- *Corresponding Author:
- J. L. Sun
Department of Anesthesia, Affiliated Hangzhou First People's Hospital, School of Medicine, Westlake University, Hangzhou 310006, China
E-mail: sjl6805@zju.edu.cn
| This article was originally published in a special issue, “New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “48-55” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The main aim of this study was to evaluate the safety and efficacy of ropivacaine in combination with varying concentrations of nalbuphine for epidural labor analgesia at reduced doses. A randomized, double-blind, prospective experimental design was used to enroll healthy full-term women who requested epidural labor analgesia at academic medical centers specializing in maternal and child health care. Participants were randomly assigned to one of the five groups with different concentrations of nalbuphine, which includes group A (0 mg/ml), group B (0.3 mg/ml), group C (0.4 mg/ml), group D (0.5 mg/ml) and group E (0.6 mg/ml), with a sample size of 30 patients per group. The effectiveness of the analgesic was defined by the visual analog scale, where score of <3 in 30 min after induction, using the Dixon-Massey sequential method. The median effective concentration of epidural ropivacaine was calculated using the Bonferroni test and the overlapping confidence interval method. Basic maternal profile data, median effective concentration, neonatal outcomes, obstetric aspects, and adverse effects were collected 30 min after injecting epidural local anesthetic. The median effective concentration values in groups C, D and E were significantly lower than those compared to groups A and B. There was no statistically significant difference in median effective concentration values between groups C, D and E. There were no statistically significant differences between the groups in neonatal outcomes, obstetric aspects and adverse effects. Ropivacaine combined with a nalbuphine concentration of 0.4 mg/ml showed the greatest clinical effect, with no additional benefit beyond 0.4 mg/ml and the combined efficacy was efficient, and the safety profile was high.
Keywords
Ropivacaine, nalbuphine, epidural labor analgesia, median effective concentration
Vaginal birth or a natural delivery is a type of birth in which the mother utilizes her own labor force and contractions to deliver the fetus. This process can be demanding, causing intense pain and discomfort due to the pressure on the pubic bone. The physical and mental strain of a natural delivery can have negative impacts on both the mother and the baby's well-being and physiology[1,2]. With the popularization of the concept of comfort care, the rate of labor analgesia has become a new indicator in medical quality management, and the clinical demand for labor analgesia as an aid to labor and to relieve maternal pain has increased significantly[3]. Therefore, efficient analgesic protocols have become a medical issue of great concern.
Uterine and vaginal contractions during labor can cause pain, where contraction pain appears as a strong pulling sensation in the smooth muscle of the uterus, often resulting in visceral pain[4]. Data from studies have shown that the degree of effectiveness of labor analgesia depends on the type, dose concentration of the epidural injection of local anesthetic, the mode of administration and whether it is combined with opioids or not[5,6]. Currently, lumbar epidural block is considered as the most effective and least intrusive labor analgesics for mother and baby, but significant side effects are observed when high concentration of doses are used[7,8]. Ropivacaine, as a long-acting amide local anesthetic, exerts inhibition of nerve conduction by modulating sodium flow in nerve cells, with little effect on motor function[9]. Low-concentration ropivacaine is used in labor analgesic drug dosing regimens, and it is often used in combination with opioids in order to further improve analgesic efficacy. It has been suggested that nalbuphine is a partial antagonist of mu (μ) receptors, an agonist of gamma (γ) receptors, highly selective for kappa (κ) receptors in the brain and spinal cord, highly effective in suppressing visceral pain, reducing opioid concentrations in labor analgesia, and improving maternal adverse effects[10,11]. Nalbuphine combined with ropivacaine analgesic regimens have been reported[12] but studies on the effect of different concentrations of nalbuphine on labor analgesia in combined regimens are scarce.
In order to investigate the clinical effect of nalbuphine combined with ropivacaine as lumbar epidural block during labor, this paper determines the safety and efficacy of the median Effective Concentration (EC50) of ropivacaine, to find and provide the best reference drug for clinical optimization.
Materials and Methods
Research design:
The start date of the study is January 15, 2021 (started research designing and collecting literature review). The study was approved by Ethics committee of Hangzhou First People’s Hospital (IIT-20210907-0032-01) and was registered in the Chinese Clinical Trial Registry on October 15, 2021 (No: ChiCTR2100052044). A randomized, double-blind, prospective experimental design was adopted to randomly select healthy full-term women who requested epidural labor analgesia at an academic medical center specializing in maternal and child health care, and a randomization protocol was generated using Microsoft Excel (Microsoft Corporation, Redmond, Washington, United States of America (USA)). The 1st patient was enrolled on October 30, 2021 and the study was ended in November 15, 2022. The subjects were randomly assigned to one of the five groups of different concentrations of nalbuphine in sequentially numbered separate opaque envelopes. The sample size was selected as 30 patients per group with nalbuphine dose as group A (0 mg/ml), B (0.3 mg/ml), C (0.4 mg/ml), D (0.5 mg/ml) and E (0.6 mg/ml). Neither the study participants nor the investigators responsible for outcome assessment were aware of the subgroup data during the implementation of the experiment. Prior to enrollment, each woman was fully informed by the investigator regarding the purpose, nature, procedure, and possible benefits and risks of the study. The subjects were fully informed to participate voluntarily and signed an informed consent form to begin the study. All information regarding the identity of the subjects was kept confidential and was not disclosed to the public beyond the scope of relevant laws and regulations.
Inclusion criteria:
Patients with grade I or II score of American Society of Anesthesiologists (ASA) classification[13]; age 20 y to 35 y; height 150-170 cm; Body Mass Index (BMI) 24-30 kg/m²; full-term singleton pregnancy (gestational week≥37 w) in primigravida or menstruating women; latent cervical dilatation 2-5 cm during labor and patients with normal heart, liver, kidney, blood and other examination indices are included in the study.
Exclusion criteria:
Patients with primary disease of important organs; maternal refusal to cooperate with endospinal puncture, e.g., history of mental illness; presence of contraindications to endospinal block, such as infection or injury at the puncture site, disruption of coagulation, severe spinal deformity and increased intracranial pressure and patients who are allergic reaction to experimental drugs are excluded from the study.
Termination criteria:
Visual Analogue Score[14] (VAS) of ≥3 after 30 min of administration was assessed as ineffective analgesia, 6 ml of 1 % lidocaine was given epidurally and the VAS was assessed after 15 min, if the score was still ≥3 the catheter was considered suspicious and the woman's data were excluded from the study analysis.
Research methods:
Operation of epidural anesthesia: The anesthesia nurse gave the anesthesiologist the uniform configuration of the maternal medication for each group according to the experimental grouping, and neither the patient nor the anesthesiologist was informed of the randomization results. The maternal upper limb venous access was opened and nasal oxygen was administered (oxygen flow rate 2-3 l/min). Maternal vital signs like Electrocardiogram (ECG), noninvasive blood pressure, temperature, respiration and pulse oximetry and fetal heartbeat were monitored throughout the procedure. The epidural operation was performed in the left lateral position, and a reinforced epidural catheter was inserted by puncture at the L2-L3 interval, with the length of 3-5 cm in the external lumen. The epidural catheter was connected to a syringe to withdraw blood and cerebrospinal fluid, and then 3 ml of 1 % lidocaine containing epinephrine (1:200 000) was injected as the test dose, and the catheter was observed for 5 min to determine whether the catheter had been placed into the vessels and whether there was a reaction to total spinal anesthesia.
Analgesic drugs and grouping: Ropivacaine hydrochloride injection (specification 100 mg per 10 ml, manufactured by AstraZeneca AB, Sweden) and Nalbuphine hydrochloride injection (specification 2 ml: 20 mg, produced by Hubei Yichang Renfu Pharmaceutical Co.).
A random number table was used to randomly divide the women who met the inclusion criteria into five groups A, B, C, D and E according to the analgesic regimen. Group A (0.1 % ropivacaine solution, 100 ml), on the basis of group A, groups B, C, D and E were combined with 0.3, 0.4, 0.5 and 0.6 mg/ml nalbuphine mixture respectively.
Drug delivery method: The drug was administered according to the Dixon-Massey sequential method. The 1st patient in each group was given a concentration of the drug according to the initial analgesic protocol, and the criterion for effective analgesia was defined as a VAS of <3 after 30 min of analgesic induction. The 1st patient in each group started the sequence with 0.1 % ropivacaine, and if the analgesia was effective then the concentration gradient of ropivacaine was decreased by one and vice versa, with a concentration gradient of 0.01 %. If the analgesia is ineffective, then 6 ml of 1 % lidocaine is given epidurally and the VAS is assessed after 15 min. If the analgesia is still ≥3 points then the analgesia is ineffective and the current concentration of ropivacaine is still used in the next patient. In case of sudden onset of severe pain during labor, which is not relieved by self-controlled analgesia, it is called explosive pain. Then the anesthesiologist first observes the catheter dislodgement and fracture, and injects 10 ml of 0.2 % ropivacaine epidurally until VAS is <3. After labor, the analgesic pump is stopped and the epidural catheter is removed. During labor analgesia, if hypotension (systolic blood pressure <20 % of the basal value or absolute value <90 mmHg) occurs, adjust the maternal position, administer fluids, and give phenylephrine, ephedrine and other vasoconstrictive drugs if necessary after excluding obstetric factors. If maternal nausea and vomiting occur, blood pressure should be measured immediately, and hypotension should be corrected promptly if it occurs. Metoclopramide and 5-Hydroxytryptamine (5-HT3) receptor antagonists can also be given.
Observed indicators:
Basic maternal information: Age, weight, height, gestational week, maternal VAS score and cervical canal dilatation; maternal sensory block (assessed using sterile cotton balls), motor block (assessed using Bromage score)[15] after 30 min of the analgesia protocol (where patients with a score of 0 can move all leg joints on their own, patients with a score of 1 can bend their knees and ankles, patients with a score of 2 can move only their ankles, and patients with a score of 3 have lost motor function in their leg joints); blood pressure (absolute value <90 mmHg defined as hypotension) and heart rate (<60 BPM defined as bradycardia), and fetal heart rate (<110 BPM defined as bradycardia), respiratory depression (oxygen saturation <90 %); maternal sedation[16] (awake with stress response defined as no sedation, awake but fatigued and sleepy defined as mild sedation, moderate sedation if awake, and severe sedation if not awake) and VAS scores; duration of the 1st and 2nd stage of labor; pH value of the umbilical artery of the newborn; Appearance, pulse, grimace, activity, and respiration (Apgar) score at 1 min and 5 min after birth; mode of delivery (vaginal vs. cesarean) and maternal nausea and vomiting, skin pruritus, and other adverse effects.
Statistical analysis:
The sample size was estimated and processed according to the pre-experimental results using GraphPad Prism version 5.0 (GraphPad Software Inc., San Diego, California, USA) and Statistical Package for Social Sciences (SPSS) version 22.0 (Internation Business Management Corp, Armonk, New York, USA) software. The Kolmogorov-Smirnov method was applied to test for normality of continuous data. Normally distributed data were presented as mean±Standard Deviation (SD) and analyzed using one-way Analysis of Variance (ANOVA). Non-normally distributed data were presented as median (interquartile range) and were tested using the Kruskal-Wallis test. Categorical data were presented as number (%), and analyzed using the Cochran-Armitage trend test and Chi-square (χ²) test. The difference of EC50 values was analyzed using one-way analysis, and the post Bonferroni test was used for pairwise comparison and a difference of p<0.05 were considered as statistically significant.
Results and Discussion
Basic maternal data was compared among the five groups as shown in Table 1. General data such as age, height, weight, gestational age, pain score (VAS) and cervical dilatation were compared among the groups (p>0.05) and were not statistically significant.
| Parameters | Group A (n=30) | Group B (n=30) | Group C (n=30) | Group D (n=30) | Group E (n=30) |
|---|---|---|---|---|---|
| Age (years) | 27±2 | 28±2 | 27±3 | 28±3 | 28±2 |
| Height (cm) | 161±4 | 160±4 | 160±5 | 161±5 | 159±6 |
| Weight (kg) | 70±7 | 70±8 | 69±8 | 71±8 | 70±8 |
| Gestational age (weeks) | 40±1 | 41±1 | 39±1 | 40±1 | 41±1 |
| VAS score (0-10) | 7 (5,8) | 7 (5,9) | 7 (6,9) | 7 (6,8) | 6 (5,8) |
| Cervical dilation (cm) | 3 (2,3) | 3 (2,3) | 3 (2,3) | 3 (2,3) | 3 (2,3) |
Note: Data are presented as mean±standard deviation or median (interquartile range)
Table 1: Comparison of basic maternal demographic data among the five groups.
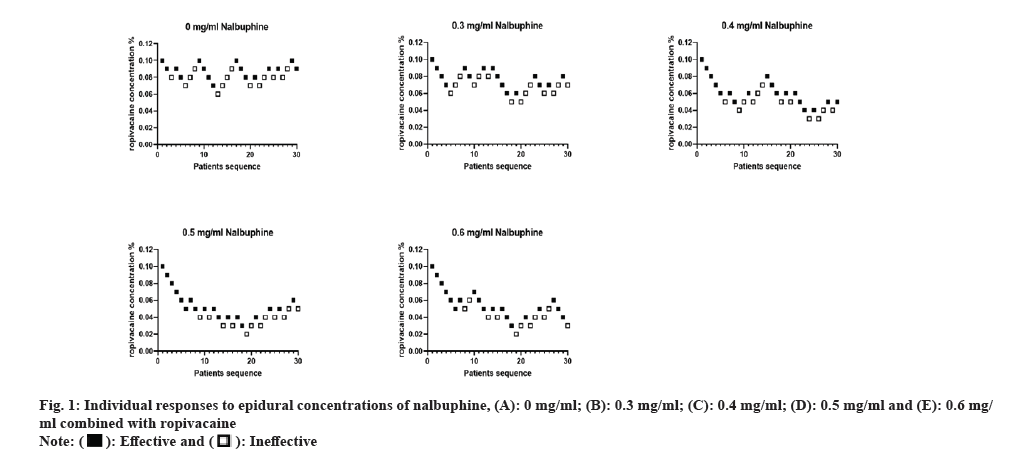
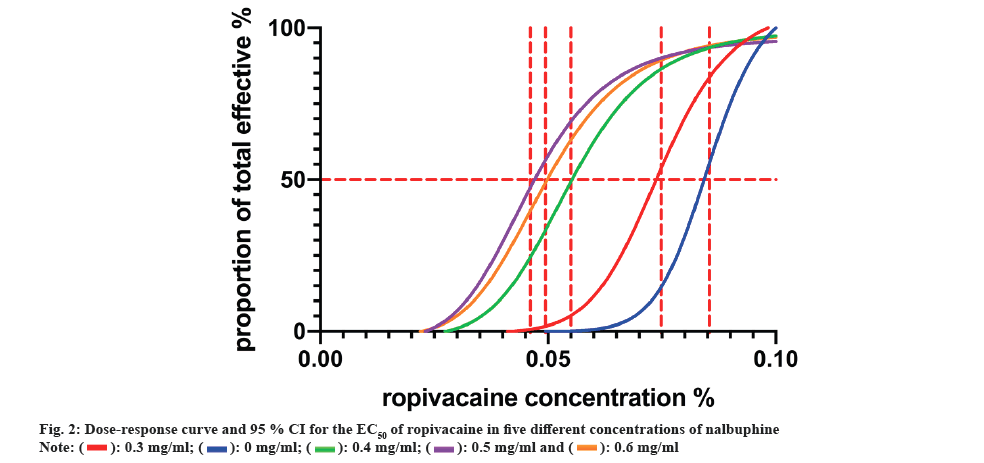
The results of the prospective study according to the Dixon-Massey sequential method are reported in fig. 1 and the EC50 values are shown in Table 2. Sensitivity tests were calculated using Bonferroni test and overlapping Confidence Interval (CI) method, and we found lower EC50 values in groups B, C, D and E compared with group A (p<0.05). There was no statistically significant difference in EC50 values between groups C, D and E (p>0.05). It was reasoned that the EC50 values of ropivacaine combined with nalbuphine were 0 mg/ml and 0.3 mg/ml. Dose response curves are shown in fig. 2. The EC50 values for epidural ropivacaine in each of the 5 randomized groups are calculated by probit analysis.
| Group (n=30) | EC50 |
|---|---|
| A | 0.082 % (95 % CI, 0.073 % to 0.090 %) |
| B | 0.071 % (95 % CI, 0.059 % to 0.081 %) |
| C | 0.050 % (95 % CI, 0.029 % to 0.061 %) |
| D | 0.041 % (95 % CI, 0.031 % to 0.049 %) |
| E | 0.042 % (95 % CI, 0.024 % to 0.051 %) |
Table 2: Comparison of EC50 among the five groups.
There were no statistically significant differences among the groups 30 min after administration of analgesic protocol in terms of sensory block, motor block, incidence of hypotension, maternal and fetal bradycardia, respiratory depression, maternal sedation, VAS scores, duration of 1st and 2nd stage of labor, neonatal umbilical artery pH, Apgar scores at 1 min and 5 min or mode of delivery, nausea and vomiting, and skin pruritus (p>0.05). None of the patients had maternal bradycardia, respiratory depression, or severe maternal sedation (Table 3).
| Characteristics | Group A (n=30) | Group B (n=30) | Group C (n=30) | Group D (n=30) | Group E (n=30) | p |
|---|---|---|---|---|---|---|
| Sensory block (dermatome level) | T8 (T7-T9) | T8 (T6-T9) | T8 (T6-T9) | T8 (T7-T9) | T8 (T6-T9) | 0.792 |
| Motor block (Bromage score=0) | 30 | 30 | 30 | 30 | 30 | - |
| Hypotension | 1 (3.33 %) | 2 (6.67 %) | 1 (3.33 %) | 3 (10.00 %) | 1 (3.33 %) | 0.715 |
| Maternal bradycardia | 0 | 0 | 0 | 0 | 0 | - |
| Fetal bradycardia | 1 (3.33 %) | 0 (0.00 %) | 1 (3.33 %) | 2 (6.67 %) | 2 (6.67 %) | 0.657 |
| Respiratory depression | 0 | 0 | 0 | 0 | 0 | - |
| Severe maternal sedation | 0 | 0 | 0 | 0 | 0 | - |
| VAS score (0-10) | 1 (0, 2) | 2 (1, 3) | 2 (1, 4) | 2 (1, 4) | 1 (0, 2) | 0.673 |
| Labor, 1st stage (min) | 428 (245-612) | 401 (306-478) | 357 (289-450) | 478 (292-538) | 452 (353-504) | 0.316 |
| Labor, 2nd stage (min) | 48 (27-87) | 54 (39-92) | 52 (34-68) | 44 (32-74) | 57 (43-72) | 0.619 |
| Umbilical artery pH | 7.29±0.08 | 7.28±0.09 | 7.30±0.09 | 7.31±0.08 | 7.29±0.09 | 0.717 |
| Apgar score, 1 min | 10 (9-10) | 10 (9-10) | 10 (9-10) | 10 (9-10) | 10 (9-10) | 0.956 |
| Apgar score, 5 min | 10 (9-10) | 10 (9-10) | 10 (9-10) | 10 (9-10) | 10 (9-10) | 0.901 |
| Cesarean delivery rate | 1 (3.33 %) | 0 (0.00 %) | 1 (3.33 %) | 0 (0.00 %) | 1 (3.33 %) | 0.728 |
| Nausea or vomiting | 4 (13.33 %) | 3 (10.00 %) | 2 (6.67 %) | 2 (6.67 %) | 3 (10.00 %) | 0.894 |
| Pruritus | 3 (10.00 %) | 2 (6.67 %) | 1 (3.33 %) | 2 (6.67 %) | 2 (6.67 %) | 0.899 |
Note: Data are presented as number (%), mean±standard deviation or median (interquartile range)
Table 3: Comparison of exploratory outcomes among the five groups.
"Fear of unknown pain in natural childbirth" is a key factor influencing natural childbirth and causing an increase in the rate of cesarean deliveries[17]. Labor pain causes a strong psychological and organic stress response, and excessive stress can weaken maternal energy reserves during labor, and prolonged pain can activate inflammatory transmitter release and oxidative stress, which can affect labor performance and pose a threat to maternal and infant health[18]. Epidural ropivacaine combined with small doses of opioids is a common clinical tool to reduce maternal labor pain[19]. We propose further experiments on how ropivacaine combined with different concentrations of nalbuphine for epidural labor analgesia can achieve an optimal ratio because of the different pain intensity and nature of labor pain in women.
In the present study, we counted the EC50 values in ropivacaine combined with different concentrations of nalbuphine and found that the EC50 values in groups C, D and E were significantly lower than those in groups A and B. The comparison of EC50 values between groups C, D and E was not statistically significant. It has been shown that the higher nalbuphine concentration in epidural anesthesia is more significant in sensory and motor blockade[20,21]. Combining the results of this study suggests that the lowest concentration of ropivacaine combined with nalbuphine for maximum effect in the clinic is 0.4 mg/ml, and that side effects may be less in the minimum effective concentration combination regimen. The opioid-produced adverse reactions to epidural labor analgesia have attracted attention, but little is known about the exact mechanism of the reduction in EC50 of epidural ropivacaine combined with nalbuphine. Our study found no significant differences in blood pressure and heart rate, respiratory depression, maternal sedation and VAS scores among the groups after 30 min of administration of the analgesic regimen. Nalbuphine has analgesic and sedative effects via agonism of κ receptors on spinal ganglia, brings antagonism to μ receptors, suppresses visceral pain and increases the duration of analgesia, suppresses the respiratory system to a lesser extent and does not alter its hemodynamic changes[22,23]. It is suggested that combined nalbuphine may reduce the maternal need for analgesia and that the clinical application is more advantageous at a combined concentration of 0.4 mg/ml. It has also been shown that labor analgesia using nalbuphine may cross the placental barrier when administered intravenously, with the potential to reduce fetal heart rate[24]. However, this theory needs further study. Therefore, we designed a study comparing neonatal outcomes between the groups and found no significant differences. It is speculated that the intraspinal dose is less than the intravenous dose, and a small amount of transplacental barrier prevents the occurrence of potential risks. With increasing nalbuphine concentration, there were no significant differences between the groups in the mode of delivery and in the duration of each stage of labor, umbilical artery blood pH, and Apgar scores at 1 min and 5 min after birth for mothers who delivered spontaneously. Significant changes in serum and amniotic fluid can be observed close to delivery, while estrogen reduces the analgesic effect of μ agonists, and pain in the 2nd stage of labor due to factors such as rapid dilatation of the uterus and vagina or assisted labor with instruments which can be effectively reduced by implementing an analgesic regimen with reasonable administration of anesthetic self-administered doses[25,26]. The effectiveness and non-differentiation of the analgesic effect of ropivacaine combined with different concentrations of nalbuphine in labor analgesia was suggested, and no serious adverse effects of the drug on maternal labor outcome or neonates were found. However, it has been suggested that there is an effect of common doses of nalbuphine on neonatal Apgar scores[27]. It is speculated that it may be related to factors such as the mode of anesthesia and sample size. The cesarean delivery rate was lower in all groups in our study, and there was no significant difference in the mode of delivery between the groups. It is speculated that this may be related to the strict standardization of criteria such as BMI, degree of cervical dilatation, vaginal assisted delivery method used by hospital obstetricians and indications for contraindication to cesarean delivery. Therefore, the low cesarean delivery rate in our study population was expected. We found no significant differences in maternal nausea and vomiting, pruritus and other side effects between the groups. It has been shown that the use of low-concentration nalbuphine can suppress nausea and vomiting, and pruritus reactions caused by opioids[28,29]. Overall, low concentration of nalbuphine compounded with ropivacaine in maternal labor analgesia can better reduce maternal stress response. However, there were shortcomings in our study; the sample size designed was not large enough to significantly express differences when measuring differences in EC50 values.
In conclusion, the analgesic effect of ropivacaine combined with nalbuphine in the epidural administration regimen for labor analgesia was significant, with the best analgesic effect at a concentration of 0.4 mg/ml, and there were no significant differences in neonatal outcome, maternal outcome and complications, with definite combined efficacy, safety and effectiveness.
Funding:
This study was supported by the Zhejiang Health Science and Technology Plan (Grant number: 2021KY860).
Conflict of interests:
The authors declared no conflict of interests.
References
- Benfield RD, Hortobágyi T, Tanner CJ, Swanson M, Heitkemper MM, Newton ER. The effects of hydrotherapy on anxiety, pain, neuroendocrine responses, and contraction dynamics during labor. Biol Res Nurs 2010;12(1):28-36.
[Crossref] [Google scholar] [PubMed]
- Deussen AR, Ashwood P, Martis R, Stewart F, Grzeskowiak LE. Relief of pain due to uterine cramping/involution after birth. Cochrane Database Syst Rev 2020;10(10):1-130.
[Crossref] [Google scholar] [PubMed]
- Nadjafizadeh M, Caron FM. Normal childbirth: Physiologic labor support and medical procedures. Guidelines of the French National Authority for Health (HAS) with the collaboration of the French College of Gynaecologists and Obstetricians (CNGOF) and the French College of Midwives (CNSF)-Newborn care in the delivery room. Gynecol Obstet Fertil Senol 2020;48(12):907-16.
[Crossref] [Google scholar] [PubMed]
- Biem SR, Turnell RW, Olatunbosun O, Tauh M, Biem HJ. A randomized controlled trial of outpatient vs. inpatient labour induction with vaginal controlled-release prostaglandin-E2: Effectiveness and satisfaction. J Obstet Gynaecol Can 2003;25(1):23-31.
[Crossref] [Google scholar] [PubMed]
- Cederholm I. Preliminary risk-benefit analysis of ropivacaine in labour and following surgery. Drug Saf 1997;16(6):391-402.
[Crossref] [Google scholar] [PubMed]
- Cai S, Zheng J, Meng Q, Chai J, Ma R, Wang Y, et al. Investigation of the minimum local analgesic concentration of epidural sufentanil combined with ropivacaine for labor analgesia. Clin Ther 2020;42(1):210-9.
[Crossref] [Google scholar] [PubMed]
- Anim‐Somuah M, Smyth RM, Cyna AM, Cuthbert A. Epidural vs. non‐epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev 2018;5(5):1-153.
[Crossref] [Google scholar] [PubMed]
- Parpaglioni R, Frigo MG, Sebastiani M, Lemma A, Barbati G, Celleno D. High volume of subarachnoid levobupivacaine decreases drug requirement in first stage labor analgesia. Minerva Anestesiol 2004;70(12):809-21.
[Google scholar] [PubMed]
- Dregalla RC, Uribe Y, Bodor M. Effect of local anesthetics on platelet physiology and function. J Orthop Res 2021;39(12):2744-54.
[Crossref] [Google scholar] [PubMed]
- Inan S, Torres-Huerta A, Jensen LE, Dun NJ, Cowan A. Nalbuphine, a kappa opioid receptor agonist and mu opioid receptor antagonist attenuates pruritus, decreases IL-31, and increases IL-10 in mice with contact dermatitis. Eur J Pharmacol 2019;864:1-41.
[Crossref] [Google scholar] [PubMed]
- Liu YY, Hsiao HT, Wang JC, Liu YC, Wu SN. Effectiveness of nalbuphine, a κ‐opioid receptor agonist and μ‐opioid receptor antagonist, in the inhibition of INa, IK(M), and IK(erg) unlinked to interaction with opioid receptors. Drug Dev Res 2019;80(6):846-56.
[Crossref] [Google scholar] [PubMed]
- Wang C, Sun S, Jiao J, Yu X, Huang S. Effects of nalbuphine on the cardiotoxicity of ropivacaine in rats. Fundam Clin Pharmacol 2022;36(5):811-7.
[Crossref] [Google scholar] [PubMed]
- Kannan T. ASA grading: A step forward. J Perioper Pract 2017;27(3):54-9.
[Crossref] [Google scholar] [PubMed]
- Chiarotto A, Maxwell LJ, Ostelo RW, Boers M, Tugwell P, Terwee CB. Measurement properties of visual analogue scale, numeric rating scale, and pain severity subscale of the brief pain inventory in patients with low back pain: A systematic review. J Pain 2019;20(3):245-63.
[Crossref] [Google scholar] [PubMed]
- Craig D, Carli F. Bromage motor blockade score-A score that has lasted more than a lifetime. Can J Anaesth 2018;65(7):837-8.
[Crossref] [Google scholar] [PubMed]
- Huang BB, Niu SK. The effectiveness and safety of ropivacaine and medium-dose dexmedetomidine in cesarean section. Evid Based Complement Alternat Med 2022;2022:1-8.
[Crossref] [Google scholar] [PubMed]
- Mohyadin E, Ghorashi Z, Molamomanaei Z. The effect of practicing yoga during pregnancy on labor stages length, anxiety and pain: A randomized controlled trial. J Complement Integr Med 2020;18(2):413-7.
[Crossref] [Google scholar] [PubMed]
- Tsuzuki Y, Yamashita Y, Hattori Y, Li GH, Akatsuka S, Kotani T, et al. Pain-reducing anesthesia prevents oxidative stress in human term placenta. J Clin Biochem Nutr 2016;58(2):156-60.
[Crossref] [Google scholar] [PubMed]
- Roelants F. The use of neuraxial adjuvant drugs (neostigmine, clonidine) in obstetrics. Curr Opin Anaesthesiol 2006;19(3):233-7.
[Crossref] [Google scholar] [PubMed]
- Schoppmann S, Spiess D, Müller D, Burch A, Zimmermann R, Simões-Wüst AP. Nalbuphine: A candidate for treatment of women overwhelmed with sudden, intense labor pain? J Matern Fetal Neonatal Med 2022;35(25):6112-4.
[Crossref] [Google scholar] [PubMed]
- Mattout HK, Fouda SM. The use of topical nalbuphine in different concentrations to control pain after photorefractive keratectomy. Int Ophthalmol 2022;42(7):2145-53.
[Crossref] [Google scholar] [PubMed]
- Ortiz MI, Cariño-Cortés R, Castañeda-Hernández G. Participation of the opioid receptor-nitric oxide-cGMP-K+ channel pathway in the peripheral antinociceptive effect of nalbuphine and buprenorphine in rats. Can J Physiol Pharmacol 2020;98(11):753-62.
[Crossref] [Google scholar] [PubMed]
- Rawal N, Wennhager M. Influence of perioperative nalbuphine and fentanyl on postoperative respiration and analgesia. Acta Anaesthesiol Scand 1990;34(3):197-202.
[Crossref] [Google scholar] [PubMed]
- Wilson CM, McClean E, Moore J, Dundee JW. A double‐blind comparison of intramuscular pethidine and nalbuphine in labour. Anaesthesia 1986;41(12):1207-13.
[Crossref] [Google scholar] [PubMed]
- Wang Y, Chen Q, Xu S, Chao S. Obstetric risk factors and serological characteristics of early-onset neonates bacterial infections. Front Surg 2022;9:1-8.
[Crossref] [Google scholar] [PubMed]
- Micevych PE, Rissman EF, Gustafsson JÅ, Sinchak K. Estrogen receptor‐α is required for estrogen‐induced μ‐opioid receptor internalization. J Neurosci Res 2003;71(6):802-10.
[Crossref] [Google scholar] [PubMed]
- Vavrinková B, Binder T, Horák J. Nalbuphine at maternal analgesia. Ceska Gynekol 2010;75(6):564-6.
[Google scholar] [PubMed]
- Hawi A, Alcorn H, Berg J, Hines C, Hait H, Sciascia T. Pharmacokinetics of nalbuphine hydrochloride extended release tablets in hemodialysis patients with exploratory effect on pruritus. BMC Nephrol 2015;16(1):1-10.
[Crossref] [Google scholar] [PubMed]
- He K, Ji W, Zhao H, Wei Y, Yang S, Wen Q. Pharmacokinetic comparison of nalbuphine with single injection and patient‐controlled analgesia mimic method in healthy Chinese volunteers. J Clin Pharm Ther 2021;46(4):1166-72.
[Crossref] [Google scholar] [PubMed]
 .
.
 .
.



