- *Corresponding Author:
- B. W. Qin
Department of Academic Research,
Chengde Nursing Vocational College,
Chengde, Hebei 067000,
China,
E-mail: bowenqin2005@163.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “40-47” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
High-risk human papillomavirus types are associated with most of cervical cancer cases. To explore the mechanism by which human papillomavirus 16 infections interferes with the progression and immune evasion of cervical cancer by acting on T helper 9 cells cytokines. Cervical cancer cells were obtained from China Center for Type Culture Collection. Cells were cultured in Roswell Park Memorial Institute Medium-1640. Through wound healing test and transwell analysis, the effect of interleukin-9 on the movement, migration and invasion of cancer cells was evaluated. The expression of epithelial-mesenchymal transition markers and programmed death-ligand 1 and programmed cell death protein 1 messenger ribonucleic acid was detected. Human papillomavirus 16 and interleukin-9 receptor had higher expression in cervical cancer tumor tissue than normal group. The proliferation of cervical cancer cells was inhibited in interleukin-9 transfection group compared with the cervical cancer group. Interleukin-9 transfection group reduced N-cadherin and vimentin expression, and increased E-cadherin expression. Interleukin-9 inhibition group increased N-cadherin and vimentin expression and decreased E-cadherin expression. Interleukin-9 transfection group reduced programmed death-ligand 1 and programmed cell death protein 1 expression, while interleukin-9 inhibition group increased programmed death-ligand 1 and programmed cell death protein 1 expression. These cytokines can limit tumor proliferation, movement, migration and invasion. They induce the up-regulation of E-cadherin expression in cervical cells and inhibit programmed death-ligand 1 and programmed cell death protein 1 expression, thereby expressing tumor antigens.
Keywords
Human papillomavirus 16, cervical cancer, T helper 9 cells cytokines, interleukin-9, programmed death-ligand 1, programmed cell death protein 1
Cervical cancer is one of the most common gynecological malignancies. 500 000 new cases are diagnosed every year worldwide, about one-third of which causes death[1]. High-risk Human Papillomavirus (HPV) types (such as HPV 16, 18 and 31) are associated with more than 90 % of cervical cancer cases. Cellmediated immunity plays a key role in the outcome of HPV disease[2]. Whether HPV infection is cleared or still persists depends to a large extent on the types of T cell subsets that constitute the tumor microenvironment. The infiltration of T cell subsets that inhibit tumor growth will prevent the development of cancer and vice versa[3]. T helper 9 (Th9) cell is a newly discovered subset of T cells, which is reported to prevent tumor growth in a variety of cancers[4]. Interleukin-9 (IL-9) as its hallmark cytokine plays a role in preventing and inhibiting the development of cancer[5]. Studies have reported that Th9 cells mediate strong tumor immunity against solid tumors through IL-9[6]. However, their role in cervical cancer immunity has not yet discovered. The signal molecules in the Programmed Death- Ligand 1 (PD-L1)/Programmed Cell Death Protein 1 (PD-1) signaling pathway are associated with tumor immune escape[7]. The interaction between PD-L1 on Antigen Presenting Cells (APC) and its receptor PD-1 on T lymphocytes leads to inhibition of T lymphocyte activation and induces T lymphocyte apoptosis or anergy[8]. The abnormally high expression of PD-L1 is common in various tumors, which is related to tumor progression and patient survival[9].
This research aims to study the mechanism by which Human Papillomavirus 16 (HPV16) infection interferes with the progression and immune evasion of cervical cancer by acting on Th9 cytokines.
Materials and Methods
Cell culture and transfection:
Cervical cancer cell line (CaSki) cells and HPVnegative epithelial cells Human Prostate Cancer Cell Line (PC3) related to HPV16 were obtained from China Center for Type Culture Collection (CCTCC) (Wuhan, China). Cells were cultured in Roswell Park Memorial Institute medium (RPMI)-1640 supplemented with 10 % newborn calf serum and 1 % penicillin/streptomycin at 37° and 5 % Carbon dioxide (CO2). The purified plasmid IL-9 and IL-9 inhibitor were stably transfected into CaSki cells (1×105/ml) using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). According to the manufacturer’s instructions, positive clones were selected based on their resistance to Gibco Geneticin reagent (G418) (800 mg/ml; North China Pharmaceutical Co., Ltd.) 2 w later and blotting was analyzed by Reverse Transcription-Polymerase Chain Reaction (RT-PCR), flow cytometry and western analysis.
Histopathological analysis and immunohistochemical staining:
Cervical cancer (60 patients) and normal cervical tissue (60 cases) samples were obtained from the obstetrics and gynecology department of our hospital between August 2019 and March 2020. The study meets the standards of the hospital ethics committee. All cervical tissue specimens were fixed with 10 % neutral formalin, embedded in paraffin, serially sectioned at 5 μm and loaded on a glass slide coated with histostick. A total of 4-5 adjacent sections were collected for histopathological analysis and immunohistochemical staining. The clinical histopathological diagnosis of all cases was performed according to World Health Organisation (WHO) standards. HPV16 and Interleukin-9 receptor (IL-9R) were immunostained using a biotin-streptavidin complex kit (Boster Biological Technology Co., Ltd., Wuhan, China). Except the primary antibody, all the following reagents are part of the kit. According to the manufacturer’s protocol, the operation steps are briefly described as follows: Use normal non-immune goat serum for dewaxing. After inactivating endogenous peroxidase activity and inhibiting cross-reaction, incubate the sections overnight in a mild primary antibody dilution at 4°. Localization of the primary antibody was achieved by the subsequent application of biotin-conjugated Immunoglobulin G (IgG) secondary antibody and streptavidin-conjugated peroxidase. The signal was visualized with diaminobenzidine and the nucleus was stained with instant hematoxylin. A negative control was established by replacing the primary antibody with a normal isotype control (kit provided). The quantitative analysis of positive staining is performed as follows: Open the image analysis system, define negative and positive standards and scan the sample. The relative brightness value is obtained using Image-Pro plus 6.0 software.
Cell Counting Kit-8 (CCK-8) determination:
Each group of cells in the log phase was prepared as single cell suspension and seeded into a 96-well plate after cell density adjustment to 1000 cells per well. Afterwards, six repeated holes were set in each group. On the 2nd d, after the cell attachment, 10 μl of CCK- 8 solution (Beyotime Biotechnology) was added to the sample and a blank control well containing only the medium and CCK-8 solution was set. After incubation for 1 h, use a microplate reader to measure and record the absorbance (Optical Density (OD)) value of each well at a wavelength of 450 nm. Finally, 4 d measurement was performed at 24 h interval.
Wound healing test determination:
Inoculate the cells in a 6-well plate with 2 ml complete medium in each well. When the fusion rate reaches 80 %-90 %, scrape the cells with a vertical tip and wash twice with Phosphate Buffered Saline (PBS). Subsequently, the complete medium was replaced with a medium without Fetal Bovine Serum (FBS), the plate was taken out after 24 h to observe scraping healing.
Transwell migration and invasion analysis:
Transwell experiment was performed using a transwell chamber (Millipore, Billerica, USA). In the migration assay, after trypsinization, the transfected Gastric Cancer (GC) cells were centrifuged at 1000 r/min for 3 min. The cells were resuspended in serum-free medium, with density adjusted to 1×105/ml. Add 200 μl cell suspension to the upper compartment of the transwell chamber. A total of 700 μl 10 % FBS-containing medium was added to the lower compartment. The cells were cultured for 24 h. Finally, remove the chamber and gently wipe the cells on the upper compartment with a cotton swab. The cells passing through the membrane were fixed and stained with crystal violet solution for 30 min and washed twice with PBS. After drying, observe the cells with a microscope and randomly select five fields (100×) for counting. The average value is regarded as the number of migratory cells. In the invasion test, 50 μl Matrigel was diluted and coated on the upper compartment, and the other experimental procedures were the same as the migration experiment.
Western blot analysis:
Add protein extraction buffer (radioimmunoprecipitation assay and phenylmethylsulfonyl fluoride; Sigma- Aldrich; Merck KGaA, Darmstadt, Germany) to the cells and use Bicinchoninic Acid (BCA) kit to quantify protein concentration. The samples were homogenized, centrifuged at 12 000 ×g for 30 min at 4° and subject to ultrasonic treatment (5 s for each action with an interval of 6 s, 5 times for each sample). The supernatant was removed by centrifugation at 4000 ×g for 20 min at 4°. The sample was used to quantify protein expression using BCA. According to the manufacturer’s protocol, 20 μg of each sample was separated by 10 % Sodium Dodecyl Sulphate- Polyacrylamide Gel Electrophoresis (SDS-PAGE) (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and transferred to a Polyvinylidene Fluoride (PVDF) membrane (EMD Millipore, Billerica, MA, USA). The PVDF membrane was incubated with blocking buffer (PBS containing 5 % skim milk and 0.1 % Tween-20) at room temperature for 1 h. The membrane was then washed 3 times with Tris-buffered saline/Tween-20, 5 min each time and incubated with primary antibody overnight at 4°. The main antibodies used herein are as follows: N-Cadherin (catalog number 13116; 1:1000 dilution; Cell Signaling Technology, Inc., Denver, Massachusetts, USA), vimentin (catalog number 5741; 1:1000 dilution; Cell Signaling Technology, Inc.), E-cadherin (catalog number 3195; 1:1,000 dilution; Cell Signaling Technology, Inc.) and human Beta (β)- actin (catalog number 3700; 1:1,000 dilution; Cell Signaling Technology, Inc.). Horseradish peroxidaseanti- rabbit IgG (catalog number 7074; 1:3000 dilution; Cell Signaling Technology, Inc.) and anti-mouse IgG (catalog number 7076; 1:3,000 dilution; Cell Signaling Technology, Inc.), horseradish peroxidaseconjugated secondary antibody was used for 2 h at room temperature. The enhanced chemiluminescence kit (Beyotime Institute of Biotechnology) was used to detect the results of western blot analysis. The relative level of each protein was the ratio of the average value of each band to the average value of β-actin. The relative density was quantified using a digital imaging analyzer ImageJ 1.4.1 (National Institutes of Health, Bethesda, Massachusetts, USA).
Ribonucleic Acid (RNA) extraction and Quantitative RT-PCR (RT-qPCR):
Total RNA was isolated from tissues and cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). According to the manufacturer’s protocol, the total RNA sample was reverse-transcribed using ReverTra AceTM qPCR RT kit (Higashiosaka Life Sciences, Osaka, Japan). Thunderbird SYBR qPCR mixture (Toyobo Life Science) and real-time PCR system (Applied Biosystems 7500; Applied Biosystems; Thermo Fisher Scientific, Inc.) were used for qPCR reaction. The relative expression of the gene was normalized to Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) expression and calculated according to the 2-ΔΔCt method (Table 1).
| Gene | Upstream primer | Downstream primer |
|---|---|---|
| PD-L1 | 5'-ACCGTTTATATAGGCCGTAAA-3' | 5'-GGTCTCGTCGACGCTAGTAAA-3' |
| PD-1 | 5'-AAGTGTCGCACCCTGGGATA-3' | 5'-ACGTTATAAGGGCCCCTTTTCA-3' |
| GAPDH | 5'-GTCTCCTCTGACTTCAACAGCG-3' | 5'-ACCACCCTGTTGCTGTAGCCAA-3' |
Table 1: RT-PCR Primer Sequence
Statistical analysis:
All results are expressed as mean±Standard Deviation (SD). Use Student’s t test and Fisher’s exact test to assess difference between the groups, p<0.05 indicates statistical significance. All data were analyzed using Statistical Package for the Social Sciences (SPSS) 20.0 software.
Results and Discussion
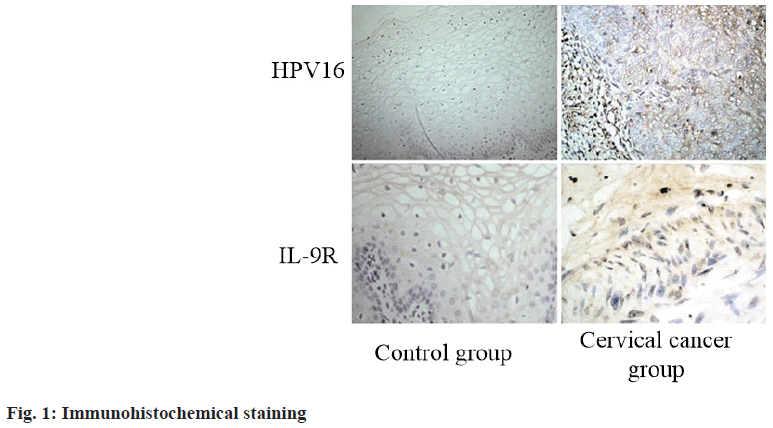
The expression of HPV16 and IL-9R in cervical cancer and normal cervical tissues was detected by immunohistochemical staining. The results showed that HPV16 and IL-9R had higher expression in cervical cancer tumor tissue than in normal control group (p<0.05) (fig. 1 and Table 2).
| Group | HPV16 | IL-9R |
|---|---|---|
| Control group (n=60) | 1.52±0.23 | 1.33±0.14 |
| Cervical cancer group (n=60) | 4.37±0.28 | 5.85±0.32 |
| t value | 6.537 | 7.524 |
| p value | 0.012 | 0.011 |
Table 2: Expression of HPV16 and IL-9R
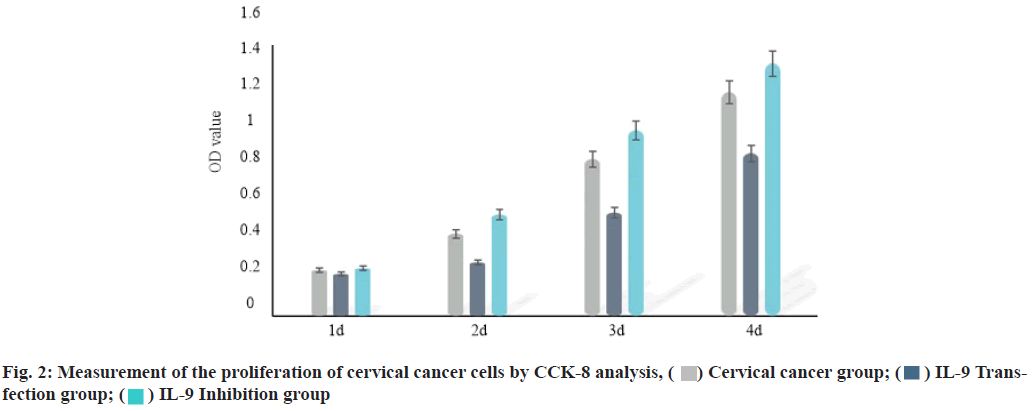
IL-9 regulates the proliferation of cervical cancer cells. Plasmid Cloning Deoxyribonucleic acid (pcDNA) vector and inhibitor were used to over-express and inhibit the expression of IL-9 in cervical cancer cells. CCK-8 analysis was performed to evaluate the effect of IL-9 on proliferation of cervical cancer cells. The results showed that compared with the cervical cancer group, the proliferation of cervical cancer cells was inhibited in IL-9 transfection group (2 d, 3 d and 4 d) (p<0.05). The proliferation of cervical cancer cells (2 d, 3 d and 4 d) was increased in IL-9 inhibition group (p<0.05) (fig. 2 and Table 3).
| Group | 1 d | 2 d | 3 d | 4 d |
|---|---|---|---|---|
| Cervical cancer group (n=60) | 0.24±0.07 | 0.43±0.06 | 0.82±0.11 | 1.17±0.16 |
| IL-9 transfection group (n=60) | 0.22±0.03 | 0.28±0.09 | 0.54±0.14 | 0.85±0.08 |
| IL-9 inhibition group (n=60) | 0.25±0.18 | 0.53±0.12 | 0.97±0.17 | 1.32±0.18 |
| F value | 13.548 | 11.138 | 12.145 | 13.264 |
| p value | 0.369 | 0.012 | 0.005 | <0.001 |
Table 3: Measurement of the Proliferation of Cervical Cancer Cells by Cck-8 Analysis Method
IL-9 regulates the movement, migration and invasion of cervical cancer cells. Through wound healing test analysis and transwell analysis, the effect of IL-9 on the movement, migration and invasion of cervical cancer cells was evaluated. The results showed that compared with the cervical cancer group, the movement, migration and invasion of cervical cancer cells were inhibited in IL-9 transfection group (p<0.05). The movement, migration and invasion of cervical cancer cells were increased in IL-9 inhibition group (p<0.05) (Table 4).
| Group | Wound width | Cell number |
|---|---|---|
| Cervical cancer group (n=60) | 1.00±0.13 | 113.58±15.43 |
| IL-9 transfection group (n=60) | 0.65±0.12 | 65.37±11.12 |
| IL-9 inhibition group (n=60) | 1.68±0.11 | 187.46±11.15 |
| F value | 12.547 | 13.457 |
| p value | 0.006 | 0.013 |
Table 4: IL-9 Regulates the Movement, Migration and Invasion of Cervical Cancer Cells
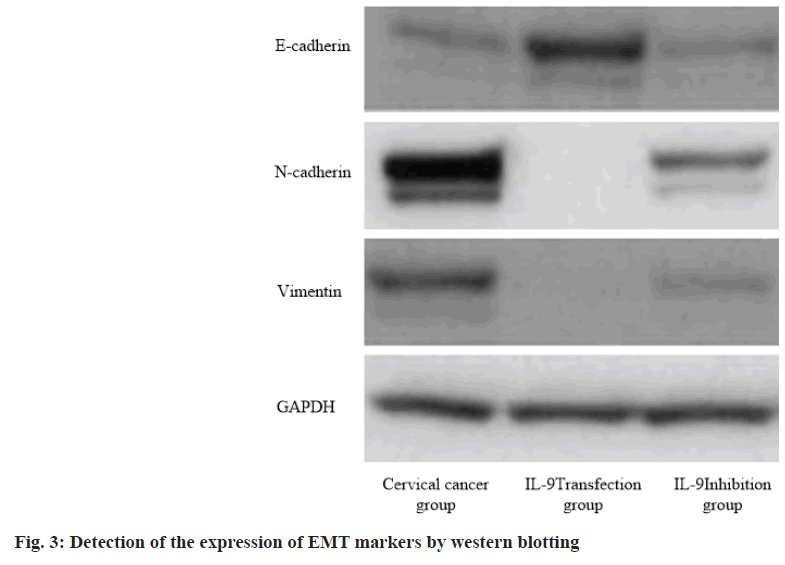
IL-9 regulates cervical cancer progression and immune evasion by regulating Epithelial-Mesenchymal Transition (EMT). EMT is one important indicator to measure tumor cell metastasis. The expression of EMT markers (E-cadherin, N-cadherin and vimentin) was detected by western blotting. The results showed that IL-9 transfection group reduced the expression of N-cadherin and vimentin, and increased the expression of E-cadherin (p<0.05). On the contrary, IL-9 inhibition group increased the expression of N-cadherin and vimentin, and decreased the expression of E-cadherin (p<0.05). This indicates that IL-9 may regulate cervical cancer progression and immune evasion by changing EMT (fig. 3 and Table 5).
| Group | E-cadherin | N-cadherin | Vimentin |
|---|---|---|---|
| Cervical cancer group (n=60) | 2.13±0.42 | 4.36±0.35 | 4.23±0.14 |
| IL-9 transfection group (n=60) | 4.45±0.15 | 0.58±0.13 | 0.66±0.08 |
| IL-9 inhibition group (n=60) | 1.25±0.48 | 6.33±0.28 | 7.42±0.34 |
| F value | 11.153 | 12.458 | 14.637 |
| p value | 0.005 | 0.012 | 0.013 |
Table 5: IL-9 Regulates Cervical Cancer Progression and Immune Evasion by Regulating EMT
IL-9 regulates cervical cancer immune evasion by regulating PD-L1/PD-1. Dysfunction of cytotoxic T lymphocytes is usually related to the activation of PD-L1/PD-1 pathway, which is a major obstacle in cancer treatment. The expression of PD-L1 and PD-1 messenger RNA (mRNA) was detected by RT-qPCR. The results showed that IL-9 transfection group reduced the expression of PD-L1 and PD-1 mRNA (p<0.05), while IL-9 inhibition group increased the expression of PD-L1 and PD-1 mRNA (p<0.05) (Table 6).
| Group | PD-L1 | PD-1 |
|---|---|---|
| Cervical cancer group (n=60) | 1.34±0.15 | 1.15±0.13 |
| IL-9 transfection group (n=60) | 0.32±0.12 | 0.41±0.13 |
| IL-9 inhibition group (n=60) | 3.57±0.21 | 4.32±0.16 |
| F value | 13.564 | 13.854 |
| p value | 0.013 | 0.006 |
Table 6: RT-qPCR Detection of PD-L1 and PD-1 mRNA
Over millions of years, papillomavirus has successfully evolved to reproduce in most mammals and birds. Today, more than 300 papillomaviruses have been identified and sequenced, including more than 200 types of HPV[10]. HPV is the most common sexual transmission medium in the world. Low-risk types can cause benign genital and skin warts, while high-risk HPV types can cause genital cancers such as cervical cancer, vaginal cancer, penile cancer and anal cancer, as well as subset of head and neck cancers, namely oropharyngeal cancer[11,12]. Among cervical cancers, 60 % are caused by HPV16 and 15 % are caused by HPV188[13]. Compared with other viruses, HPV replicates very slowly. Therefore, it is necessary to develop an effective immune escape mechanism to make the infection last long enough, thus completing the virus replication cycle[14]. Natural immune control of HPV infection is achieved through innate and adaptive immune responses (including specific antibodies and effector T1 cells).
HPV can interfere with cell growth, apoptosis and differentiation, which also affects the host’s antiviral/ tumor immune response. Previous studies reported that HPV16 affects the innate immune response by down-regulating the expression of interferon response genes, which will affect the subsequent activation of adaptive immune response[15]. Lymphocyte-mediated cytotoxicity involving cytotoxic T lymphocytes is the most effective mechanism to control and eliminate viral infections. Compared with normal cervical tissues, higher levels of HPV16 and IL-9R expression are observed in cervical cancer tissues. According to reports, the anti-tumor effect of IL-9 is a result of the up-regulation of immune suppression mediated by Regulatory T cells (Treg) and mast cells. Previous reports indicate that IL-9 and Interleukin-21 (IL-21) are the hallmark cytokines of Th9, which play an important role in preventing and inhibiting the development of various cancers. To study the potential role of Th9 in the pathogenesis of cervical cancer, we tested the effect of overexpression and inhibition of its hallmark cytokine IL-9 on cervical cell apoptosis and proliferation. It is found that the overexpression of IL-9, a Th9 hallmark cytokine, can inhibit the proliferation, movement, migration and invasion of cervical cells.
EMT is a process in which epithelial cells undergo a mesenchymal phenotype transition, which is characterized by E-cadherin function loss via activation of one or more factors (for example, N-cadherin, Snail Family Transcriptional Repressor 1 (SNAIL), Snail Family Transcriptional Repressor 2 (SLUG), Zinc Finger E-Box-Binding Homeobox (ZEB) and Twist- Related Protein (TWIST))[16,17]. Cancer cells receiving EMT have increased migration ability and invasiveness, which causes primary tumor cell metastasis and forms systemic secondary tumors[18]. To enable effective immunotherapy, the markers that expose the tumor to immune surveillance must increase expression. Our results show that when IL-9 is overexpressed, the observed expression of N-cadherin and vimentin in HPV cervical cell lines is down-regulated, while the expression of E-cadherin is up-regulated, indicating that Th9 exhibits immunosuppressive activity. Therefore, these cells can examine the evasion mechanism by which tumor cells evades immune surveillance. Therefore, our research shows that Th9 contributes to anti-tumor immune response with the help of multiple mechanisms.
The activation of T lymphocytes requires two signals, namely T cell receptor recognition peptide (represented by the major histocompatibility complex molecule on APC) and the second signal provided by costimulatory molecules[19]. The balance between positive and negative signals is crucial in maximizing the ability of adaptive immune response to defend the host and/ or maintain self-tolerance. One of the previously identified T lymphocyte inhibitory molecules is PD-L1. PD-L1 on APC binds to the receptor PD-1 on activated T lymphocytes, resulting in inhibition of T lymphocyte activity. The abnormally high expression of PD-L1 is common in various tumor tissues, which is related to tumor progression. Studies have shown that during chronic viral infection and cancer, PD-1 expressed on the surface of T cell membranes encounters PD-L1 on infected or tumor cells, which is related to the failure of virus-specific Cluster of Differentiation 8 (CD8+) T cells[20]. The results of this study indicate that IL-9 transfection group can reduce the expression of PD-L1 and PD-1 mRNA, while IL-9 inhibition group increases the expression of PD-L1 and PD-1 mRNA. This suggests that Th9 cytokines may promote lymphocyte proliferation and cytotoxic activity by down-regulating PD-L1 and PD-1, thereby inhibiting immune escape of tumor cells.
To conclude, we found that IL-9R is overexpressed in cervical cancer cells to mediate the IL-9 anti-tumor immune response secreted by Th9 cells. These cytokines can limit tumor proliferation, movement, migration and invasion, and induce the up-regulation of E-cadherin expression in cervical cells, inhibit the expression of PD-L1 and PD-1, thereby expressing tumor antigens and unable to escape immunoassay. Therefore, this study helps us to better understand the role of the newly discovered Th9 cells in cervical cancer and their future use as potential therapeutic targets for patients with cervical cancer.
Conflict of interests:
The authors declared no conflict of interest.
References
- Gao M, Li YH, Li LH, Deng XZ, Nie Y, Li F, et al. Prevalence of anal HPV infection among HIV positive men who have sex with men. J Trop Med 2019;19(6):785-8.
[Crossref] [Google Scholar] [PubMed]
- Santegoets SJ, van Ham VJ, Ehsan I, Charoentong P, Duurland CL, van Unen V, et al. The anatomical location shapes the immune infiltrate in tumors of same etiology and affects survival. Clin Cancer Res 2019;25(1):240-52.
[Crossref] [Google Scholar] [PubMed]
- Morgan EL, Macdonald A. Autocrine STAT3 activation in HPV positive cervical cancer through a virus-driven Rac1-NFκB-IL-6 signalling axis. PLoS Pathog 2019;15(6):e1007835.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Zheng H, Zhao S, Yang X. Overexpression of LATS1 suppresses proliferation and invasion of head and neck squamous cell carcinoma B88 cells and its clinical significance. Tumor 2019;39(1):1-9.
- Xu P, Wang Y, Jiang WW. Bioinformatics analysis of Aurora-A in head and neck squamous cell carcinoma. Chin Clin Oncol 2020;25(7):577-83.
- Xiao R, An Y, Ye W, Derakhshan A, Cheng H, Yang X, et al. Dual antagonist of cIAP/XIAP ASTX660 sensitizes HPV− and HPV+ head and neck cancers to TNFα, TRAIL, and radiation therapy. Clin Cancer Res 2019;25(21):6463-74.
[Crossref] [Google Scholar] [PubMed]
- Dai Y, Somoza RA, Wang L, Welter JF, Li Y, Caplan AI, et al. Exploring the trans‐cleavage activity of CRISPR‐Cas12a (cpf1) for the development of a universal electrochemical biosensor. Angew Chem Int Ed Engl 2019;131(48):17560-6.
[Crossref] [Google Scholar] [PubMed]
- Yang X, Cheng H, Chen J, Wang R, Saleh A, Si H, et al. Head and neck cancers promote an inflammatory transcriptome through coactivation of classic and alternative NF-kB Pathways. Cancer 2019;7:1-5.
[Crossref] [Google Scholar] [PubMed]
- Haiyan Z, Zhiyong J, Qingyan S, Jing L. The correlation between high-risk HPV infection and immune function and oncogene expression in patients with cervical cancer. Chin J Microecol 2019;31(11):1322-5.
- Tang C, Lin L, Zhou W, Liu X, Fu Y, Zhang L, et al. CDK6 inhibits lymphoid cell infiltration and represents a prognostic marker in HPV+ squamous cell carcinoma of head and neck. Chin J Cancer Res 2019;31(6):901-9.
[Crossref] [Google Scholar] [PubMed]
- Aggarwal C, Cohen RB, Morrow MP, Kraynyak KA, Sylvester AJ, Knoblock DM, et al. Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin Cancer Res 2019;25(1):110-24.
[Crossref] [Google Scholar] [PubMed]
- Feng J. Effect of maixuekang capsule combined with cerebroside and carnosine injection on neurological function of patients with ischemic stroke in convalescent period. Clin Res 2020;28(3):107-9.
- Lyu X, Zhang M, Li G, Cai Y, Li G, Qiao Q. Interleukin‐6 production mediated by the IRE 1‐XBP 1 pathway confers radioresistance in human papillomavirus‐negative oropharyngeal carcinoma. Cancer Sci 2019;110(8):2471-84.
[Crossref] [Google Scholar] [PubMed]
- Campbell-Tofte J, Vrahatis A, Josefsen K, Mehlsen J, Winther K. Investigating the aetiology of adverse events following HPV vaccination with systems vaccinology. Cell Mol Life Sci 2019;76(1):67-87.
[Crossref] [Google Scholar] [PubMed]
- Dick S, Kremer WW, De Strooper LM, Lissenberg-Witte BI, Steenbergen RD, Meijer CJ, et al. Long-term CIN3+ risk of HPV positive women after triage with FAM19A4/miR124-2 methylation analysis. Gynecol Oncol 2019;154(2):368-73.
[Crossref] [Google Scholar] [PubMed]
- Li TT, Wu L, Lu X, Xia JH. Research progress on infection status and immunotherapy of HPV. J Pract Med 2019;35(18):2862-7.
- Gong X, Tian T, Liang SM. Effects of whole body radiotherapy on ICOS and ICOSL of immune cell of tumor micro-environmental of tumor-bearing mice with U14. China Med Equip 2019;16(8):138-42.
- Kremer WW, Van Zummeren M, Breytenbach E, Richter KL, Steenbergen RD, Meijer CJ, et al. The use of molecular markers for cervical screening of women living with HIV in South Africa. AIDS 2019;33(13):2035-42.
[Crossref] [Google Scholar] [PubMed]
- Su L, Han J. Clinical significance of multiple high-risk HPV infection in cervical squamous cell lesions. J Clin Med Lit 2020;7(63):60-1.
- Luo C, Yan X, Lin Q. Effect of polyunsaturated fatty acids on immune enhancement in patients with malignant tumor. China Health Care Nutr 2020;30(26):75-6.