- *Corresponding Author:
- J. Liu
Department of Integrated Chinese and Western Medicine,
Jiangsu Health Vocational College,
Nanjing 210029,
China
E-mail: liujia402@163.com
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3) Spl Issue “152-161” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Drug resistance is the leading cause of death in prostate cancer patients. Therefore, exploring the mechanism underlying drug resistance of prostate cancer and identifying novel therapeutic targets are urgently needed. Long non-coding ribonucleic acid disrupted in renal carcinoma 3 acts as a tumor suppressor gene in several tumor types. However, to date, no study has investigated the biological function of long non-coding ribonucleic acid disrupted in renal carcinoma 3 in prostate cancer. Gene expression was detected by reverse transcription-quantitative polymerase chain reaction assay and Western blot. Cell viability was detected by cell counting kit-8 assay and cell apoptosis was analyzed by flow cytometry. The interactions between long non-coding ribonucleic acid and microRNA, microRNA and messengerRNA were analyzed by bioinformatics analysis and confirmed by a dual-luciferase reporter gene assay. Long non-coding ribonucleic acid disrupted in renal carcinoma 3 was down-regulated in drug-resistant prostate cancer tissues and cell lines. Over expression of long non-coding ribonucleic acid disrupted in renal carcinoma 3 overcome the drug resistance of PC-3/R and DU-145/R to docetaxel by down-regulating drugresistance related lung resistance protein, P-glycoprotein and multidrug resistance-associated protein. Long non-coding ribonucleic acid disrupted in renal carcinoma 3 sponged microRNA-24-3p in PC-3/R and DU-145/R to up-regulate KIT ligand. MicroRNA-24-3p/KIT ligand mediated the effect of long noncoding ribonucleic acid disrupted in renal carcinoma 3 overexpression on docetaxel-resistance of prostate cancer cells. Long non-coding ribonucleic acid disrupted in renal carcinoma 3 overcome drug resistance of docetaxel-tolerated prostate cancer cells to docetaxel via the microRNA-24-3p/KIT ligand axis. Our study revealed that long non-coding ribonucleic acid disrupted in renal carcinoma 3 was a potential therapeutic target in overcoming docetaxel resistance for prostate cancer patients.
Keywords
Micro RNA-24-3p/KIT ligand, prostate cancer, docetaxel, Western blot
As one of the most prevalent malignant tumors, Prostate Cancer (PCa) has a high degree of aggressiveness and leads to great deaths in the male population[1]. The most common etiology of PCa is gene mutations or epigenetic alterations in the Androgen Receptor (AR) signaling or growth pathway activation[2,3]. Therefore, Androgen Deprivation Therapies (ADT) could stop the response of PCa to androgen and inhibit the aggressive progression[3]. However, although most patients benefit from ADT treatment, PCa cancer will evolve to have castration resistance after 2-3 y[4,5]. At this stage, AR targeted next generation drugs, such as enzalutamide and abiraterone and chemical drugs Docetaxel (DTX) and cabazitaxel are the only options for PCa patients. But the drug resistance for these drugs is also a prominent problem. Therefore, identifying new targets to inhibit the progression of PCa and overcome drug resistance is urgently needed.
In the past decade, long non-coding Ribonucleic Acid (lncRNA) have been demonstrated to participate in various physiological and pathological processes and display irreplaceable functions, such as embryonic development, organism aging and tumorigenesis[6,7]. Transcriptome sequencing and function investigation have identified numerous lncRNAs that are aberrantly expressed in multiple tumor types and play crucial roles in tumor cell proliferation, differentiation, metastasis and drug resistance[7,8]. As for PCa, reports have found that some lncRNAs play important roles in the progression.
For example, HOXD cluster Antisense RNA 1 (HOXD- AS1) is up-regulated in Castration Resistant Prostate Cancer (CRPC) and closely correlated with an enhanced disease progression[9]. Knockdown of HOXD-AS1 restores chemo-sensitivity by recruiting WD Repeat Domain 5 (WDR5) to regulate chemo-resistance- related genes[9]. Knockdown of HOXA Transcript at the Distal Tip (HOTTIP) inhibits the inactivation of Wnt/Beta (β)-catenin pathway in PCa, further inducing proliferation, inhibition and enhancing cell sensitivity to cisplatin[10]. However, our knowledge of lncRNA function in PCa still remains very poor. Therefore, it is greatly beneficial to identify novel lncRNAs involved in PCa progression and chemo-resistance.
LncRNA Disrupted in Renal Carcinoma 3 (DIRC3), located at chromosome 2q35, is firstly identified in 2003 to form a DIRC3-HSPB1 Associated Protein 1 (HSPBAP1) fusion transcript in renal cell cancer, which may be involved in regulating chromatin remodeling and stress response signals[11]. The subsequent study reveals that lncRNA DIRC3 inhibits the proliferation and metastasis of Differentiated Thyroid Cancers (DTC) and melanoma by activating the expression of its neighboring Insulin-Like Growth Factor Binding Protein 5 (IGFBP5), a tumor suppressor that regulates chromosomal remodeling[12,13]. Despite these advances, the function and action mechanism of lncRNA DIRC3 in other tumor types, such as PCa, are still largely unknown.
The present study investigated the expression of lncRNA DIRC3 in drug-sensitive and resistant PCa tumor tissues and cells. Gain-of-function experiment was performed to assess the effects of lncRNA DIRC3 on PCa cell proliferation and DTX resistance. Finally, the down-stream micro RNA (miRNA)-messenger RNA (mRNA) signals network of lncRNA DIRC3 was also explored. Our results suggested that overexpression of lncRNA DIRC3 in PCa could restore the DTX sensitivity in PCa chemotherapy.
Materials and Methods
Tumor tissues collection:
Seventy patients with PCa were recruited from Jiangsu Province Hospital of Chinese medicine. The patients received DTX based chemotherapy without any other treatment. According to the treatment outcome, PCa patients included the DTX resistant group (n=34) and the DTX sensitive group (n=36). Tumor tissues were collected and subsequently stored in liquid nitrogen. These experiments were approved by the ethics committee of Jiangsu Province Hospital of Chinese medicine (No. NL-129-02) and patients signed informed consent documents.
Cell culture:
The PCa cell lines, PC3 and DU145 (American Type Culture Collection) were maintained in Modified Eagle Medium (DMEM) (Gibco, United States of America (USA)) with 10 % fetal bovine serum (Gibco) at 37° and 5 % Carbon dioxide (CO2). To generate DTX-resistant PCa cells, PC3 and DU145 cells were incubated with stepwise increased doses of DTX for 6 mo as described in a previous study[14]. In brief, the initial concentration of DTX was 5 nmol/l. The medium was replaced every 2-3 d. When drug resistance occurred, live cells were continuously maintained and drug concentration was stepwise increased to 80 nmol/l. Finally, PCa cells were maintained in 10 nm paclitaxel to keep drug resistance.
Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR):
Trizol (Takara, Dalian, China) agents were used for RNA extraction from cells. Then about 1 µg of total RNA was transformed to complementary Deoxyribonucleic Acid (cDNA) by a Superscript™ III transcriptase (Invitrogen). RT-qPCR reaction was performed by a fluorescence quantitative PCR kit (Takara, Dalian, China). Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) or U6 was used as an internal control. Data was analyzed using 2−ΔΔCt method[15]. The primers used in this study were as follows:
DIRC3-F:5’-CCCACCCCACCACTATTCAC-3’; DIRC3-R: 5’-CCACTGGGACAACCATCTCC-3’; microRNA-24-3p (miR-203a-3p)-F: 5’-GGGGTGAAATGTTTAGGAC-3’;miR-203a- 3p-R: 5’-CAGTGCGTGTCGTGGAGT-3’; KITLG-F: 5’-AGCCTTATACTGGAAGAAGAGAC-3’; KITLG-R: 5’-GTTGATACAAGCCACAATTACAC-3’; GAPDH-F: 5’-GACCCCTTCATTGACCTCAAC-3’; GAPDH-R: 5’-CTTCTCCATGGTGGTGAAGA-3’; U6-F: 5’-GCTTCGGCAGCACATATACTAAAAT-3’ and U6-R: 5’-CGCTTCACGAATTTGCGTGTCAT-3’.
Cell transfection:
MPC-Negative Control (NC), pc-DIRC3, NC mimic, miR-203a-3p mimic, NC inhibitor, miR-203a-3p inhibitor and pc-KITLG were purchased from Gene Pharma (Shanghai, China). These vectors were transfected into PC-3/R and DU-145/R cells using Lipofectamine™ 3000 (Invitrogen, USA) according to the instructions.
Cell Counting Kit-8 (CCK-8) assay:
PC-3/R and DU-145/R cells were transfected as above for 48 h. Then cells were digested and suspended into DMEM medium at a density of 2×104 cells/ml. 100 µl of cell solution was added into each well of 96-well plates. For drug cytotoxicity assay, PC-3/R and DU- 145/R cells were maintained in 30 nm of DTX. After 48 h, 10 µl of CCK-8 reagent was added into each well of 96-well plate and incubated at 37° for 1 h. The absorbance at 490 nm was detected.
Flow cytometry analysis:
The transfected PC-3/R and DU-145/R cells were detected by an Annexin V-Enhanced Green Fluorescent Protein (V-EGFP) apoptosis detection kit (Beyotime, Haimen, China)[10]. In brief, 1×106 cells were suspended into 1 ml of binding buffer. Then cells were stained with 5 µl of Annexin V-Fluorescein Isothiocyanate (V-FITC) and 5 µl of Propidium iodide (PI) for 30 min in the dark. The apoptotic cells were separated using flow cytometry.
Western blot analysis:
The protocol of Western blot was described in a previous study[16]. After cell transfection and DTX incubation, total proteins of PC-3/R and DU-145/R cells were extracted by Radioimmunoprecipitation Assay (RIPA) lysis buffer. Protein separation was performed using Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) electrophoresis and the bands were transferred onto Polyvinylidene Fluoride (PVDF) membranes. The protein membranes were blocked and incubated with primary antibody at 4° for 12 h. The primary antibodies contained anti- Lung Resistance Protein 1 (LRP1) (1:1000, ab92544, Abcam, USA), anti-P-glycoprotein (P-gp) (1:1000, ab261736, Abcam, USA), anti-Multidrug Resistance- Associated Protein 1 (MRP1) (1:1000, ab233 383, Abcam, USA) and anti-β-actin (1:1000, ab8226, Abcam, USA). The secondary antibodies (1:10 000, Abcam, USA) was incubated with protein bands for 1 h at room temperature. The visualization of proteins was performed by an Enhanced Chemiluminescence (ECL) detection system (GE Healthcare).
Dual-luciferase reporter gene assay:
We constructed a dual-luciferase reporter gene plasmid
containing the Wild Type (WT) and mutated binding sites on lncRNA DIRC3. Briefly, PC-3/R and DU-145/R cells were co-transfected with miR-203a-3p mimic and pGL3 Luciferase (pGL3-Luc) plasmid containing the potential binding sites of lncRNA DIRC3 located at the upstream of a firefly luciferase expression vector. NC mimic was used as a NC. The luciferase activity was then measured using a dual-luciferase assay kit (Promega, USA). Relative luciferase activity was calculated as firefly/Renilla luciferase activities. The interaction between miR-203a-3p and KITLG was detected as similar as above. The relative luciferase activity was calculated as firefly/Renilla luciferase activity[17].
Statistical analysis:
All experimental data were expressed as mean±Standard Deviation (SD) from three independent experiments. The difference was analyzed using a student’s t-test or a one-way analysis of variance. p<0.05 was considered a significant difference.
Results and Discussion
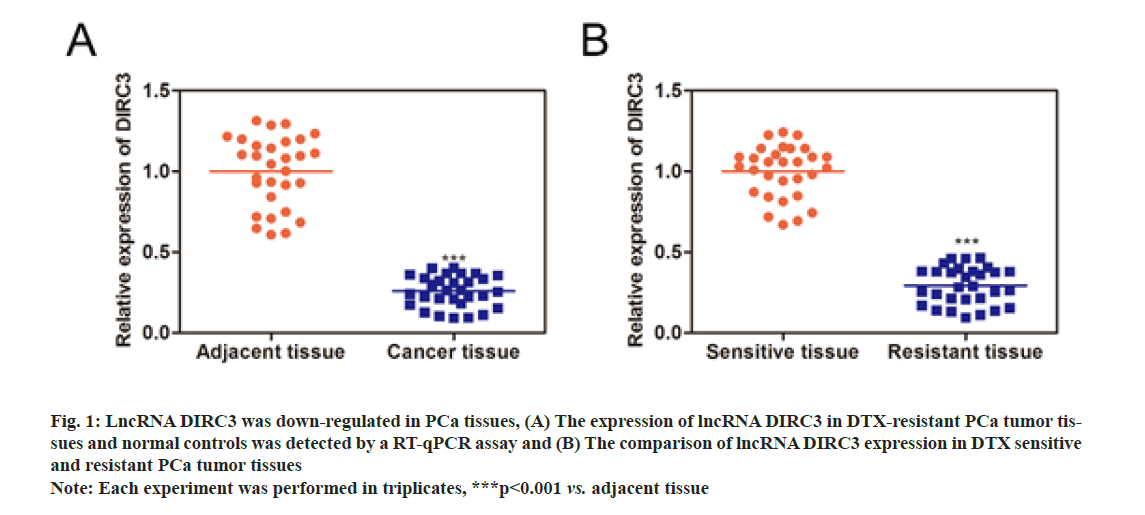
As shown in fig. 1A, lncRNA DIRC3 expression was significantly decreased in PCa tissues, compared with normal controls. Furthermore, we detected the expression of lncRNA DIRC3 in drug-sensitive and resistant PCa tissues to examine the association between lncRNA DIRC3 and drug resistance in PCa (fig. 1B). The results showed that, compared with drug-sensitive tissues, lncRNA DIRC3 was highly expressed in drug- resistant tissues.
Fig. 1: LncRNA DIRC3 was down-regulated in PCa tissues, (A) The expression of lncRNA DIRC3 in DTX-resistant PCa tumor tissues and normal controls was detected by a RT-qPCR assay and (B) The comparison of lncRNA DIRC3 expression in DTX sensitive and resistant PCa tumor tissues
Note: Each experiment was performed in triplicates, ***p<0.001 vs. adjacent tissue
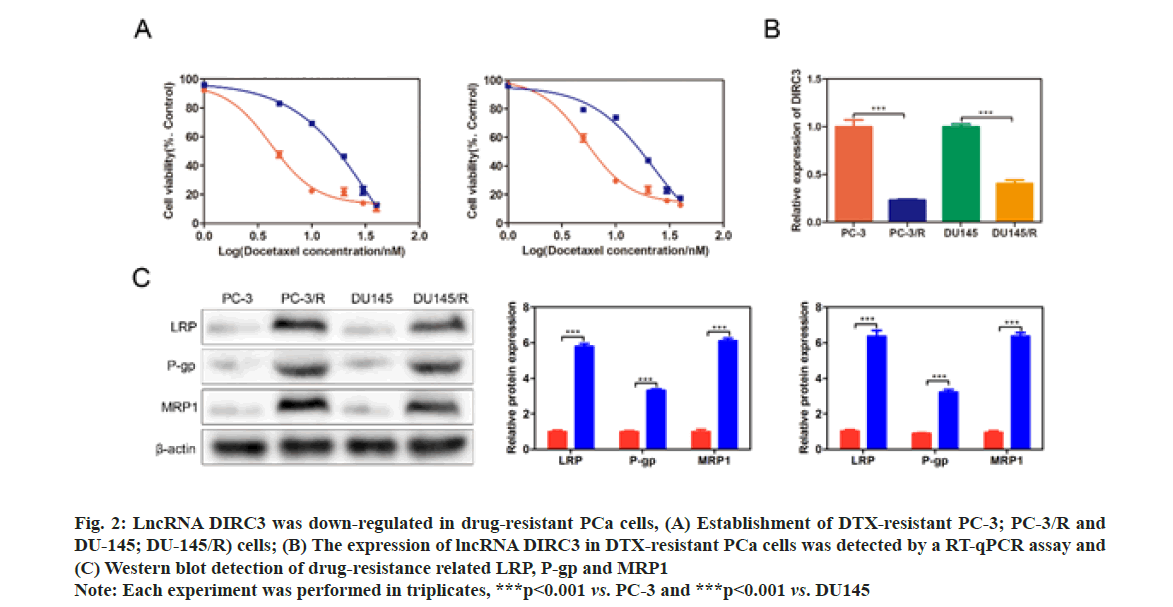
The Half-Maximal Inhibitory Concentration (IC50) DTX of PC-3/R and DU-145/R were respectively at 8 and 4 fold higher than their parental cells, demonstrating a successful establishment of drug-resistant PCa cell lines (fig. 2A). qRT-PCR results showed that lncRNA DIRC3 was significantly down-regulated in PC-3/R and DU-145/R cells, compared with their control groups (p<0.001, fig. 2B). Furthermore, we found that drug-resistance related genes, including LRP, P-gp and MRP1, were significantly increased in PC-3/R and DU-145/R cells, compared with their control groups (p<0.05, fig. 2C).
Fig. 2: LncRNA DIRC3 was down-regulated in drug-resistant PCa cells, (A) Establishment of DTX-resistant PC-3; PC-3/R and DU-145; DU-145/R) cells; (B) The expression of lncRNA DIRC3 in DTX-resistant PCa cells was detected by a RT-qPCR assay and (C) Western blot detection of drug-resistance related LRP, P-gp and MRP1
Note: Each experiment was performed in triplicates, ***p<0.001 vs. PC-3 and ***p<0.001 vs. DU145
Taken together, these results suggested that drug- resistant PCa tissues and cell lines displayed a low expression of lncRNA DIRC3.
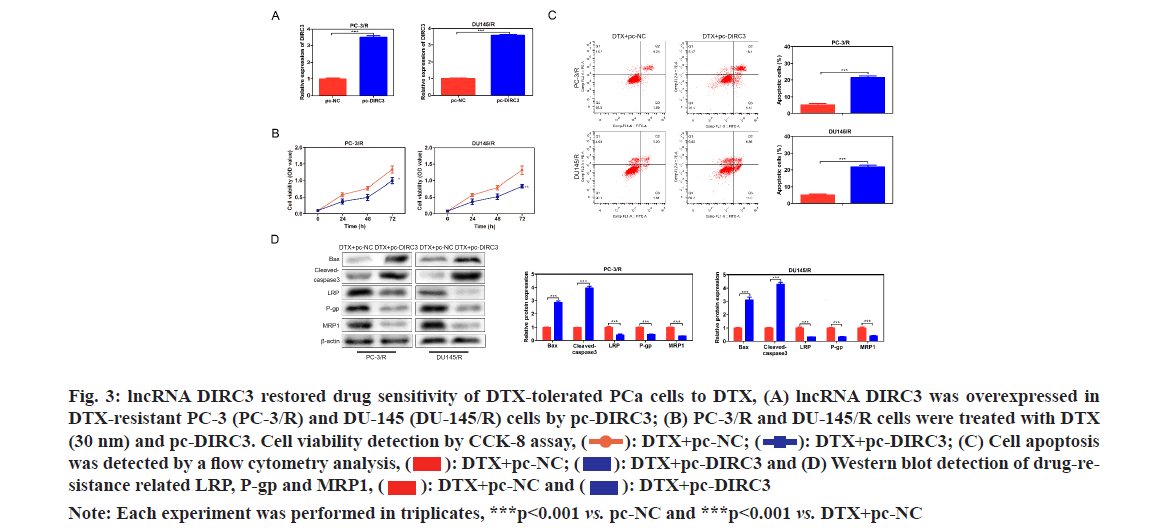
In order to evaluate whether lncRNA DIRC3 affected the drug assistance of PCa cells, lncRNA DIRC3 was amplified in PC-3/R and DU-145/R cells using a plasmid cloning DNA (pcDNA) 3.1 vector as shown in fig. 3A. Cell proliferation analysis showed that, below 40 nm of DTX, the cell viability of the pc- DIRC3 group was decreased than that in the NC group as shown in fig. 3B. The cell apoptosis rate of the pc-DIRC3 group was approximately 20 %, while the NC group was 6 % as shown in fig. 3C. To evaluate whether the newly acquired DTX sensitivity was associated with drug-resistance related genes, we detected their expression using Western blot assay. The results showed that overexpression of lncRNA DIRC3 led to a significant decrease of drug-resistance related genes LRP, P-gp and MRP1, companied by an increase of pro-apoptosis proteins Bcl-2-Associated X Protein (BAX) and cleaved-caspase 3 as shown in fig. 3D. These results suggested that overexpression of lncRNA DIRC3 restored DTX sensitivity of PCa cells by down- regulating LRP, P-gp and MRP1.
Fig. 3: lncRNA DIRC3 restored drug sensitivity of DTX-tolerated PCa cells to DTX, (A) lncRNA DIRC3 was overexpressed in DTX-resistant PC-3 (PC-3/R) and DU-145 (DU-145/R) cells by pc-DIRC3; (B) PC-3/R and DU-145/R cells were treated with DTX (30 nm) and pc-DIRC3. Cell viability detection by CCK-8 assay,  DTX+pc-DIRC3; (C) Cell apoptosis was detected by a flow cytometry analysis,
DTX+pc-DIRC3; (C) Cell apoptosis was detected by a flow cytometry analysis,  DTX+pc-DIRC3 and (D) Western blot detection of drug-resistance related LRP, P-gp and MRP1,
DTX+pc-DIRC3 and (D) Western blot detection of drug-resistance related LRP, P-gp and MRP1, 
Note: Each experiment was performed in triplicates, ***p<0.001 vs. pc-NC and ***p<0.001 vs. DTX+pc-NC
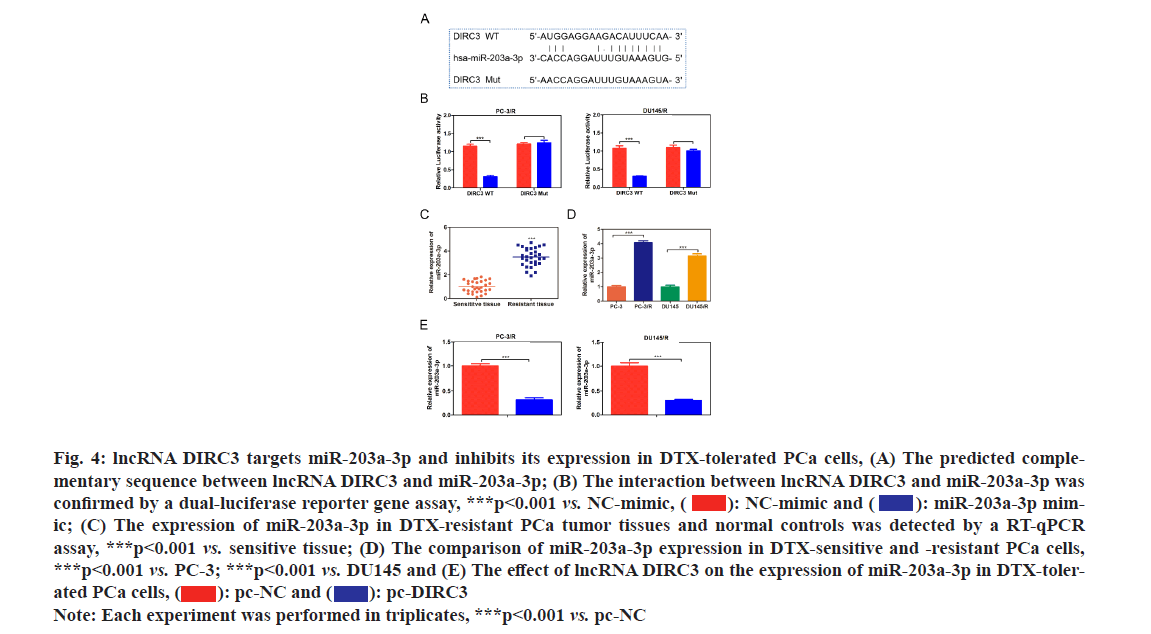
Various studies have revealed that lncRNAs could indirectly regulate protein expression by sponging downstream miRNAs and impeding their function[18-20]. The predicted binding sites between lncRNA DIRC3 and miR-203a-3p as shown in fig. 4A. Then we constructed luciferase expression vectors containing WT or mutant binding site of lncRNA DIRC3 and co-transfected them with NC mimic or miR-203a-3p mimic into HEK293T cells. The results showed that miR-203a-3p mimic could significantly reduce the luciferase activity of WT lncRNA DIRC3, but had no effect on mutant lncRNA DIRC3 as shown in fig. 4B, which demonstrated the direct interaction between lncRNA DIRC3 and miR-203a-3p. Furthermore, compared with normal controls, miR-203a-3p was significantly up-regulated in drug-resistant PCa tumor tissues and cell lines as shown in fig. 4C and fig. 4D. Moreover, overexpression of lncRNA DIRC3 resulted in a decrease of miR-203a-3p in PCa cells as shown in fig. 4E. These results suggested that miR-203a-3p was a direct target of lncRNA DIRC3 in PCa cells.
Fig. 4: lncRNA DIRC3 targets miR-203a-3p and inhibits its expression in DTX-tolerated PCa cells, (A) The predicted complementary sequence between lncRNA DIRC3 and miR-203a-3p; (B) The interaction between lncRNA DIRC3 and miR-203a-3p was confirmed by a dual-luciferase reporter gene assay, ***p<0.001 vs. NC-mimic,  miR-203a-3p mimic; (C) The expression of miR-203a-3p in DTX-resistant PCa tumor tissues and normal controls was detected by a RT-qPCR assay, ***p<0.001 vs. sensitive tissue; (D) The comparison of miR-203a-3p expression in DTX-sensitive and -resistant PCa cells, ***p<0.001 vs. PC-3; ***p<0.001 vs. DU145 and (E) The effect of lncRNA DIRC3 on the expression of miR-203a-3p in DTX-tolerated PCa cells,
miR-203a-3p mimic; (C) The expression of miR-203a-3p in DTX-resistant PCa tumor tissues and normal controls was detected by a RT-qPCR assay, ***p<0.001 vs. sensitive tissue; (D) The comparison of miR-203a-3p expression in DTX-sensitive and -resistant PCa cells, ***p<0.001 vs. PC-3; ***p<0.001 vs. DU145 and (E) The effect of lncRNA DIRC3 on the expression of miR-203a-3p in DTX-tolerated PCa cells,
Note: Each experiment was performed in triplicates, ***p<0.001 vs. pc-NC
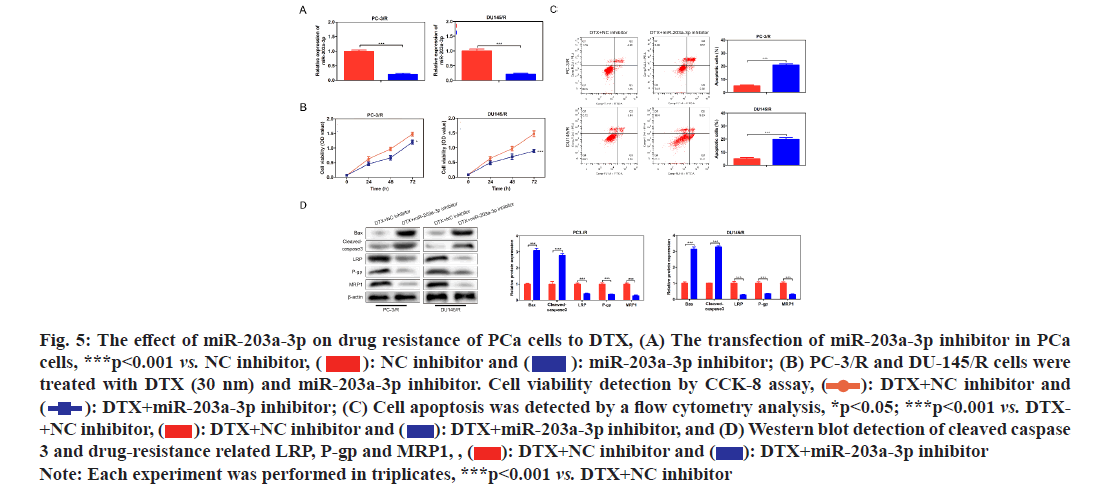
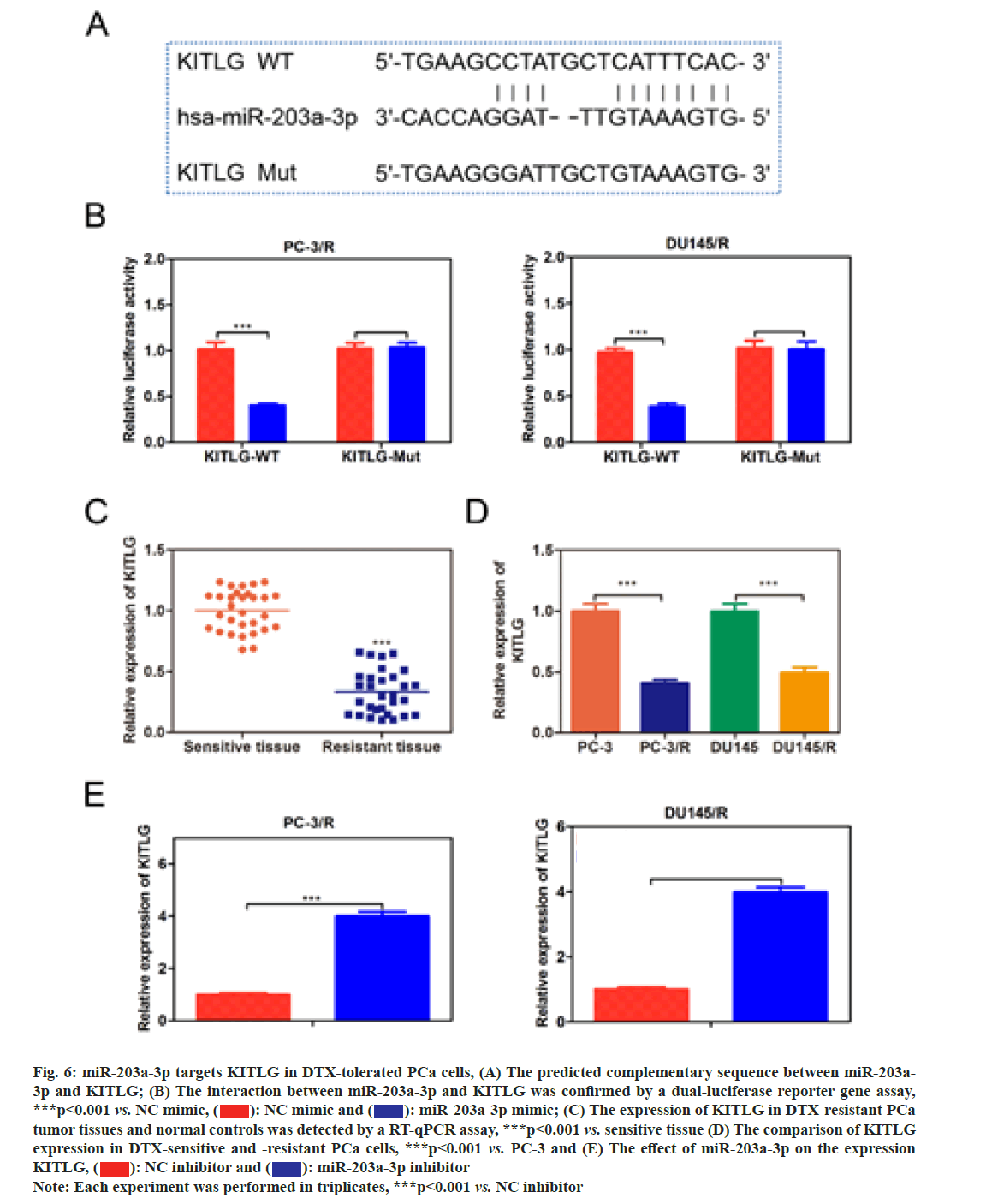
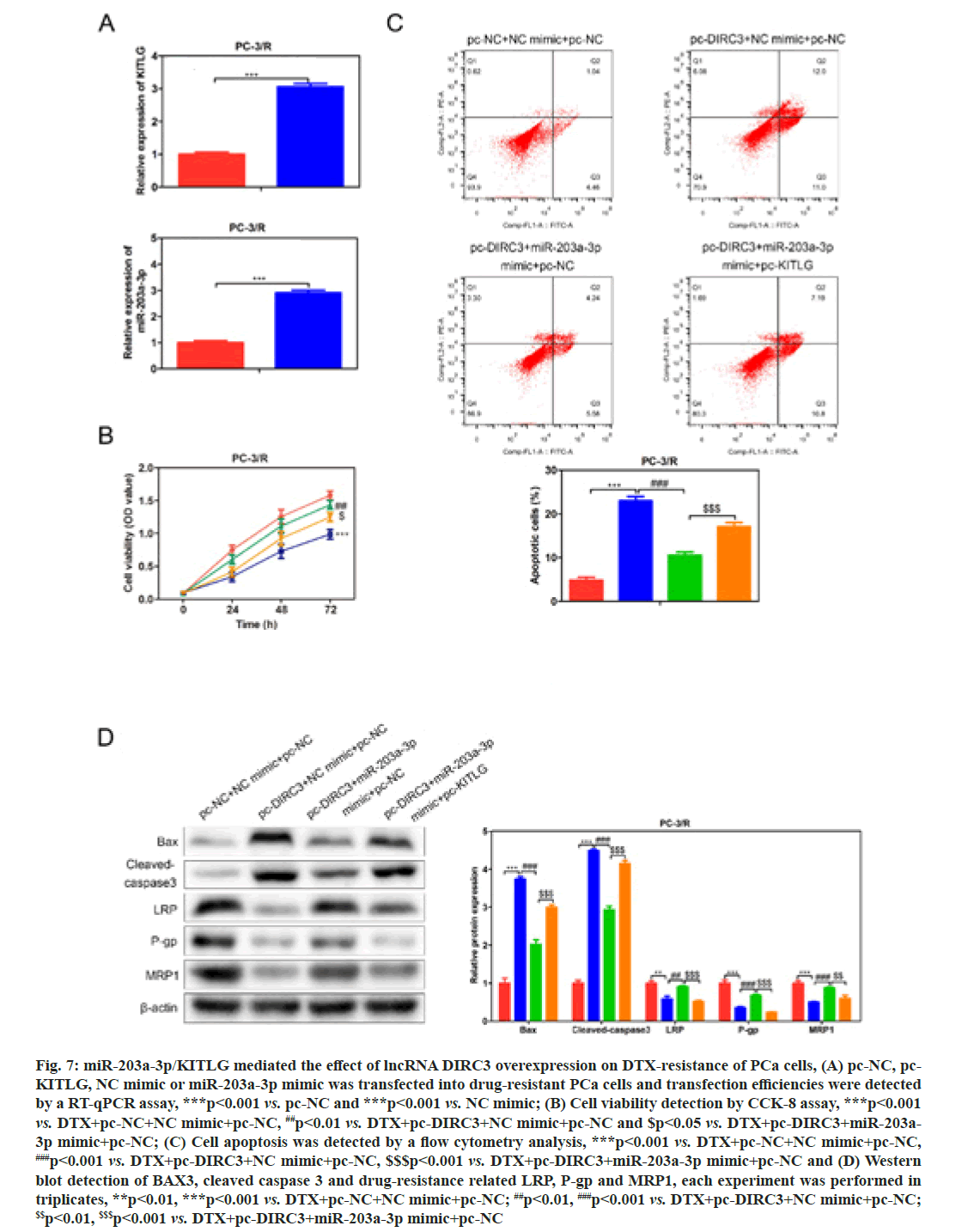
In order to investigate whether miR-203a-3p affected the DTX resistance in PCa cells, we synthesized a miRNA inhibitor targeting miR-203a-3p in PC-3/R and DU-145/R cells as shown in fig. 5A. Survival analysis results suggested that, below 40 nm of DTX, the cell viability of the miR-203a-3p inhibitor group was significantly decreased (fig. 5B, p<0.05). Furthermore, flow cytometry analysis results indicated that the apoptosis percentage of DTX+NC group was 6 %, while the DTX+miR-203a-3p inhibitor increased to -20 % (fig. 5C, p<0.001). MiR-203a-3p inhibitor also restored the DTX sensitivity of PC-3/R and DU- 145/R cells. In terms of action mechanism, we found that miR-203a-3p inhibitor inhibited the protein expression of LRP, P-gp and MRP1, companied by increased pro-apoptosis proteins BAX and cleaved- caspase 3 as shown in fig. 5D. It is well known that miRNAs regulate cellular function by binding to the 3’ Untranslated Regions (3’UTR) region of target mRNA and impeding its translation. The star base prediction indicated that KITLG had a complementary sequence with miR-203a-3p at its 3’UTR region as shown in fig. 6A. Then we verified this interaction by co-transfecting WT or mutant-binding site of KITLG with miR-203a- 3p mimic or NC mimic into PCa cells. Dual-luciferase reporter assay suggested that miR-203a-3p mimic could significantly decrease the fluorescence activity of WT- KITLG report gene, but had no effect on mutant-KITLG as shown in fig. 6B. Analysis of clinical samples showed that, compared with drug-sensitive tissues, KITLG was significantly down-regulated in drug-resistant tissues as shown in fig. 6C. Besides, KITLG expression was significantly decreased in PC-3/R and DU-145/R cells, compared with drug-sensitive PCa cells as shown in fig. 6D. Finally, the Western blot assay demonstrated that miR-203a-3p inhibitor significantly increased KITLG expression in PC-3/R and DU-145/R cells as shown in fig. 6E. Taken together, these results suggested that miR-203a-3p could target KITLG and inhibit its expression in PCa cells. To investigate the role of miR- 203a-3p/KITLG in lncRNA DIRC3-regulated DTX- resistance of PCa cells, we overexpressed miR-203a-3p and KITLG in lncRNA DIRC3-expressed PC-3/R and DU-145/R cells (fig. 7A, p<0.001). The results showed that, below 40 nm of DTX, pc-DIRC3 transfection inhibited cell viability compared with NC group. In comparison, co-transfection of pc-DIRC3 and miR- 203a-3p mimic restored cell viability (fig. 7B, p<0.001). Furthermore, cell viability was further decreased when pc-DIRC3, miR-203a-3p mimic and pc-KITLG were co-transfected into PCa cells with DTX resistance (fig. 7B, p<0.05). Cell apoptosis percentage of pc-DIRC3 group was ~21 % (NC group, 8 %) and co-transfection of pc-DIRC3 and miR-203a-3p mimic decreased cell apoptosis to ~12 % (fig. 7C, p<0.05). Overexpression of KITLG restores cell apoptosis to ~17 % on the basis of pc-DIRC3 and miR-203a-3p mimic (fig. 7C, p<0.05). Western blot assays also suggested that miR- 203a-3p mimic partly reversed the effects of pc-DIRC3 expression on drug-resistance related genes LRP, P-gp and MRP1 and pro-apoptosis proteins BAX and cleaved-caspase 3 (fig. 7D, p<0.05). Overexpression of KITLG reduced the expression of LRP, P-gp and MRP1 and increased BAX and cleaved caspase 3 on the basis of pc-DIRC3 and miR-203a-3p mimic (fig. 7D, p<0.05). Taken together, miR-203a-3p/KITLG could reverse the effects of lncRNA DIRC3 overexpression on DTX resistance of PCa cells. The tumor-suppressive functions of lncRNA DIRC3 have been reported in several tumor types. In melanoma, Microphthalmia associated Transcription Factor (MITF)-SRY-Box Transcription Factor 10 (SOX10) acts as an important transcription factor that regulates the expression of multiple signal proteins controlling melanocyte development and malignant transformation[21]. LncRNA DIRC3, as a down-stream target gene repressed by MITF-SOX10, up-regulates the transcription of its adjacent IGFBP5 by repressing the binding of SOX10 on IGFBP5[13]. Subsequently, lncRNA DIRC3-activated IGFBP5 regulates the expression of multiple genes that were involved in the proliferation and metastasis of melanoma cells[13]. Similarly, lncRNA DIRC3 is down-regulated in DTC compared with normal controls and patients with low expression of lncRNA DIRC3 have a higher frequency of capsular invasion or metastasis[12]. Cell model experiments show that lncRNA DIRC3 silencing promotes cell viability, migration and invasiveness of DTC by down-regulating tumor suppressor IGFBP5 and inhibiting Insulin-Like Growth Factor-1 (IGF- 1) signaling pathway[12]. Although great progress has been achieved in the function and action mechanism of lncRNA DIRC3, whether lncRNA DIRC3 is associated with drug resistance in tumor is still unknown. In this study, we reported the tumor suppressor role of lncRNA DIRC3 in PCa. The results showed that lncRNA DIRC3 was down-regulated in DTX resistant PCa tissues compared with DTX-sensitive tissues and overexpression of lncRNA DIRC3 restored DTX sensitivity of PCa cells by inhibiting cell proliferation and inducing apoptosis. Furthermore, drug-resistance related genes, including LRP, P-gp and MRP1, were significantly up-regulated by pc-DIRC3. As far as we know, this is the first study to reveal the positive function of lncRNA DIRC3 in overcoming the drug-resistance in tumor. Numerous studies have demonstrated that lncRNAs regulate the expression of protein-coding genes through interaction with miRNAs[9,22]. LncRNA could serve as a competitive endogenous RNA (ceRNA) to disrupt the binding of miRNA with its target mRNA, therefore relieving the depression of mRNA[9,23,24]. Here we found that lncRNA DIRC3 targets miR-203a-3p and inhibits its expression. Furthermore, miR-203a-3p inhibitor also restored the DTX sensitivity of PC-3/R and DU-145/R cells by inhibiting protein expression of drug-resistance related genes LRP, P-gp and MRP1, companied by increase of pro-apoptosis proteins BAX and cleaved caspase 3. Recent studies have reported that miR-203a-3p is down-regulated in ovarian cancer[25], human non-small-cell lung cancer[26] and nasopharyngeal carcinoma[27], but up-regulated in colorectal cancer[28] and hepatocellular carcinoma[29]. Function investigation reveals that miR-203a-3p inhibits the proliferation and cell cycle progression by inactivating IGF-1R- Protein Kinase B (AKT) signaling pathway in gastric cancer[30]. miR-203a-3p functions as a tumor suppressor in ovarian cancer through regulating AKT/Glycogen Synthase Kinase 3 β (GSK-3β)/snail signaling pathway by targeting Ataxia Telangiectasia Mutated (ATM) [25]. Inversely, another report demonstrates that miR- 203a-3p promotes the progression of colorectal cancer through Phosphodiesterase 4D (PDE4D)[28]. Here we demonstrated that miR-203a-3p inhibitor inhibited the proliferation and induced cell apoptosis of DTX resistant PCa cells, which demonstrated that pro-tumor role of miR-203a-3p in PCa. Furthermore, KITLG is a target of miR-203a-3p in PCa cells. And rescue experiments demonstrated that the antagonistic effect of lncRNA DIRC3 on drug resistance was mediated by miR-203a-3p/KITLG. KITLG have been demonstrated to play an oncogenic function in multiple tumor types[31]. Here our results suggested that overexpression of KITLG inhibited the proliferation and survival of drug-resistant PCa cells, restoring dug sensitivity. In conclusion, our study provided the first evidence that overexpression of lncRNA DIRC3 restored drug sensitivity of DTX- tolerated PC cells to DTX via the miR-203a-3p/KITLG axis, elucidating the action mechanism of how lncRNA DIRC3 affected drug resistance in PCa cells. The results revealed that lncRNA DIRC3 was a potential therapeutic target in overcoming DTX resistance for PCa patients.
Fig. 5: The effect of miR-203a-3p on drug resistance of PCa cells to DTX, (A) The transfection of miR-203a-3p inhibitor in PCa cells, ***p<0.001 vs. NC inhibitor,  miR-203a-3p inhibitor; (B) PC-3/R and DU-145/R cells were treated with DTX (30 nm) and miR-203a-3p inhibitor. Cell viability detection by CCK-8 assay,
miR-203a-3p inhibitor; (B) PC-3/R and DU-145/R cells were treated with DTX (30 nm) and miR-203a-3p inhibitor. Cell viability detection by CCK-8 assay,  DTX+NC inhibitor and
DTX+NC inhibitor and DTX+miR-203a-3p inhibitor; (C) Cell apoptosis was detected by a flow cytometry analysis, *p<0.05; ***p<0.001 vs. DTX- +NC inhibitor,
DTX+miR-203a-3p inhibitor; (C) Cell apoptosis was detected by a flow cytometry analysis, *p<0.05; ***p<0.001 vs. DTX- +NC inhibitor,  DTX+miR-203a-3p inhibitor, and (D) Western blot detection of cleaved caspase 3 and drug-resistance related LRP, P-gp and MRP1, ,
DTX+miR-203a-3p inhibitor, and (D) Western blot detection of cleaved caspase 3 and drug-resistance related LRP, P-gp and MRP1, ,  DTX+miR-203a-3p inhibitor
DTX+miR-203a-3p inhibitor
Note: Each experiment was performed in triplicates, ***p<0.001 vs. DTX+NC inhibitor
Fig. 6: miR-203a-3p targets KITLG in DTX-tolerated PCa cells, (A) The predicted complementary sequence between miR-203a- 3p and KITLG; (B) The interaction between miR-203a-3p and KITLG was confirmed by a dual-luciferase reporter gene assay, ***p<0.001 vs. NC mimic,  miR-203a-3p mimic; (C) The expression of KITLG in DTX-resistant PCa tumor tissues and normal controls was detected by a RT-qPCR assay, ***p<0.001 vs. sensitive tissue (D) The comparison of KITLG expression in DTX-sensitive and -resistant PCa cells, ***p<0.001 vs. PC-3 and (E) The effect of miR-203a-3p on the expression KITLG,
miR-203a-3p mimic; (C) The expression of KITLG in DTX-resistant PCa tumor tissues and normal controls was detected by a RT-qPCR assay, ***p<0.001 vs. sensitive tissue (D) The comparison of KITLG expression in DTX-sensitive and -resistant PCa cells, ***p<0.001 vs. PC-3 and (E) The effect of miR-203a-3p on the expression KITLG, miR-203a-3p inhibitor
miR-203a-3p inhibitor
Note: Each experiment was performed in triplicates, ***p<0.001 vs. NC inhibitor
Fig. 7: miR-203a-3p/KITLG mediated the effect of lncRNA DIRC3 overexpression on DTX-resistance of PCa cells, (A) pc-NC, pc- KITLG, NC mimic or miR-203a-3p mimic was transfected into drug-resistant PCa cells and transfection efficiencies were detected by a RT-qPCR assay, ***p<0.001 vs. pc-NC and ***p<0.001 vs. NC mimic; (B) Cell viability detection by CCK-8 assay, ***p<0.001 vs. DTX+pc-NC+NC mimic+pc-NC, ##p<0.01 vs. DTX+pc-DIRC3+NC mimic+pc-NC and $p<0.05 vs. DTX+pc-DIRC3+miR-203a- 3p mimic+pc-NC; (C) Cell apoptosis was detected by a flow cytometry analysis, ***p<0.001 vs. DTX+pc-NC+NC mimic+pc-NC, ###p<0.001 vs. DTX+pc-DIRC3+NC mimic+pc-NC, $$$p<0.001 vs. DTX+pc-DIRC3+miR-203a-3p mimic+pc-NC and (D) Western blot detection of BAX3, cleaved caspase 3 and drug-resistance related LRP, P-gp and MRP1, each experiment was performed in triplicates, **p<0.01, ***p<0.001 vs. DTX+pc-NC+NC mimic+pc-NC; ##p<0.01, ###p<0.001 vs. DTX+pc-DIRC3+NC mimic+pc-NC; $$p<0.01, $$$p<0.001 vs. DTX+pc-DIRC3+miR-203a-3p mimic+pc-NC
Ethics approval and consent to participate:
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Ethics Committee of Jiangsu Province Hospital of Chinese Medicine (No: NL-129- 02). All patients signed informed consent documents.
Availability of data and material:
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Funding:
This work was supported by Natural Science Foundation of Jiangsu Province (BK20191498).
Acknowledgments:
Jie Zhu and Feng Lei Li have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interest.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65(1):5-29.
[Crossref] [Google Scholar] [Pub Med]
- Wong YN, Ferraldeschi R, Attard G, de Bono J. Evolution of androgen receptor targeted therapy for advanced prostate cancer. Nat Rev Clin Oncol 2014;11(6):365-76.
[Crossref] [Google Scholar] [Pub Med]
- Katzenwadel A, Wolf P. Androgen deprivation of prostate cancer: Leading to a therapeutic dead end. Cancer Lett 2015;367(1):12-7.
[Crossref] [Google Scholar] [Pub Med]
- Tilki D, Schaeffer EM, Evans CP. Understanding mechanisms of resistance in metastatic castration-resistant prostate cancer: The role of the androgen receptor. Eur Urol Focus 2016;2(5):499-505.
[Crossref] [Google Scholar] [Pub Med]
- Lohiya V, Aragon-Ching JB, Sonpavde G. Role of chemotherapy and mechanisms of resistance to chemotherapy in metastatic castration-resistant prostate cancer. Clin Med Insights Oncol 2016;10:57-66.
[Crossref] [Google Scholar] [Pub Med]
- Zhao Z, Sun W, Guo Z, Zhang J, Yu H, Liu B. Mechanisms of lncRNA/microRNA interactions in angiogenesis. Life Sci 2020;254:116900.
[Crossref] [Google Scholar] [Pub Med]
- Wang Y, Fang Z, Hong M, Yang D, Xie W. Long-noncoding RNAs (lncRNAs) in drug metabolism and disposition, implications in cancer chemo-resistance. Acta Pharm Sin B 2020;10(1):105-12.
[Crossref] [Google Scholar] [Pub Med]
- Ghafouri-Fard S, Esmaeili M, Taheri M. H19 lncRNA: Roles in tumorigenesis. Biomed Pharmacother 2020;123:109774.
[Crossref] [Google Scholar] [Pub Med]
- Gu P, Chen X, Xie R, Han J, Xie W, Wang B, et al. lncRNA HOXD-AS1 regulates proliferation and chemo-resistance of castration-resistant prostate cancer via recruiting WDR5. Mol Ther 2017;25(8):1959-73.
[Crossref] [Google Scholar] [Pub Med]
- Jiang H, Xiong W, Chen L, Lv Z, Yang C, Li Y. Knockdown of the long noncoding RNA HOTTIP inhibits cell proliferation and enhances cell sensitivity to cisplatin by suppressing the Wnt/β-catenin pathway in prostate cancer. J Cell Biochem 2019;120(6):8965-74.
[Crossref] [Google Scholar] [Pub Med]
- Bodmer D, Schepens M, Eleveld MJ, Schoenmakers EF, Geurts van Kessel A. Disruption of a novel gene, DIRC3 and expression of DIRC3-HSPBAP1 fusion transcripts in a case of familial renal cell cancer and t (2; 3)(q35; q21). Genes Chromosomes Cancer 2003;38(2):107-16.
[Crossref] [Google Scholar] [Pub Med]
- Wysocki PT, Kolanowska M, Nowis D. 22P Functional characterization of DIRC3 long non-coding RNA in differentiated thyroid cancer. Ann Oncol 2021;32:S367.
- Coe EA, Tan JY, Shapiro M, Louphrasitthiphol P, Bassett AR, Marques AC, et al. The MITF-SOX10 regulated long non-coding RNA DIRC3 is a melanoma tumour suppressor. PLoS Genet 2019;15(12):e1008501.
[Crossref] [Google Scholar] [Pub Med]
- Xue D, Lu H, Xu HY, Zhou CX, He XZ. Long noncoding RNA MALAT1 enhances the docetaxel resistance of prostate cancer cells via miR-145-5p-mediated regulation of AKAP12. J Cell Mol Med 2018;22(6):3223.
[Crossref] [Google Scholar] [Pub Med]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001;25(4):402-8.
[Crossref] [Google Scholar] [Pub Med]
- Luo J, Wang K, Yeh S, Sun Y, Liang L, Xiao Y, et al. LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat Commun 2019;10(1):1-7.
[Crossref] [Google Scholar] [Pub Med]
- Bai T, Liu Y, Li B. LncRNA LOXL1-AS1/miR-let-7a-5p/EGFR-related pathway regulates the doxorubicin resistance of prostate cancer DU-145 cells. IUBMB Life 2019;71(10):1537-51.
- Chen R, Zhou S, Chen J, Lin S, Ye F, Jiang P. LncRNA BLACAT1/miR-519d-3p/CREB1 axis mediates proliferation, apoptosis, migration, invasion and drug-resistance in colorectal cancer progression. Cancer Manag Res 2020;12:13137.
[Crossref] [Google Scholar] [Pub Med]
- Fang Q, Chen X, Zhi X. Long non-coding RNA (LncRNA) urothelial carcinoma associated 1 (UCA1) increases multi-drug resistance of gastric cancer via downregulating miR-27b. Med Sci Monit 2016;22:3506.
- Fu D, Lu C, Qu X, Li P, Chen K, Shan L, et al. LncRNA TTN-AS1 regulates osteosarcoma cell apoptosis and drug resistance via the miR-134-5p/MBTD1 axis. Aging (Albany NY) 2019;11(19):8374.
[Crossref] [Google Scholar] [Pub Med]
- Laurette P, Strub T, Koludrovic D, Keime C, Le Gras S, Seberg H, et al. Transcription factor MITF and remodeller BRG1 define chromatin organisation at regulatory elements in melanoma cells. Elife 2015;4:e06857.
[Crossref] [Google Scholar] [Pub Med]
- Ma Y, Fan B, Ren Z, Liu B, Wang Y. Long noncoding RNA DANCR contributes to docetaxel resistance in prostate cancer through targeting the miR-34a-5p/JAG1 pathway. Onco Targets Ther 2019;12:5485.
[Crossref] [Google Scholar] [Pub Med]
- Liu B, Jiang HY, Yuan T, Zhou WD, Xiang ZD, Jiang QQ, et al. Long non-coding RNA AFAP1-AS1 facilitates prostate cancer progression by regulating miR-15b/IGF1R axis. Curr Pharm Des 2021;27(41):4261-9.
[Crossref] [Google Scholar] [Pub Med]
- Li X, Han X, Wei P, Yang J, Sun J. Knockdown of lncRNA CCAT1 enhances sensitivity of paclitaxel in prostate cancer viaregulating miR-24-3p and FSCN1. Cancer Biol Ther 2020;21(5):452-62.
[Crossref] [Google Scholar] [Pub Med]
- Liu HY, Zhang YY, Zhu BL, Feng FZ, Zhang HT, Yan H, et al. MiR-203a-3p regulates the biological behaviors of ovarian cancer cells through mediating the Akt/GSK-3β/Snail signaling pathway by targeting ATM. J Ovarian Res 2019;12(1):1-3.
[Crossref] [Google Scholar] [Pub Med]
- Liang J, Sun T, Wang G, Zhang H. Clinical significance and functions of miR-203a-3p/AVL9 axis in human non-small-cell lung cancer. Per Med 2020;17(4):271-82.
[Crossref] [Google Scholar] [Pub Med]
- Jiang N, Jiang X, Chen Z, Song X, Wu L, Zong D, et al. MiR-203a-3p suppresses cell proliferation and metastasis through inhibiting LASP1 in nasopharyngeal carcinoma. J Exp Clin Cancer Res 2017;36(1):138.
[Crossref] [Google Scholar] [Pub Med]
- Chen L, Gao H, Liang J, Qiao J, Duan J, Shi H, et al. miR-203a-3p promotes colorectal cancer proliferation and migration by targeting PDE4D. Am J Cancer Res 2018;8(12):2387-401.
- Huo W, Du M, Pan X, Zhu X, Gao Y, Li Z. miR-203a-3p. 1 targets IL-24 to modulate hepatocellular carcinoma cell growth and metastasis. FEBS Open Bio 2017;7(8):1085-91.
[Crossref] [Google Scholar] [Pub Med]
- Wang Z, Zhao Z, Yang Y, Luo M, Zhang M, Wang X, et al. MiR-99b-5p and miR-203a-3p function as tumor suppressors by targeting IGF-1R in gastric cancer. Sci Rep 2018;8(1):1-2.
[Crossref] [Google Scholar] [Pub Med]
- Yang S, Li W, Sun H, Wu B, Ji F, Sun T, et al. Resveratrol elicits anti-colorectal cancer effect by activating miR-34c-KITLG in vitro and in vivo. BMC Cancer 2015;15(1):1.
[Crossref] [Google Scholar] [Pub Med]