- *Corresponding Author:
- Ding Zhu ChenDepartment of Cardiac and Thoracic, Zhangzhou Hospital Affiliated to Fujian Medical University, No. 59, Shenglixi Road, Zhangzhou, Fujian 363000
P. R. China
E-mail: chendingzhufjmu@sohu.com
| This article was originally published in a special issue, “Therapeutic Perspectives in Biomedical Research and Pharmaceutical Sciences and their Nursing Methods” |
| Indian J Pharm Sci 2021:83(4)Spl issue “144-152” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the molecular mechanism of long noncoding RNA cancer susceptibility candidate 9 involved in gefitinib resistance in non-small cell lung cancer cells by regulating microRNA-382-5p/ phosphoinositidedependent protein kinase-1/mammalian target of rapamycin complex 2 signal axis. 10 cases of serum before treatment and 10 cases of drug resistant serum after gefitinib treatment were collected from December 2019 to December 2020. The expression levels in non-small cell lung cancer tissues or cell lines were detected by real-time quantitative reverse transcription polymerase chain reaction. Cell counting kit 8, transwell and cell flow cytometry were used to detect the sensitivity of non-small cell lung cancer cells to gifetinib. Double luciferase reporter assay was used to verify the targeting relationship and the regulatory relationship was detected by western blotting and real-time quantitative reverse transcription polymerase chain reaction. Cancer susceptibility candidate 9 was highly expressed in non-small cell lung cancer tissues and drug-resistant HCC827/G cells. Overexpression of cancer susceptibility candidate 9 can significantly promote the proliferation, invasion and inhibit apoptosis of HCC827/G cells. At the same time, dual luciferase reporter assay confirmed that cancer susceptibility candidate 9 could negatively regulate the expression of microRNA-382-5p and microRNA-185-5p could bind to the 3'UTR of phosphoinositidedependent protein kinase-1 and mammalian target of rapamycin complex 2 and negatively regulate the expression of phosphoinositide-dependent protein kinase-1 and mammalian target of rapamycin complex 2. Further experiments showed that cancer susceptibility candidate 9 could up regulate the drug resistance of HCC827/G cells to gefitinib by down regulating the inhibition of microRNA-382-5p on phosphoinositide-dependent protein kinase-1 and mammalian target of rapamycin complex 2, thereby promoting the proliferation, invasion and inhibiting apoptosis of HCC827/G cells. Cancer susceptibility candidate 9 induces gefitinib resistance in non-small cell lung cancer cells by regulating microRNA-382- 5p/phosphoinositide-dependent protein kinase-1/mammalian target of rapamycin complex 2 signal axis.
Keywords
Non-small cell lung cancer, gefitinib, microRNA-382-5p, rapamycin
Lung cancer is one of the most common malignant tumors in clinic and its mortality rate ranks the first[1]. The incidence rate and mortality rate of lung cancer show a sharp upward trend[2]. Lung cancer is mainly divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) and NSCLC is the main phenotype. NSCLC has the characteristics of high recurrence rate and easy metastasis. Gefitinib is a common chemotherapy drug in the treatment of NSCLC, but more and more patients show significant drug resistance[3]. It is the focus of clinical and basic research to study the mechanism of drug resistance of NSCLC cells and provide more effective chemosensitization methods. Research found that long non coding RNA (lncRNA) was abnormally expressed in drug-resistant and drug sensitive lung cancer cells, which may lead to drug resistance of patients[4]. Long noncoding RNA cancer susceptibility candidate 9 (lncRNA CASC9) was up-regulated in bladder cancer and melanoma tissues and cells, and its expression level was related to the proliferation, migration, invasion and apoptosis of tumor cells[5,6]. Lncbase predicted v.2 predicted that there were complementary binding sites between MicroRNA-382-5p (miR-382-5p) and CASC9. Mir-382-5p was low expressed in NSCLC[7] and overexpression of miR-382-5p can increase the chemosensitivity of pancreatic ductal adenocarcinoma cells to gemcitabine[8]. However, the expression of CASC9 and miR-382-5p in HCC827/G, the relationship between them and the mechanism of their effect on drug sensitivity of HCC827 cells are still unclear. Therefore, this study was to investigate the effects of CASC9 and miR-382-5p on the proliferation, invasion, migration and gefitinib sensitivity of HCC827 cells and the underlying mechanism, so as to provide a new research direction for the drug resistance of NSCLC.
Materials and Methods
Materials:
HCC827 cell line was purchased from Shanghai Institute of cells, Chinese Academy of Sciences. HCC827/G cells were constructed by continuously stimulating HCC827 cells with low concentration of gefitinib. 10 cases of serum before treatment and 10 cases of drug-resistant serum after gefitinib treatment were collected from December 2019 to December 2020, including 12 males and 8 females, aged 49-73 y.
Drugs, reagents and instruments:
Small interfering RNA (siRNA) and mimics of CASC9, miR-382-5p, phosphoinositide-dependent protein kinase-1 (PDK1) and mammalian target of rapamycin complex 2 (mTORC2) were purchased from Shanghai Gemma Company. Dulbecco's modified eagle medium (DMEM) and fetal bovine serum were purchased from biological industries company. Penicillin and streptomycin were purchased from Beijing Regan Biotechnology Co., Ltd. Annexin V-FITC/PI apoptosis detection kit was purchased from ebioscience company and cell counting kit-8 (CCK-8) kit was purchased from Wuhan Huamei Bioengineering Co., Ltd. Transwell small room was purchased from Corelle, USA, DNA enzyme and LipofectamineTM 2000 and reverse transcriptase kit were purchased from Japan TaKaRa company, high purity total RNA rapid extraction kit purchased from Beijing hundred Tek Biotechnology Co., Ltd., full protein extraction kit, nuclear protein and cytoplasm protein extraction kit, sodium dodecyl sulfate polyacrylamide gel electrophoresis gel rapid preparation kit were purchased from Bio-Rad company of America. Both western blotting anti-1 and anti-secondary were purchased from CST Company of the United States; dual luciferase reporter gene kit and reporter gene vector were purchased from Promega Company. Enzyme labelling, fluorescence quantitative PCR, electrophoresis and gel imaging systems were purchased from the Thermo Fisher Scientific Company of the United States. The ultraspeed centrifuge and electrophoresis tank were purchased from Beijing 61 Biotechnology Co., Ltd.
Cell culture:
HCC827 cells were cultured in the DMEM medium containing 10 % fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin in 37°, 5 % CO2 incubator.
Establishment of drug resistant NSCLC cell line:
The HCC827 cells were treated with gefitinib at a beginning concentration of 0.001 μg/ml for 48 h, then the medium was discarded and DMEM medium was added to continue the culture. The cell viability was observed. If there was no obvious death, the cells were selected to gradually increase the concentration of gefitinib. The cells were subcultured repeatedly until they could survive stably in gefitinib at the concentration of 1 μg/ml, which named HCC827/G cells.
Cell transfection:
NSCLC HCC827 cells in logarithmic growth phase were digested with trypsin and then the density was adjusted to 1×105/ml using the DMEM medium. Then, 2 ml cell suspension were seeded on a 6-well plate and cultured in a 5 % CO2 incubator at 37° for 24 h. Then CASC9 siRNA and pcDNA-CASC9 were transfected to the cells. The transfection method was according to the instructions of LipofectamineTM 2000 transfection reagent. 48 h after transfection, the transfection effect was observed under fluorescence microscope. Meanwhile, HCC827/G cells in logarithmic growth phase were digested with trypsin and then the density was adjusted to 1×105/ml using the DMEM medium. Then, 2 ml cell suspension were seeded on a 6-well plate and cultured in a 5 % CO2 incubator at 37° for 24 h. Then CASC9 siRNA, si-PDK/mTORC2, pcDNA-CASC9 and miR-382-5p mimics were transfected to the cells. The transfection method was according to the instructions of LipofectamineTM 2000 transfection reagent. 48 h after transfection, the transfection effect was observed under fluorescence microscope.
Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR):
Clinical samples and NSCLC HCC827 and HCC827/G cells 48 h after transfection were collected and total RNA was extracted from tissues and cultured cells by Trizol for qRT-PCR. U6 and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as the control and the primer sequences were shown as follows: PDK1, F: 5’-CTACCAGCCATGTCAGAGGATG-3’, R: 5’-AGGCTGGTTTCCACCGTAGACA-3’; mTORC2, F: 5’-CTTCCTATCCGTCTTGGCAGAC-3’, R: 5’-CTCCAGACAGATGGCAATCAGG-3’; CASC9, F: 5’-CTCAGTAGTGGTATGTGCTCAG-3’, R: 5’-GGGTACAGTATGTCAGCACAG-3’; miR- 382-5p, F: 5,-CAAGCATCATGCAGCTCTTC-3, R:5’-TCCCAGAATTTCCAGGCTTA-3’; U6, F: 5’-CAGTGTGAGGTCCTTTCCATCC-3’, R: 5’-GCCATAGATGCTTGCGACTGTG-3’; GAPDH, F: 5’-CTCAGTAGTGGTATGTGCTCA G-3’, R: 5’-GGGTACAGTATGTCAGCACAG-3’. The PCR thermal cycle parameters were: 95° for 5 min, followed by three steps: 94° denaturation for 30 s and 60° annealing for 30 s. A total of 45 cycles were carried out. The results were analyzed by 2-ΔΔCt method.
Western blotting:
After the protein was extracted, the protein concentration was detected according to the guidance of diquinoline formic acid kit. The extracted protein was added with loading buffer, heated to 95° for 10 min. The drug loading of each well sample was 30 μg, adding 10 % polyacrylamide gel to isolate protein. The voltage of gel electrophoresis was controlled at 80~120 V and the pressure of wet transport and membrane transfer was controlled at 100 mV for 45~70 min. After polyvinylidene fluoride (PVDF) was transferred, the protein was sealed in 5 % bovine serum protein for 1 h and then one antibody was added to the membrane and then cultured overnight at 4°. The protein was washed with membrane washing buffer for 3 times, 5 min each time and then the second antibody was added and cultured for 1 h. After washing the membrane three times again, the chemiluminescence reagent developer protein was added. GAPDH was used as the control and Image J software was used to analyze the gray level of the target protein band.
CCK-8 assay:
HCC827 and HCC827/G cells in logarithmic growth phase were seeded on 96 well plates, with 104 cells in each well that containing 100 μl medium. Add 10 μl CCK-8 solution to each well 1 h before the test. The culture plates were incubated in the incubator for 1~4 h. The optical density (d) at 450 nm was measured by microplate to analyze the proliferation activity of NSCLC cells.
Transwell assay:
The transfected cells were selected as the experimental group and the non-transfected cells as the control group. The cells of each treatment group were digested with trypsin and inoculated into 24 well plate of transwell chamber. Add 100 μl cell suspension (2×105/ml) to the upper chamber and add 250 μl medium containing 10 % fetal bovine serum to the lower chamber. After incubated for 48 h in 5 % CO2 at 37°, the chamber was taken out. The cells in the upper chamber of the microporous membrane were wiped off with cotton swabs and the cells in the upper and lower chambers were carefully washed with phosphate buffered saline (PBS) twice. The cells that invaded and adhered to the microporous membrane of the chamber were fixed with 4 % paraformaldehyde for 15 min and stained with crystal violet for 15 min. After then, the chamber was washed with PBS, dried and observed under 100 fold inverted microscope.
Cell flow cytometry:
Flow cytometry was used to detect cell apoptosis. HCC827 and HCC827/G cells in transfection group and non-transfection group were selected and cultured to logarithmic growth phase. The cells were washed twice with PBS. Mix cells evenly with 500 μ L precooling 1×combined buffer and 5 μl Annexin- V-FITC, incubate at room temperature for 15 min. Then, add 2.5 μl propidium iodide (PI) staining 5 min before operation to detect the apoptosis of HCC827 and HCC827/G cells.
Dual luciferase reporter assay:
Firstly, the candidate target molecule miR-382- 5p 3'UTR of CASC9 or the candidate target genes PDK1 and mTORC2 3'UTR of miR-382-5p were inserted into the downstream of firefly luciferase gene. The expression vector pcDNA-enhanced green fluorescent protein (EGFP)-pre-CASC9 and its target gene miR-382-5p verification vector pmirglo-CASC9-miR-382-5p 3'UTR, as well as the expression vector pcDNA-egfp-pre-miR-382-5p and its target gene PDK1 and mTORC2 verification vector pmirglo-miR-382-5p-PDK1-mTORC2 3'UTR were cotransfected into HCC827 cells respectively, and the empty vector was cotransfected with CASC9 or miR-382-5p expression vector. In 24 well plates, 200 ng of pmir glo Mir gene 3'UTR, 600 ng of pcDNA EGFP pre miRNA and 30 pmol/l of negative control were transfected into each well. 50 μl opti minimal essential medium (MEM) I medium diluted 2 μl LipofectamineTM 2000, incubated at room temperature for 5 min; The DNA to be transfected and diluted LipofectamineTM 2000 were mixed. After incubation at room temperature for 20 min, the complex was directly added to the cells containing 0.4 ml opti MEM I medium and the culture plate was gently shaken to mix. The cells were cultured in 5 % CO2 incubator at 37° for 8 h. 0.5 ml of normal DMEM medium containing 10 % fetal bovine serum and no antibiotics was replaced. The cells were cultured in 5 % CO2 incubator at 37° for 48 h. Luciferase was detected according to the instructions of dual luciferase reporter gene kit. The fluorescence values of firefly and sea cucumber were detected by microplate reader and the fluorescence values of sea cucumber were used as internal reference.
Statistical methods:
Statistical package for the social sciences (SPSS) 20.0 software was used for statistical analysis, t test was used for comparison between the two groups and Graphpad prism 8 was used to draw relevant pictures of the experimental data. p<0.05 or p<0.01 meant the difference was statistically significant.
Results and Discussion
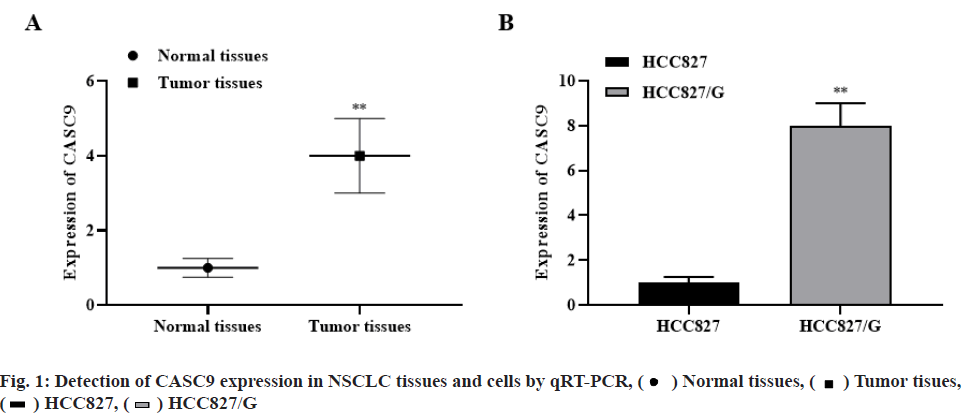
The expression level of CASC9 in NSCLC was significantly higher than that in normal tissues (p<0.01, fig. 1A). Meanwhile, the expression level of CASC9 in HCC827/G cells was significantly higher than that in HCC827 cells (p<0.01, fig. 1B). The results indicated that the abnormal expression of CASC9 may be related to the occurrence and development of NSCLC and gefitinib resistance.
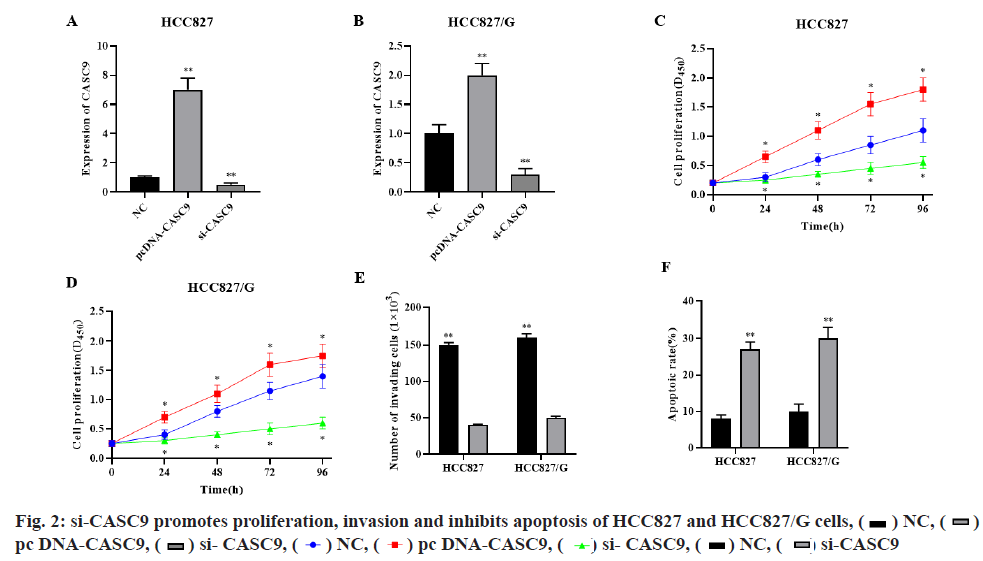
qRT-PCR showed that si-CASC9 could significantly decrease while pcDNA-CASC9 could significantly increase the CASC9 expression level in HCC827 and HCC827/G cells (fig. 2A-fig. 2B, p<0.01, respectively). CCK-8 assay showed that silence of CASC9 could significantly inhibit the proliferation of HCC827 and HCC827/G cells, while overexpression of CASC9 could significantly promote the proliferation of HCC827 and HCC827/G cells (fig. 2C-fig. 2D, p<0.05, respectively). Transwell assay indicated that silencing CASC9 could significantly inhibit the invasion ability of HCC827 and HCC827/G cells (fig. 2E, p<0.01). Cell flow cytometry showed that si-CASC9 could significantly promote the apoptosis of HCC827 and HCC827/G cells (fig. 2F, p<0.01). These results demonstrated that silencing CASC9 could significantly promote gefitinib induced apoptosis and inhibit cell proliferation and invasion in HCC827 and HCC827/G cells.
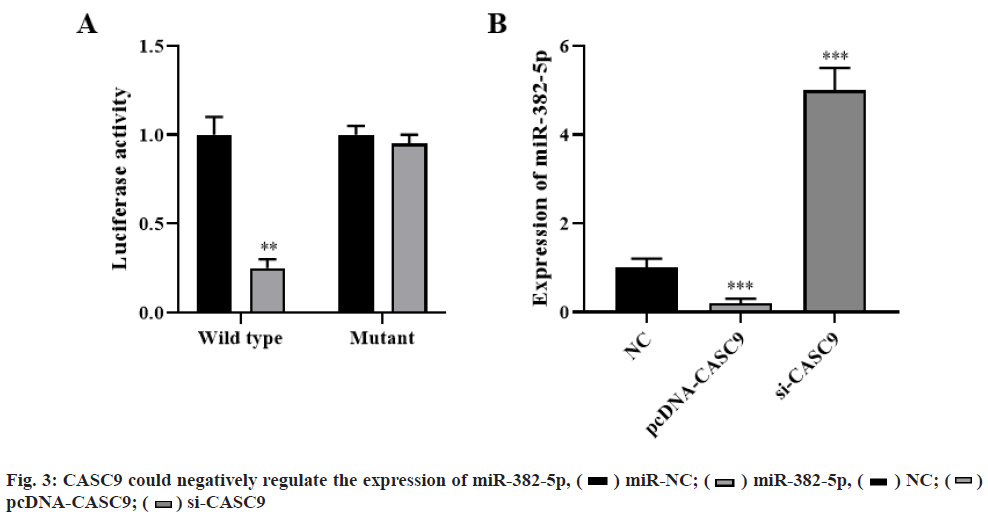
Dual luciferase reporter assay showed that overexpression of miR-382-5p could significantly decrease luciferase activity, while co-transfection of miR-382-5p mimics and pmirglo-CASC9-mut vector with mutation of target site resulted in loss of inhibition effect of miR-382-5p on luciferase activity (p<0.01, fig. 3A). qRT-PCR analysis showed that CASC9 overexpression significantly inhibited the expression of miR-382-5p, while CASC9 knockdown significantly promoted the expression of miR-382- 5p (p<0.001, fig. 3B). These results indicated that CASC9 could negatively regulate the expression of miR-382-5p.
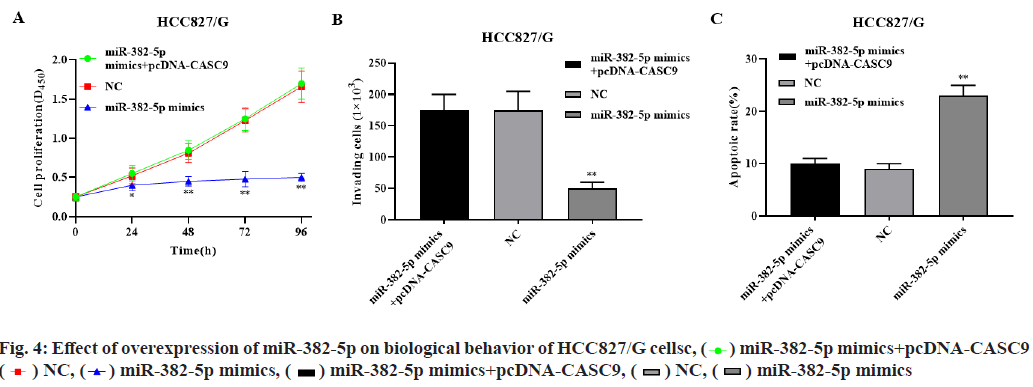
Overexpression of miR-382-5p significantly inhibited the proliferation of HCC827/G cells, while overexpression of miR-382-5p and CASC9 significantly restored the proliferation of HCC827/G cells (p<0.01, fig. 4A). Overexpression of miR- 382-5p significantly inhibited the invasion and promoted the apoptosis of HCC827/G cells, while overexpression of miR-382-5p and CASC9 simultaneously had no significant effect on the invasion and apoptosis of HCC827/G cells (p<0.01, fig. 4B- fig. 4C). These results indicated that CASC9 can promote the proliferation, invasion and inhibit apoptosis of HCC827/G cells by down regulating miR-382-5p.
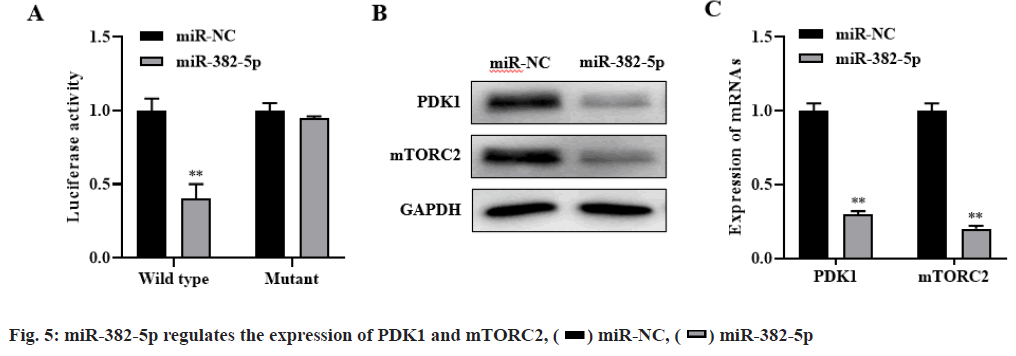
The bioinformatics database targets can predict that PDK1 and mTORC2 were candidate target genes of miR-382-5p. Dual luciferase reporter assay showed that miR-382-5p could negatively regulate the expression of PDK1 and mTORC2 (p<0.01, fig. 5A). Besides, western blot and RT-PCR results showed that overexpression of miR-382-5p significantly inhibit the expression of PDK1 and mTORC2 in HCC827/G cells (p<0.01, fig. 5B-fig. 5C). These results indicated that PDK1 and mTORC2 are the target genes of miR- 382-5p and miR-382-5p can negatively regulate the expression of PDK1 and mTORC2.
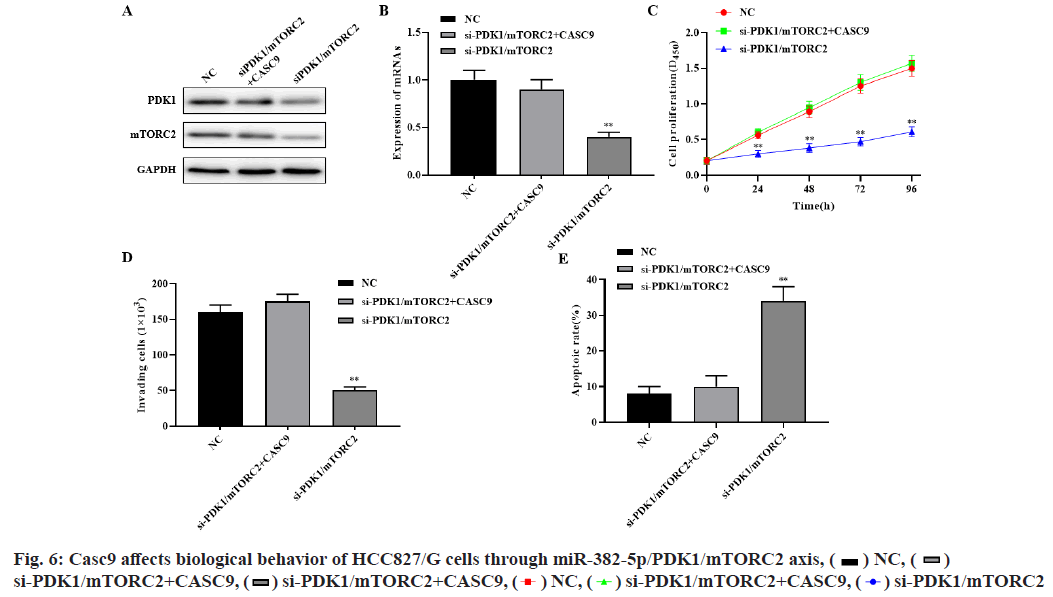
Western blot and RT-PCR results showed that silencing PDK1 and mTORC2 significantly inhibited the expression of PDK1 and mTORC2, while simultaneously silencing CASC9, PDK1 and mTORC2 significantly restored the expression of PDK1 and mTORC2 (p<0.01, fig. 6A-fig. 6B). Compared with the control group, si-mTORC2 significantly inhibited cell proliferation and invasion and promoted cell apoptosis. However, silencing the expression of CASC9, PDK1 and mTORC2 at the same time could significantly restore the effect of si-mTORC2 on the proliferation, invasion and apoptosis of HCC827/G cells (p<0.01, fig. 6C-fig. 6E). These results indicated that CASC9 up regulates the drug resistance of HCC827/G cells to gefitinib by down regulating the inhibitory effect of miR-382- 5p on PDK1 and mTORC2, thereby promoting the proliferation, invasion and inhibiting apoptosis of HCC827/G cells.
NSCLC accounts for about 80 % to 85 % of lung cancer and most patients have lost the chance of surgical treatment because they are in advanced stage at the time of diagnosis. Chemotherapy is the most important treatment, but the obvious side effects limit the role of chemotherapy in the treatment of advanced NSCLC. Recent studies have proved that[9], due to the tolerance of NSCLC to chemotherapy drugs, the expected curative effect cannot be achieved[10, 11]. In recent years, lncRNA has been confirmed to play an important regulatory role in the occurrence and development of tumor and drug resistance[12,13]. However, the role of lncRNA in multi-drug resistance of NSCLC remains unclear. Therefore, it is urgent to find the upstream key molecules of the genes related to drug resistance in NSCLC and further study their molecular mechanisms, so as to provide more evidence for the clinical treatment of NSCLC.
In recent years, in addition to structural non-coding RNAs and various small RNAs (miRNAs), researchers have also found a class of lncRNAs with a length of more than 200 nucleotides. LncRNA regulates gene expression in a variety of ways, including epigenetic regulation, transcriptional regulation and post transcriptional regulation. In a word, lncRNAs regulate the occurrence and development of NSCLC, thyroid cancer, colon cancer, liver cancer and other tumors and the process of chemotherapy drug resistance by participating in a variety of biological mechanisms such as X chromosome silencing, genomic imprinting and DNA damage response[14]. Recently, it has been reported that lncRNA metallothionein 1 J pseudogene (MT1JP)[15], lncRNA Actin filament associated protein 1 (AFAP1)[16], lncRNA small nucleolar RNA host gene 8 (SNHG8) [17] have significant effects on the proliferation, migration and epithelial mesenchymal transition of lung cancer cells, suggesting that lncRNA may be closely related to the occurrence and development of lung cancer. However, the molecular mechanism of lncRNA CASC9 resistance to gefitinib in NSCLC cells has not been reported.
At the same time, recent studies have found that the abnormal regulation of miRNAs is considered to be a key factor in the occurrence and development of many diseases, including NSCLC. For example, miR-382-5p inhibits the proliferation and migration of NSCLC cells by down regulating the expression of Tripartite motif containing 29 (TRIM29)[18]. Zhang et al.[19] confirmed that miR-382-5p can be used as an independent prognostic marker for NSCLC. At the same time, some studies have shown that miR-382-5p has significant effects on the proliferation, migration and epithelial mesenchymal transition of lung cancer[20], colorectal cancer[21], pancreatic cancer[22] and liver cancer[23]. However, the mechanism of miR- 382-5p on NSCLC has not been reported.
According to bioinformatics tools, PDK1 and mTORC2 are predicted to be possible target genes of miR-382-5p. PDK1 and mTORC2 are members of cell cycle family and their abnormal expression may lead to abnormal proliferation of cells. Studies have found that PDK1 and mTORC2 are abnormally expressed in a variety of tumor tissues, such as cervical cancer, non-small cell lung cancer, non-small cell lung cancer and prostate cancer. Chen et al.[24] reported that miR-615 inhibits the proliferation and invasion of prostate cancer cells by down regulating of PDK1 and mTORC2. In addition, miR-1297[25], miR-154[26] and miR-497[27] have been reported to down regulate PDK1 and mTORC2 to inhibit the proliferation and migration of tumor cells. Therefore, we speculate that miR-382-5p may regulate the proliferation and invasion of NSCLC cells through targeting PDK1 and mTORC2.
In this study, CASC9 was highly expressed in NSCLC tissues and drug-resistant HCC827/G cells. Overexpression of CASC9 can significantly promote the proliferation, invasion and inhibit apoptosis of HCC827/G cells. At the same time, dual luciferase reporter assay confirmed that CASC9 could negatively regulate the expression of miR-382-5p and miR-185-5p could bind to the 3'UTR of PDK1 and mTORC2 and negatively regulate the expression of PDK1 and mTORC2. Further experiments showed that CASC9 could up regulate the drug resistance of HCC827/G cells to gefitinib by down regulating the inhibition of miR-382-5p on PDK1 and mTORC2, thereby promoting the proliferation, invasion and inhibiting apoptosis of HCC827/G cells.
Acknowledgement:
This work was supported by the Science and Technology Project of Fujian Natural Science Foundation (No. 2018J01204).
Conflicts of interest:
The authors report no conflicts of interest.
References
- Roth A, Diederichs S. Long Noncoding Rnas in Lung Cancer. Curr Top Microbiol Immunol 2016;394:57-110.
- Dai J, Xu LJ, Han GD, Jiang HT, Sun HL, Zhu GT, et al. Down-regulation of long non-coding RNA ITGB2-AS1 inhibits osteosarcoma proliferation and metastasis by repressing Wnt/β-catenin signalling and predicts favourable prognosis. Artif Cells Nanomed Biotechnol 2018;46(3):S783-90.
- Wang L, Ma L, Xu F, Zhai W, Dong S, Yin L, et al. Role of long non‐coding RNA in drug resistance in non‐small cell lung cancer. Thorac Cancer 2018;9(7):761-8.
- Yang M, Qin Q, Zhu J, Guo Y, Yin T, Wu H, Wang C. Long noncoding RNA ITGB2‐AS1 promotes growth and metastasis through miR‐4319/RAF1 axis in pancreatic ductal adenocarcinoma. J Cell Physiol 2020.
- Pietas A, Schlüns K, Marenholz I, Schäfer BW, Heizmann CW, Petersen I. Molecular cloning and characterization of the human S100A14 gene encoding a novel member of the S100 family. Genomics 2002;79(4):513-22.
- Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language?. Cell 2011;146(3):353-8.
- Chheang S, Brown K. Lung cancer staging: clinical and radiologic perspectives. Semin Interv Radiol 2013;30(2):99-113.
- Qian J, Ding F, Luo A, Liu Z, Cui Z. Overexpression of S100A14 in human serous ovarian carcinoma. Oncol Lett 2016;11(2):1113-9.
- Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res 2017;77(15):3965-81.
- Katono K, Sato Y, Kobayashi M, Saito K, Nagashio R, Ryuge S, et al. Clinicopathological significance of S100A14 expression in lung adenocarcinoma. Oncol Res Treat 2017;40(10):594-602.
- Li DY, Chen WJ, Luo L, Wang YK, Shang J, Zhang Y, et al. Prospective lncRNA-miRNA-mRNA regulatory network of long non-coding RNA LINC00968 in non-small cell lung cancer A549 cells: a miRNA microarray and bioinformatics investigation. Int J Mol Med 2017;40(6):1895-906.
- Liang Y, Chen X, Wu Y, Li J, Zhang S, Wang K, et al. LncRNA CASC9 promotes esophageal squamous cell carcinoma metastasis through upregulating LAMC2 expression by interacting with the CREB-binding protein. Cell Death Differ 2018;25(11):1980-95.
- Ding F, Wang D, Li XK, Yang L, Liu HY, Cui W, et al. Overexpression of S100A14 contributes to malignant progression and predicts poor prognosis of lung adenocarcinoma. Thorac Cancer 2018;9(7):827-35.
- Pouyanrad S, Rahgozar S, Ghodousi ES. Dysregulation of miR-335-3p, targeted by NEAT1 and MALAT1 long non-coding RNAs, is associated with poor prognosis in childhood acute lymphoblastic leukemia. Gene 2019;692:35-43.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 2018;68(6):394-424.
- Hu X, Li Y, Kong D, Hu L, Liu D, Wu J. Long noncoding RNA CASC9 promotes LIN7A expression via miR‐758‐3p to facilitate the malignancy of ovarian cancer. J Cell Physiol 2019;234(7):10800-8.
- Yu L, Hao Y, Xu C, Zhu G, Cai Y. LINC00969 promotes the degeneration of intervertebral disk by sponging miR‐335‐3p and regulating NLRP3 inflammasome activation. IUBMB life 2019;71(5):611-8.
- Zhao X, Tang DY, Zuo X, Zhang TD, Wang C. Identification of lncRNA–miRNA–mRNA regulatory network associated with epithelial ovarian cancer cisplatin‐resistant. J Cell Physiol 2019;234(11):19886-94.
- Zhang J, Wang Q, Quan Z. Long non-coding RNA CASC9 enhances breast cancer progression by promoting metastasis through the meditation of miR-215/TWIST2 signaling associated with TGF-β expression. Biochem Biophys Res Commun 2019;515(4):644-50.
- Luo K, Geng J, Zhang Q, Xu Y, Zhou X, Huang Z, et al. LncRNA CASC9 interacts with CPSF3 to regulate TGF-β signaling in colorectal cancer. J Exp Clin Cancer Res 2019;38(1):1-6.
- Li M, Chen Y, Zhu J, Gao Z, Wang T, Zhou P. Long noncoding RNA CASC15 predicts unfavorable prognosis and exerts oncogenic functions in non-small cell lung cancer. Am J Transl Res 2019;11(7):4303-14.
- Jiao P, Hou J, Yao M, Wu J, Ren G. SNHG14 silencing suppresses the progression and promotes cisplatin sensitivity in non-small cell lung cancer. Biomed Pharmacother 2019;117:109164.
- Ren Y, Zhang H, Jiang P. MicroRNA-382 inhibits cell growth and migration in colorectal cancer by targeting SP1. Biol Res 2018;51(1):1-8.
- Chen L, Nan A, Zhang N, Jia Y, Li X, Ling Y, et al. Circular RNA 100146 functions as an oncogene through direct binding to miR-361-3p and miR-615-5p in non-small cell lung cancer. Mol Cancer 2019;18(1):1-8.
- Wang Y, Xue J, Kuang H, Zhou X, Liao L, Yin F. microRNA-1297 inhibits the growth and metastasis of colorectal cancer by suppressing cyclin D2 expression. DNA Cell Biol 2017;36(11):991-9.
- Zhu C, Shao P, Bao M, Li P, Zhou H, Cai H, et al. miR-154 inhibits prostate cancer cell proliferation by targeting CCND2. Urol Oncol 2014;32(1):31-e9.
- Zhong H, Yang J, Zhang B, Wang X, Pei L, Zhang L, et al. LncRNA GACAT3 predicts poor prognosis and promotes cell proliferation in breast cancer through regulation of miR-497/CCND2. Cancer Biomark 2018;22(4):787-97.