and micellar solution was obtained by self-assembly in aqueous solution[14,15]. However, micelles are not suitable for storage in aqueous solution. Therefore, the solution in the dialysis bag is distilled under reduced pressure at normal temperature and the solvent (DMSO) was removed[16,17]. It was then diluted with double distilled water to 50 ml, filtered with microporous filter membrane and freeze-dried to obtain PEG-HA-MEL lyophilized powder. Fig. 4B showed the 1H NMR spectrum of the micellar solution of PEG-HA-MEL. It can be seen that there was no peak at 7.0 ppm in Deuterium oxide (D2O) with the characteristic peaks of benzene. This is due to the fact that PEG-HA-MEL was an amphiphilic polymer. It self-assembled in water to form a core-shell structure. MEL on the other hand is lipophilic and the observed hydrophilic property of PEG was strong in the process of self-assembled formation of PEG-water-based shell in which MEL was wrapped. So it did not respond in the 1H NMR spectrum graph.
Statistical analysis:
Results were expressed as mean±Standard Deviation (SD); the differences between groups were analyzed by one-way analysis of variance and the Student-Neumann-Keuls t-test. Statistical significance was defined as p<0.05.
Results and Discussion
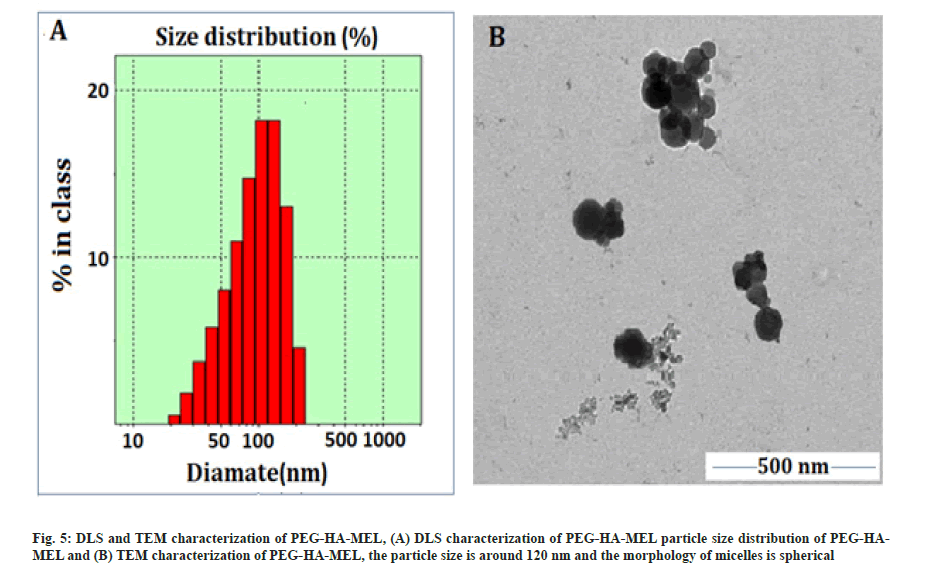
The synthetic route, its optimal conditions and the best synthesis solution of PEG-HA-MEL modified HA with activated PEG has been determined in this paper. The cross-linked HA (PEG-HA) was then connected to MEL. The prodrug PEG-HA-MEL generates micelles by self-assembly in aqueous solution. Therefore, chemical modification of MEL was achieved to improve the drug efficacy in tumor cells. The compounds were characterized by NMR and infrared spectroscopy. Particle size distribution of PEG-HA-MEL was determined by Dynamic Light Scattering (DLS). PEG-HA-MEL size distribution was shown in fig. 5A. The figure showed that the size of nano particles was evenly distributed in between 100~130 nm and most of them were about 116.4 nm. PEG-HA-MEL was dissolved in DMSO and sample morphology, dispersion degree and particle size information was obtained through TEM. From fig. 5B, it was found that the particle size was around 120 nm and the morphology of micelles is spherical, which was consistent with that of DLS.
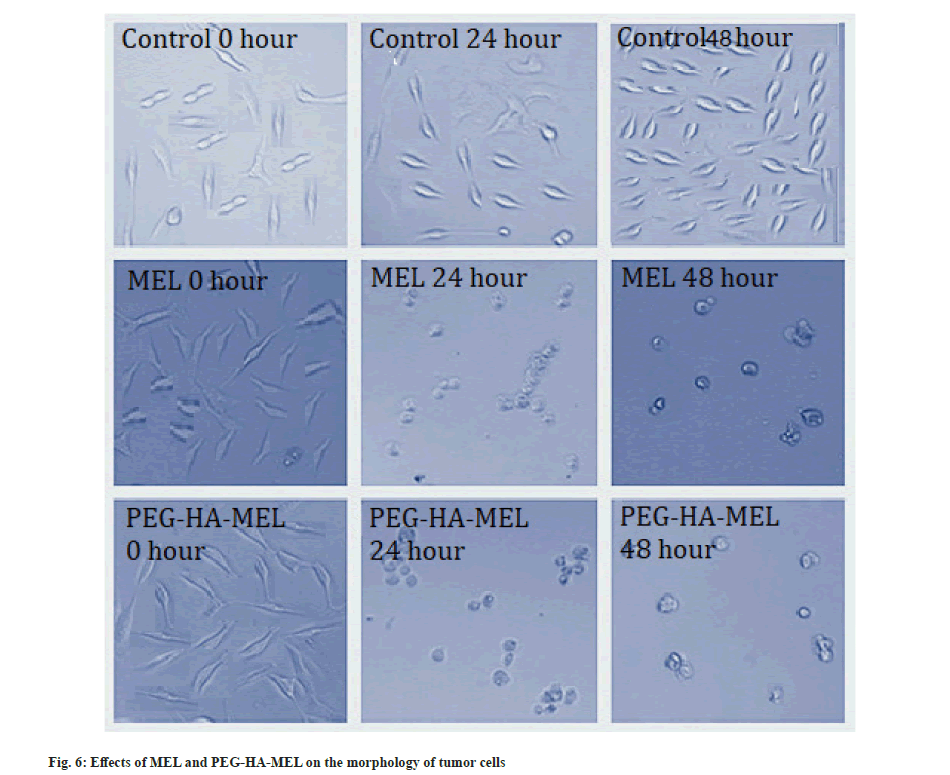
Cell cytotoxicity and morphological analysis was explained here. As shown in Table 1, SK-OV-3 cells treated with MEL (10−7, 10−6 mol/l) and PEG-HA-MEL (10−7, 10−6 mol/l) respectively showed significant changes in cell proliferation compared with the control group (p<0.05, p<0.01). MEL and PEG-HA-MEL inhibited the changes in cell proliferation of SK-OV-3 cells, PEG-HA-MEL half-maximal Inhibitory Concentration (IC50) value was 0.53 μM/48 h and MEL IC50 value was 0.88 μM/48 h. This inhibitory effect is concentration dependent from 10−7 to 10−6 mol/l. Fig. 6 showed that the control cells demonstrated normal growth at 0 h, 24 h and 48 h. The cells treated with MEL (10−6 mol/l) and PEG-HA-MEL (10−6 mol/l) were condensed and rounded, aggregated into clumps, and the number of cells were significantly reduced at 24 h and 48 h respectively.
| Group | Concentration | Optical Density (OD) value | |
|---|---|---|---|
| 24 h | 48 h | ||
| Control | - | 0.48±0.02 | 0.96±0.04 |
| MEL | 10−8 mol/l | 0.43±0.05 | 0.94±0.06 |
| 10−7 mol/l | 0.46±0.07 | 0.74±0.05* | |
| 10−6 mol/l | 0.28±0.04** | 0.46±0.02** | |
| PEG-HA-MEL | 10−8 mol/l | 0.42±0.04 | 0.89±0.08 |
| 10−7 mol/l | 0.41±0.02 | 0.64±0.02* | |
| 10−6 mol/l | 0.28±0.07** | 0.24±0.04** | |
Note: Mean±SD (n=3), *p<0.05 vs. control group and **p<0.01 vs. control group respectively
Table 1: Effects of MEL and PEG-HA-MEL on the Proliferation of SK-OV-3 Tumor Cells.
Previous studies have shown that there are many proteins that can specifically bind to HA in human and animals, which are mainly distributed on cell matrix and cell membrane. These proteins are called HA-Binding Proteins (HABP)[18]. CD44 is HABP (receptor) which has been studied extensively and is considered the most important HA receptor on the cell surface[19]. Specifically, the content of CD44 on the surface of tumor cells (such as SK-OV-3) is many times higher than that of normal cells, which can better combine with HA[20]. Therefore, HA as a carrier can achieve the target effect on tumor cells. HA is selected as a carrier and is suitable for malignant tumor drug delivery system with high expression of CD44 ligand. At the same time, there are some shortcomings of HA such as low water solubility. Therefore, PEG is used to chemically modify it and to improve its water solubility and biodegradability, reduce its degradation rate and prolong its retention time in vivo[21].
This study was to prepare and characterize the new prodrug, PEG-HA-MEL to improve the inadequacy of MEL. It has important clinical significance to use chemistry, materials science and life science research to solve the shortcomings of MEL in order to improve the effective concentration at the tumor site and improve its efficacy[22,23]. HA was modified with activated PEG to prepare PEG-HA to increase its relative molecular mass. PEG-HA was bonded with MEL to obtain targeted drug delivery system and carry out relevant evaluation. It was characterized by NMR and infrared spectroscopy[14]. Then PEG-HA-MEL was prepared into micelles by dialysis. Particle size distribution and the sample morphology of PEG-HA-MEL were determined by DLS and TEM. It was found that the morphology of micelles is spherical and the particle size is about 120 nm.
When the PEG-HA-MEL prodrug enters the body, it binds to cancer cells in a targeted manner. Then, PEG-HA will break with MEL and MEL will free out, thus exerting the drug effect. Cytotoxicity test of the tumor cells treated with PEG-HA-MEL indicated that cells were condensed and rounded, aggregated into clumps, and the number of cells were significantly reduced at 24 h and 48 h respectively. PEG-HA-MEL could inhibit tumor growth via regulating CD44 receptor and PEG-HA effect. This is a preliminary study and theoretical analysis, and further research is needed.
The PEG-HA-MEL prodrug was synthesized successfully and characterized by FTIR spectroscopy, 1H NMR, DLS and TEM. It can form micelle with the average size of about 100-130 nm. It was demonstrated that these natural, biocompatible and biodegradable particles can be used as drug delivery system, and found that this compound play potent inhibitory role on tumor cells, and indicated that the PEG-HA-MEL prodrug could be very useful on the release of control targeted drug.
Acknowledgements:
The authors wish to thank the Research Funds of Wuhan International Cooperation (Grant No. 201171034316) for financial support.
Conflict of interests:
The authors declared no conflict of interest.
References
- Oh EJ, Park K, Kim KS, Kim J, Yang JA, Kong JH, et al. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J Control Release 2010;141(1):2-12.
[Crossref] [Google scholar] [PubMed]
- Bildstein L, Dubernet C, Couvreur P. Prodrug-based intracellular delivery of anticancer agents. Adv Drug Deliv Rev 2011;63(2):3-23.
[Crossref] [Google scholar] [PubMed]
- Alexander A, Saraf S, Saraf S. A comparative study of chitosan and poloxamer based thermosensitive hydrogel for the delivery of PEGylated melphalan conjugates. Drug Dev Ind Pharm 2015;41(12):1954-61.
[Crossref] [Google scholar] [PubMed]
- Bourguignon LY, Zhu H, Chu A, Iida N, Zhang L, Hung MC. Interaction between the adhesion receptor, CD44 and the oncogene product, p185HER2, promotes human ovarian tumor cell activation. J Biol Chem 1997;272(44):27913-8.
[Crossref] [Google scholar] [PubMed]
- Pan Y, Du ZW, Leng WC, Zhou JW, Wang YJ, Sheng MJ, et al. MicroRNA-mediated silencing of RhoC inhibits tumor invasion and increases chemosensitivity to paclitaxel in SKOV3 cells in vitro. Chem Res Chin Univ 2011;27(1):70-4.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci 2007;80(21):1921-43.
[Crossref] [Google scholar] [PubMed]
- Lenormand H, Deschrevel B, Vincent JC. pH effects on the hyaluronan hydrolysis catalysed by hyaluronidase in the presence of proteins: Part I. Dual aspect of the pH-dependence. Matrix Biol 2010;29(4):330-7.
[Crossref] [Google scholar] [PubMed]
- Chen J, Fan D, Xu Z, Gu Q. Research on rheological property of hyaluronic acid derivative cross-linked by polyethylene glycol. J Tongji Univ 2006;34(1):111-5.
- Luo Y, Prestwich GD. Hyaluronic acid-N-hydroxysuccinimide: A useful intermediate for bioconjugation. Bioconjug Chem 2001;12(6):1085-8.
[Crossref] [Google scholar] [PubMed]
- Arimura H, Ouchi T, Kishida A, Ohya Y. Preparation of a hyaluronic acid hydrogel through polyion complex formation using cationic polylactide-based microspheres as a biodegradable cross-linking agent. J Biomater Sci Polym Ed 2005;16(11):1347-58.
[Crossref] [Google scholar] [PubMed]
- Wieland JA, Houchin-Ray TL, Shea LD. Non-viral vector delivery from PEG-hyaluronic acid hydrogels. J Control Release 2007;120(3):233-41.
[Crossref] [Google scholar] [PubMed]
- Chen J, Chen J, Xu Z. Rheological and biological characteristics of hyaluronic acid derivative modified by polyethylene glycol. J Wuhan Univ Technol Mater Sci Ed 2008;23(5):617-21.
- Brightman K, Finlay G, Jarvis I, Knowlton T, Manktelow CT. A stability-indicating method for the determination of melphalan and related impurity content by gradient HPLC. J Pharm Biomed Anal 1999;20(3):439-47.
[Crossref] [Google scholar] [PubMed]
- Maroda M, Bodnár M, Berkó S, Bakó J, Erős G, Csányi E, et al. Preparation and investigation of a cross-linked hyaluronan nanoparticles system. Carbohydr Polym 2011;83(3):1322-9.
- Shen Y, Li Q, Tu J, Zhu J. Synthesis and characterization of low molecular weight hyaluronic acid-based cationic micelles for efficient siRNA delivery. Carbohydr Polym 2009;77(1):95-104.
- Hahn SK, Oh EJ, Miyamoto H, Shimobouji T. Sustained release formulation of erythropoietin using hyaluronic acid hydrogels crosslinked by Michael addition. Int J Pharm 2006;322(2):44-51.
[Crossref] [Google scholar] [PubMed]
- Ouasti S, Donno R, Cellesi F, Sherratt MJ, Terenghi G, Tirelli N. Network connectivity, mechanical properties and cell adhesion for hyaluronic acid/PEG hydrogels. Biomaterials 2011;32(27):6456-70.
[Crossref] [Google scholar] [PubMed]
- Lima-Sousa R, de Melo-Diogo D, Alves CG, Costa EC, Ferreira P, Louro RO, et al. Hyaluronic acid functionalized green reduced graphene oxide for targeted cancer photothermal therapy. Carbohydr Polym 2018;200:93-9.
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990;61(7):1303-13.
[Crossref] [Google scholar] [PubMed]
- Tirella A, Kloc-Muniak K, Good L, Ridden J, Ashford M, Puri S, et al. CD44 targeted delivery of siRNA by using HA-decorated nanotechnologies for KRAS silencing in cancer treatment. Int J Pharm 2019;561:114-23.
[Crossref] [Google scholar] [PubMed]
- Jose G, Lu YJ, Chen HA, Hsu HL, Hung JT, Anilkumar TS, et al. Hyaluronic acid modified bubble-generating magnetic liposomes for targeted delivery of doxorubicin. J Magn Magn Mater 2019;474:355-64.
- Zhong L, Xu L, Liu Y, Li Q, Zhao D, Li Z, et al. Transformative hyaluronic acid-based active targeting supramolecular nanoplatform improves long circulation and enhances cellular uptake in cancer therapy. Acta Pharm Sin B 2019;9(2):397-409.
- Long H, Wu Z, Dong Q, Shen Y, Zhou W, Luo Y, et al. Effect of polyethylene glycol on mechanical properties of bamboo fiber‐reinforced polylactic acid composites. J Appl Polym Sci 2019;136(26):47709.