- *Corresponding Author:
- Fang Zhou
Department of Hematology, The 960th Hospital of the People’s Liberation Army (Formerly the General Hospital of Jinan Military Region), Jinzhou Medical University, Jinan, Shandong 250031, China
E-mail: hsrlw@163.com
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “247-254” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Cluster of differentiation 47-thrombospondin 1 is newly discovered pathway as T-cell stimulatory signal can induce immune tolerance that provides a new idea for the therapy of acute graft vs. host disease after transplantation haploid. However, numerous studies have found the regulation of cluster of differentiation 47-thrombospondin 1 on the immune system is bi-directional. Currently the specific mechanisms of cluster of differentiation 47-thrombospondin 1 pathway promotes immune tolerance is unclear. The acute graft vs. host disease model with cluster of differentiation 47 monoclonal antibody was occurred to test the function of the cluster of differentiation 47-thrombospondin 1 in acute graft vs. host disease. The consequences of the investigation affirmed that the application of cluster of differentiation 47 monoclonal antibody lessened the pathological manifestations and associated clinical symptoms of acute graft vs. host disease. Cluster of differentiation 47 monoclonal antibody has no significant impact on the proliferation of donor T lymphocytes, but it promotes the apoptosis of donor T lymphocytes. It favors the secretion of interleukin-4 and diminishes the secretion of interferon gamma and interleukin-17a. These data exhibit that the mechanisms by which cluster of differentiation 47-thrombospondin 1 inhibits the progress of acute graft vs. host disease are multifaceted, including promoting the secretion of inhibited inflammatory factors, discouraging the secretion of inflammatory factors, and promoting and frustrating the expression of transcription factors. Cluster of differentiation 47 therapeutic antibodies can convert a distinct way of treating acute graft vs. host disease by inhibiting the development of inflammation. Cluster of differentiation 47 therapeutic antibodies can improve a distinct idea for the therapy of acute graft vs. host disease by inducing immune tolerance of donor T cells.
Keywords
Transplantation, cluster of differentiation 47, acute graft vs. host disease, cluster of differentiation 47-thrombospondin 1
Haploid transplantation has been used in the treatment of malignant blood diseases widely such as leukemia[1]. However, the occurrence of acute Graft vs. Host Disease (aGVHD) seriously affects the survival rate and quality of life of patients after transplantation. aGVHD is a complicated organ injury disease based on cascade inflammation[1]. The pathological background of aGVHD is the heavy immune attack of donor T cells on recipient tissue after activation[2].
Consequently, one of the crucial processes to control aGVHD is to inhibit the donor T cell response or induce its energy. In current years, it has been observed that Cluster of Differentiation 47-Thrombospondin-1 (CD47-TSP1) is recently recognized T cell stimulatory signal pathway that can remarkably reduce the threshold of T cell activation with a large number of in-depth investigations on CD47 and its ligand molecules[3], that providing a new idea for the therapeutic method of aGVHD.
CD47 is an integral transmembrane protein that broadly expressed on a variety of cells. CD47 was discovered to play a significant role in immune regulation, aplastic anemia, stress and tumor development in mice[4]. TSP1 is the principal secretory ligand of CD47 that binds to CD47 through its C-terminal Virtual Machine Manager (VMM)[5]. TSP1 is an effective anti-inflammatory factor that can quickly infiltrate the blood into the injured tissue without causing pathogens[6].The antiinflammatory effect of TSP1 is mainly through Antigen Presenting Cell (APC) inhibiting the secretion of pro-inflammatory factors and promoting phagocytosis of phagocytes[7,8]. Transforming Growth Factor beta (TGF-β) plays a vital role in the differentiation of T cells and TSP1 is the essential agonist of TGF-β[9]. CD47 on activated T cells binds to TSP1 and promotes to inhibit the secretion of the pathway and multiple proinflammatory factors.
The anti-CD47 monoclonal antibody B6H12 has been shown a good anti-immunotherapy effect in multiple research groups. Investigations found B6H12 can block CD47/Signal Regulatory Protein-alpha (SIRPα) via inhibition of tumor extension in the tumor model subject, enhances phagocytosis of macrophages to the tumor[10]. While B6H12 inhibits the binding of CD47 to TSP1, it can also recombine the C-terminal structure of TSP1 as an agonist to mimic the effect of TSP1[11]. Various investigations have authenticated that B6H12 not only mimics the biological function of TSP1, but also blocks the binding of SIRPα[12,13]. Complementary studies have observed that the impact of B6H12 on CD47 is independent of TSP1 and SIRPα. Therefore, B6H12 can be estimated as its pharmacological agonist[14].
At present, the detailed mechanism of CD47-TSP1 pathway inducing effector T cells to promote immune tolerance is still unclear and needs further experiments to investigate. In the later investigation, we will use CD47 monoclonal antibody in the established haploid transplanted aGVHD in mice model to explore the influence of CD47-TSP1 on aGVHD mice model and its specific mechanism.
Materials and Methods
Mice:
Rat C57BL/6 (H-2b) (Specific Pathogen Free (SPF) grade, weight 20±2 g) and mice CB6F1 (H-2 b/d) (SPF grade, weight 20±2 g) were purchased from the Animal Experimental Center of Shandong University. All animal experiments comply with the regulations and requirements of the Laboratory Animal Center of Shandong University.
aGVHD model in mice:
4-6 h before transplantation, all the recipient CB6F1 mice were irradiated for 14 min in the irradiation range with a linear accelerator and the total irradiation amount was about 8.5 Gy. After systemic irradiation of the recipient mice, a suspension (1 ml) of 2×106 spleen cells and 1×107 bone marrow cells from C57BL/6 male donor mice was administered to the A1 group for 4-6 h. A suspension (0.1 ml) of 2×106 spleen cells and 1×107 bone marrow cells from C57BL/6 male donor mice was injected into the A2 group and then immediately infused with 100 μl CD47 monoclonal antibodies (B6H12). The mental state, position change, fur, body weight and stool of the recipient mice and the survival rate were observed daily. D 0, D 1, D 3, D 7, D 11 and D 15 were taken from the tail vein blood of mice for peripheral blood picture detection. The liver, lung, small intestine and skin of mice were subject to pathological examination. The presence or absence of aGVHD in each group was observed and the PAFS score was performed.
T cell proliferation and apoptosis:
After 3 d of transplantation, take mice spleen cells to adjust the cell concentration as 5×106/ml. The Carboxyfluorescein Succinimidyl Ester (CFSE) peak of MHC-I+CD3+ cells was detected by up flow cytometry. After 10 d of transplantation, take mouse spleen cells to adjust the cell concentration as 5×106/ml. Add Annexin Fluorescein Isothiocyante (V-FITC) after labeling splenocytes with CD3, MHC-I+CD3+ cells were detected by the flow cytometry.
Influence on the polarization of T helper (Th) subgroups:
After 10 d of transplantation, the peripheral blood supernatants of the two groups of mice were collected and stored in a refrigerator at -80°. The cytokine Interferon gamma (IFN-γ) secreted by Th1 cells, the cytokine Interleukin (IL)-4 secreted by Th2, IL-10 and the cytokine IL-17a secreted by Th17 were measured by Enzyme-Linked Immunoassay (ELISA).
Effect on the signaling pathway of Th cells differentiation:
On the 10th d after transplantation, the spleens of each group of recipient mice were obtained, Ribonucleic Acid (RNA) was extracted and the expression of T-Box Transcription factor (T-BeT) messenger RNA (mRNA), GATA binding protein 3 (GATA3), C-maf in groups A1 and A2 was determined by quantitative Polymerase Chain Reaction (PCR) technology and verified by Western blotting technology. The detailed operation method can be inquired on the website.
Results and Discussion
A vast amount of data indicates that the combination of CD47 and TSP1 plays a principal role in cellular stress and basic metabolic processes[15]. TSP1 can be immediately released into the blood in damaged tissues.
TSP1 is an endogenous T cell inhibitor that can exert its immunosuppressive effect by inducing Regulatory T cells (Tregs). Investigations have found that CD47/ TSP1 can also directly inhibit the occurrence of inflammation and its mechanism of action is to induce the expression of BNIP3 ((B-Cell Lymphoma 2) Bcl- 2/adenovirus E1B 19kDA-acting protein) and mediate T cell apoptosis[16]. The principal function of TSP1 on T cells is to inhibit T-Cell Receptor (TCR) signal transduction. It has also been reported that CD47 and Heparin Sulfate Proteoglycans (HSPGs) also inhibit the activation of T cells[17]. B6H12 is a CD47 monoclonal antibody that can mimic the biological function of TSP1 while blocking SIRP binding. B6H12 was used to explore the mechanism of CD47/TSP1 on the progression of aGVHD.
aGVHD is still one of the principal reasons of death after allogeneic Hematopoietic Stem Cell Transplantation (allo-HSCT)[18]. The pathological process of aGVHD can be divided into 3 stages tissue damage caused by pretreatment (including intestinal mucosal epithelial cells and liver cells); the antigenpresenting cell presents the antigen which causes the activation of donor T cells and the expansion of clonal T cells; activated donor T cells and other immune cells release inflammatory cytokines and cause damage to target organs, causing specific clinical manifestations of tissues and organs such as skin, liver and intestines[19].
Cytokine storms mainly occur in the early stage after donor lymphocyte infusion, so cytokines and their receptors may become potential diagnostic biomarkers for aGVHD[20]. Based on a large-sample clinical study, Paczesny uses an antibody microarray technology to detect the combined detection of four cytokines (IL-2α, Tumor Necrosis Factor Receptor 1 (TNFR1), IL-8 and Hepatocyte Growth Factor (HGF)) as a biomarker for the diagnosis of aGVHD, which is highly specific. It is the first time to use it as a reliable clinical evidence for the onset and prognosis of aGVHD[21]. Studies have shown that both Th1 and Th17 cells can mediate the occurrence of aGVHD. Th2 cytokines IL-4 and IL-10 inhibit the secretion of IFN-γ and reduce the severity of aGVHD[22]. T cells can promote immune response by expressing pro-inflammatory cytokines and participate in the process of humoral immunity and cellular immunity. Its common characteristic molecules include T-bet, GATA-3 and so on. T-bet as a Th1-specific transcription factor that plays an important role in the development of Th1 cells and inhibits the synthesis of Th2 cytokines. GATA-3 is the specific transcription factor of Th2. c-Maf is a transcription factor with basic leucine zipper (b-Zip), which protects tissues from infection or inflammation[23].
After the hybrid mice (CB6F1) were irradiated with myeloablative treatment, one group of mice underwent haploidentical transplantation with C57BL/6 donors and the other group underwent the same haploidentical transplantation with B6H12 infusion. Observing the aGVHD-related data of the two groups of mice after transplantation found that the mice in the B6H12 group had a longer survival time than the control group. B6H12 group with lighter signs such as arched back and hair loss, and weakened the pathological manifestations of the target organs. The apoptosis of donor T lymphocytes in group A2 was faster, but there was no significant difference in the proliferation of donor T lymphocytes between the two groups. The cytokine IL-4 secreted by Th2 in the blood of group A2 increased, but the cytokines IFN-γ and IL-17a secreted by Th1 and Th7 decreased, and the expression of related transcription factors T-BeT and C-maf mRNA also decreased, but the expression level of GATA3 has been increased. Experiments show that the mechanism of aGVHD reduction in group A2 may be to accelerate the apoptosis of T cells, the secretion of Th1 and Th17 pro-inflammatory cytokines decreases, and the secretion of Th2 anti-inflammatory cytokines increases. The mechanism may be inhibiting the differentiation of Th1 and Th17 cells or inhibiting the secretion of related cytokines.
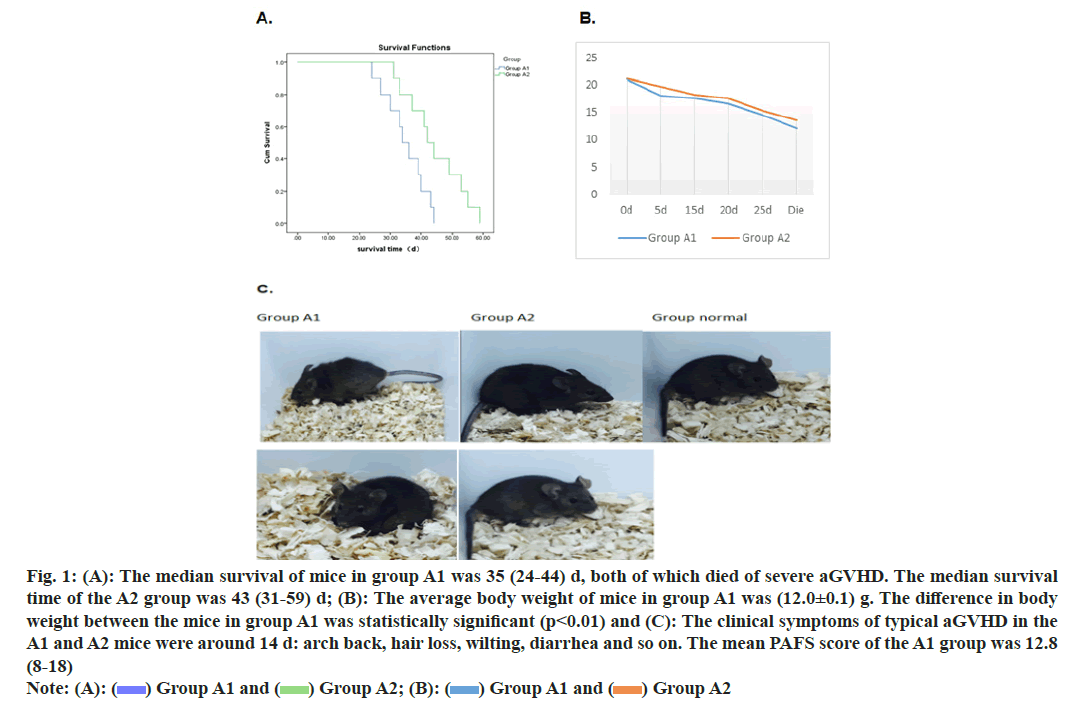
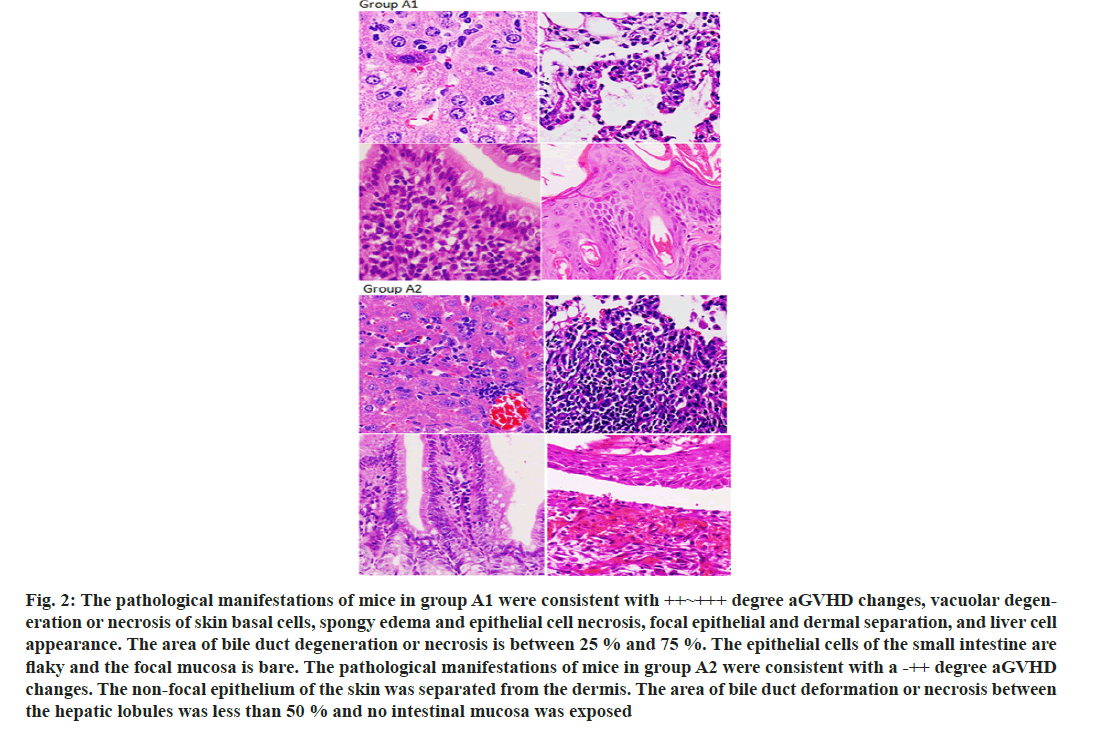
After establishing a stable haploid transplanted aGVHD in mice model, the mice were treated with infusion of CD47 monoclonal antibody to monitor the relevant data of the occurrence of aGVHD in mice. We observe that CD47 monoclonal antibody prolong the survival of aGVHD mice (fig. 1A) and alleviate the clinical symptoms of aGVHD in mice (fig. 1B and fig. 1C). Simultaneously, CD47 monoclonal antibody reduces the pathological manifestations of aGVHD in mice (fig. 2A and fig. 2B).
Fig. 1: (A): The median survival of mice in group A1 was 35 (24-44) d, both of which died of severe aGVHD. The median survival time of the A2 group was 43 (31-59) d; (B): The average body weight of mice in group A1 was (12.0±0.1) g. The difference in body weight between the mice in group A1 was statistically significant (p<0.01) and (C): The clinical symptoms of typical aGVHD in the A1 and A2 mice were around 14 d: arch back, hair loss, wilting, diarrhea and so on. The mean PAFS score of the A1 group was 12.8 (8-18) Note: (A): ( ) Group A1 and (
) Group A1 and ( ) Group A2; (B): (
) Group A2; (B): ( ) Group A1 and (
) Group A1 and ( ) Group A2
) Group A2
Fig. 2: The pathological manifestations of mice in group A1 were consistent with ++~+++ degree aGVHD changes, vacuolar degeneration or necrosis of skin basal cells, spongy edema and epithelial cell necrosis, focal epithelial and dermal separation, and liver cell appearance. The area of bile duct degeneration or necrosis is between 25 % and 75 %. The epithelial cells of the small intestine are flaky and the focal mucosa is bare. The pathological manifestations of mice in group A2 were consistent with a -++ degree aGVHD changes. The non-focal epithelium of the skin was separated from the dermis. The area of bile duct deformation or necrosis between the hepatic lobules was less than 50 % and no intestinal mucosa was exposed
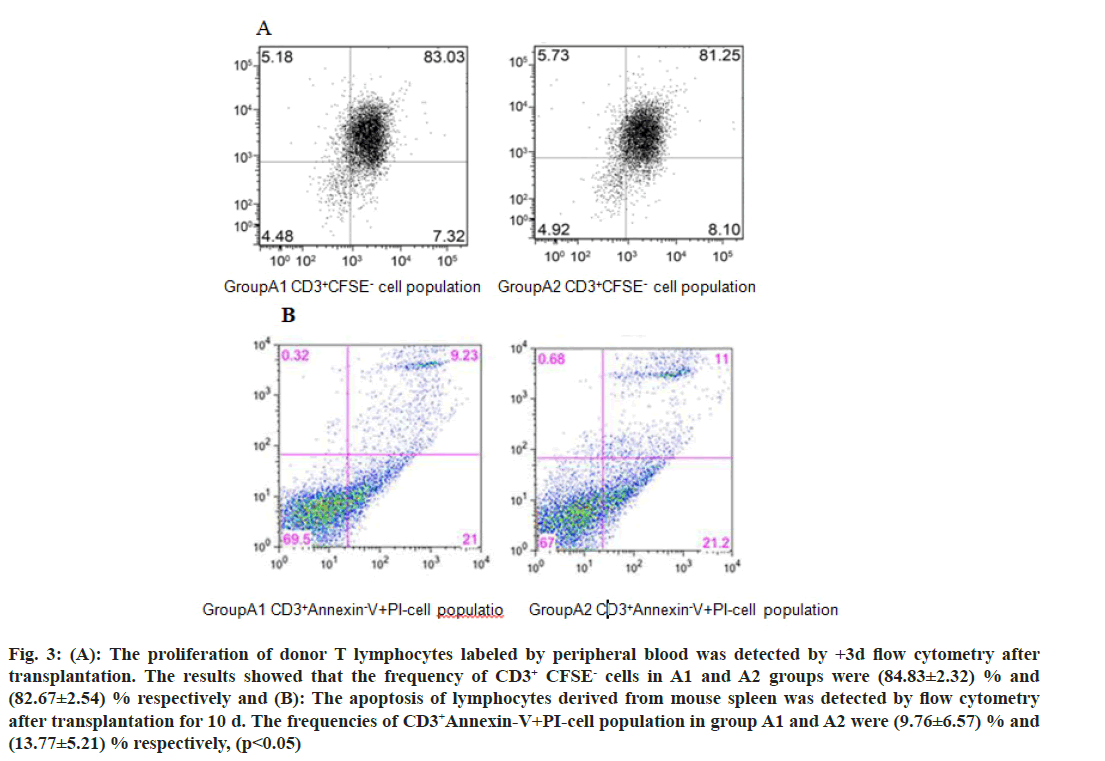
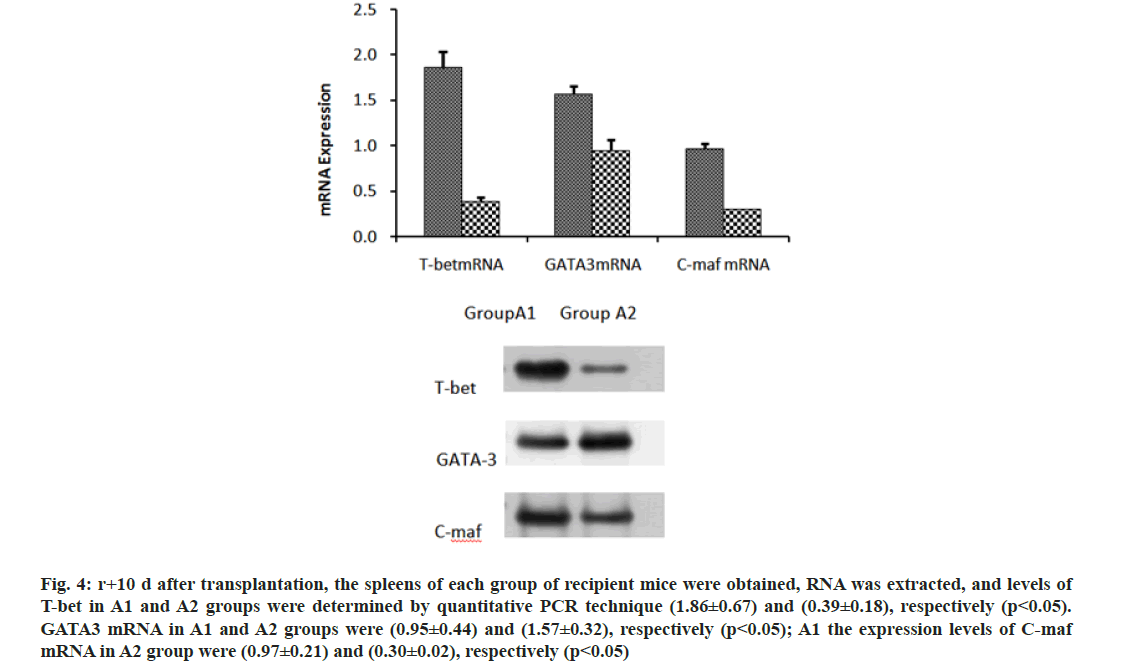
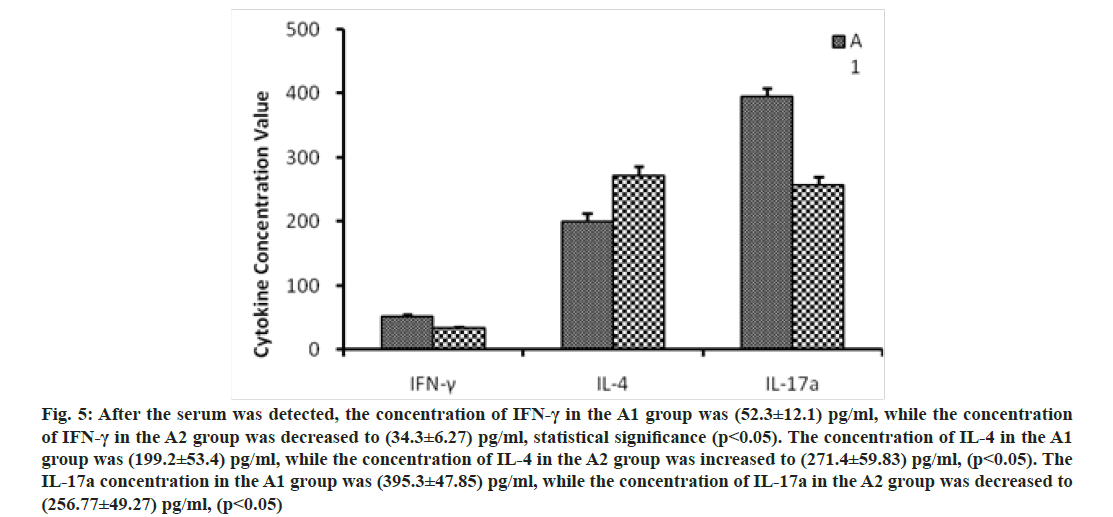
We further investigated the mechanism of CD47-TSP1 in aGVHD. CD47 monoclonal antibody had no notable influence on the proliferation of donor T lymphocytes during aGVHD intervention (fig. 3A), but promoted the apoptosis of donor T lymphocytes (fig. 3B). CD47 monoclonal antibody promoted the secretion of IL-4 by Th2 cells, reducing Th-1 cytokine IFN-γ, Th-17 cytokine IL-17a secretion and decreased the transcription factors T-bet, C-maf mRNA expression and increased the expression of GATA-3 (fig. 4 and fig. 5).
Fig. 3: (A): The proliferation of donor T lymphocytes labeled by peripheral blood was detected by +3d flow cytometry after transplantation. The results showed that the frequency of CD3+ CFSE- cells in A1 and A2 groups were (84.83±2.32) % and (82.67±2.54) % respectively and (B): The apoptosis of lymphocytes derived from mouse spleen was detected by flow cytometry after transplantation for 10 d. The frequencies of CD3+Annexin-V+PI-cell population in group A1 and A2 were (9.76±6.57) % and (13.77±5.21) % respectively, (p<0.05)
Fig. 4: r+10 d after transplantation, the spleens of each group of recipient mice were obtained, RNA was extracted, and levels of T-bet in A1 and A2 groups were determined by quantitative PCR technique (1.86±0.67) and (0.39±0.18), respectively (p<0.05). GATA3 mRNA in A1 and A2 groups were (0.95±0.44) and (1.57±0.32), respectively (p<0.05); A1 the expression levels of C-maf mRNA in A2 group were (0.97±0.21) and (0.30±0.02), respectively (p<0.05)
Fig. 5: After the serum was detected, the concentration of IFN-γ in the A1 group was (52.3±12.1) pg/ml, while the concentration of IFN-γ in the A2 group was decreased to (34.3±6.27) pg/ml, statistical significance (p<0.05). The concentration of IL-4 in the A1 group was (199.2±53.4) pg/ml, while the concentration of IL-4 in the A2 group was increased to (271.4±59.83) pg/ml, (p<0.05). The IL-17a concentration in the A1 group was (395.3±47.85) pg/ml, while the concentration of IL-17a in the A2 group was decreased to (256.77±49.27) pg/ml, (p<0.05)
The allogeneic immune response caused by the histocompatibility antigen caused by the APC of the donor allogeneic lymphocytes that identify the recipient is the pathological basis of aGVHD. The intervention of donor T cell activation is one of the essential methods for the treatment of aGVHD. The activation of donor T cells requires the participation of antigen and stimulatory molecules. CD47-TSP1 is used as a stimulatory signal of T cells, and its influence on aGVHD and its mechanism need to be further explored.
CD47 was previously confirmed to perform a significant role in the immune processes such as mediate leukocyte adhesion and migration, T cell activation and induction of immune tolerance. However, the performance and mechanism of CD47 and its ligand TSP1 in aGVHD are still unclear.
Some investigations have observed that CD47 monoclonal antibody has inhibitory activity. The anti- CD47 monoclonal antibody B6H12 has been proved by several research groups to have good anti-immunity and immune tolerance induction treatment effects. In this investigation, the recipient mice were infused with anti- CD47 monoclonal antibody B6H12 to induce immune tolerance. It was observed that CD47 monoclonal antibodies can significantly prolong the survival time of aGVHD mice and reduce aGVHD symptoms. Under the intervention of CD47 monoclonal antibody, the mRNA expression of T-bet, C-maf and GATA3 is reduced. By inhibiting the secretion of IFN-γ, it promotes the secretion of IL-4, decreases the conversion of Th0 to Th1 cells and reduces the occurrence and development of aGVHD. At the same time, ELISA results showed that under the intervention of CD47 monoclonal antibody, Th2 cytokine IL-4 expression increased, Th-1 cytokine IFN-γ, Th-17 cytokine IL- 17a expression decreased, once again verified CD47 monoclonal antibody can decrease the occurrence of aGVHD by interfering with the expression of cytokines.
Although CD47-TSP1 is a stimulatory signal of T cells, studies have shown that the regulatory mechanism of TSP-1 and CD47 binding in vivo is bidirectional. When TSP-1 is structurally intact, the immune response can be negatively regulated by binding to CD47[24]. The main function of TSP1 on T cells is to inhibit TCR signal transduction. It has also been reported that CD47 and HSPGs inhibit the activation of T cells[25]. TSP-1 binds to CD47 and plays a regulatory role, which affect initial T cell differentiation, inhibit early T cell activation and induce naive T cell energy. Immune tolerance principally depends on promoting T cell energy and producing Treg cell. CD47-TSP1 inhibit IL-12 mediated differentiation of naive T cells into Th1 subgroup but has no inhibitory effect on Th2 subgroup[26]. The Th1 subset of T cells mainly secretes cytokines that promote inflammation such as IL-2 and IFN-γ.
Furthermore, TSP1 is an inhibitor of TCR signal transduction and an agonist of TGF-β[27]. TSP1 activates TGF-β principally through the KRFK sequence[28]. In vitro experiments determined that Treg inhibit naive T cells. TGF-β is a cytokine with diverse functions, but must be activated from its dormant precursor state to conduct signal transduction. The TGF-β precursor can be transformed into an activated state through a variety of mechanisms, including proteolysis, binding with integrin’s, interacting with the cytoskeletonextracellular matrix mechanical force combined with TSP1, etc.[29]. The specific mechanism of activation is through the combination of KRFK sequence in TSR1. The N-terminal region repeating sequence LSKL of the precursor TGF-β Latent Related Peptide (LAP), which disrupts the interaction of the LAP mature domain and exposes the receptor-binding mature domain site, so that TGF can activate its biological function[30].
TSP-1 increases rapidly and transiently in stressed or damaged tissues or in response to inflammatory signals. TSP1 promote the secretion of cytokines IL-6 and IL-1β, and inhibiting TSP1 reduce the secretion level of cytokines in inflammation[31]. At the same time, TSP1 induce apoptosis of T cells and B cells to reduce inflammation. This function is produced by the combination of the carboxyl end of TSP1 and CD47. Apoptosis signals mediate the translocation of CD47-bound BNIP3 to mitochondria to trigger cell apoptosis. TSP1 promote the secretion of TGF-β from monocytes[32]. In addition, CD47-TSP1 promotes the differentiation of Treg cells through TGF-β dependently, inhibits the proliferation of Th0, Th1 and Th2 cells and the production of related cytokines to reduce inflammation[33]. CD47-TSP1 can also inhibit IL-12 secretion by macrophages. The combination of CD47 on the surface of APC and TSP1 inhibit the secretion of inflammasomes and IL-1b, and also inhibit T cell proliferation, activation and cytotoxicity[34]. The interaction between TSP-1 and CD47 reduces inflammation.
The differentiation of CD4+ T cells is mainly affected by the signals provided by cytokines and APC[35,36]. The CD47 binding peptide derived from TSP1 inhibit the secretion of IL-2R and inhibit the secretion of IFN-γ from DC, thereby inhibiting the differentiation of Th1. Exogenous TSP-1 inhibits the differentiation of Th17 and stimulates the differentiation of CD4+ T cells into Treg cells[37]. Dendritic cells produce TSP-1, which binds to CD47 on its surface as an autocrine factor, activates downstream signaling pathways[38]. The communication between CD47 and its ligands mediates various immune regulation processes, including induction of immune tolerance and intervention of inflammatory response. However, the role of other ligands of CD47 in aGVHD and whether the ligands affect each other is still unclear.
In conclusion, the research on the pathway of CD47 and its ligands will surely open up unspoiled ideas for the future targeted therapy of aGVHD.
Conflict of interests:
The authors have declared no conflict of interests.
References
- Zeiser R, Blazar BR. Acute graft-vs.-host disease—biologic process, prevention and therapy. N Engl J Med 2017;377(22):2167-79.
[Crossref] [Google Scholar] [PubMed]
- Nguyen HD, Chatterjee S, Haarberg KM, Wu Y, Bastian D, Heinrichs J, et al. Metabolic reprogramming of alloantigen-activated T cells after hematopoietic cell transplantation. J Clin Invest 2016;126(4):1337-52.
[Crossref] [Google Scholar] [PubMed]
- Vallejo AN, Mügge LO, Klimiuk PA, Weyand CM, Goronzy JJ. Central role of thrombospondin-1 in the activation and clonal expansion of inflammatory T cells. J Immunol 2000;164(6):2947-54.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Fan J, Ju D. Insights into CD47/SIRPα axis-targeting tumor immunotherapy. Antibody Ther 2018;1(2):37-42.
[Crossref] [Google Scholar] [PubMed]
- Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, et al. Differential interactions of thrombospondin-1, 2 and 4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 2009;284(2):1116-25.
[Crossref] [Google Scholar] [PubMed]
- Baboolal K, Jones GA, Janezic A, Griffiths DR, Jurewicz WA. Molecular and structural consequences of early renal allograft injury. Kidney Int 2002;61(2):686-96.
[Crossref] [Google Scholar] [PubMed]
- Doyen V, Rubio M, Braun D, Nakajima T, Abe J, Saito H, et al. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J Exp Med 2003;198(8):1277-83.
[Crossref] [Google Scholar] [PubMed]
- Li Z, He L, Wilson KE, Roberts DD. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J Immunol 2001;166(4):2427-36.
[Crossref] [Google Scholar] [PubMed]
- Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-β-dependent and independent mechanisms. Matrix Biol 2012;31(3):178-86.
[Crossref] [Google Scholar] [PubMed]
- Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci 2012;109(17):6662-7.
[Crossref] [Google Scholar] [PubMed]
- Barazi HO, Li Z, Cashel JA, Krutzsch HC, Annis DS, Mosher DF, et al. Regulation of integrin function by CD47 ligands: Differential effects on αvβ3 and α4β1 integrin-mediated adhesion. J Biol Chem 2002;277(45):42859-66.
[Crossref] [Google Scholar] [PubMed]
- Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, et al. Anti-CD47 antibody–mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci 2013;110(27):11103-8.
[Crossref] [Google Scholar] [PubMed]
- Rodríguez-Jiménez P, Chicharro P, Llamas-Velasco M, Cibrian D, Trigo-Torres L, Vara A, et al. Thrombospondin-1/CD47 interaction regulates Th17 and Treg differentiation in psoriasis. Front Immunol 2019;10:1268.
[Crossref] [Google Scholar] [PubMed]
- Kaur S, Elkahloun AG, Singh SP, Chen QR, Meerzaman DM, Song T, et al. A function-blocking CD47 antibody suppresses stem cell and EGF signaling in triple-negative breast cancer. Oncotarget 2016;7(9):10133-52.
[Crossref] [Google Scholar] [PubMed]
- Soto-Pantoja DR, Kaur S, Roberts DD. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol 2015;50(3):212-30.
[Crossref] [Google Scholar] [PubMed]
- Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. J Immunol 2007;178(9):5930-9.
[Crossref] [Google Scholar] [PubMed]
- Li Z, He L, Wilson KE, Roberts DD. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J Immunol 2001;166(4):2427-36.
[Crossref] [Google Scholar] [PubMed]
- Gatza E, Choi SW. Approaches for the prevention of graft-vs.-host disease following hematopoietic cell transplantation. Int J Hematol Oncol 2014;4(3):113-26.
[Crossref] [Google Scholar] [PubMed]
- Blazar BR, Murphy WJ, Abedi M. Advances in graft-vs.-host disease biology and therapy. Nat Rev Immunol 2012;12(6):443-58.
[Crossref] [Google Scholar] [PubMed]
- Toubai T, Tanaka J, Paczesny S, Shono Y, Reddy P, Imamura M. Role of cytokines in the pathophysiology of Acute Graft-vs.-Host Disease (GVHD)–are serum/plasma cytokines potential biomarkers for diagnosis of acute GVHD following allogeneic hematopoietic cell transplantation (Allo-HCT)? Curr Stem Cell Res Ther 2012;7(3):229-39.
[Crossref] [Google Scholar] [PubMed]
- Paczesny S, Krijanovski OI, Braun TM, Choi SW, Clouthier SG, Kuick R, et al. A biomarker panel for acute graft-vs.-host disease. Blood 2009;113(2):273-8.
[Crossref] [Google Scholar] [PubMed]
- Ferrara JL. The cytokine modulation of acute graft-vs.-host disease. Bone Marrow Transplant 1998;21:S13-5.
[Google Scholar] [PubMed]
- Rutz S, Noubade R, Eidenschenk C, Ota N, Zeng W, Zheng Y, et al. Transcription factor c-Maf mediates the TGF-β-dependent suppression of IL-22 production in TH17 cells. Nat Immunol 2011;12(12):1238-45.
[Crossref] [Google Scholar] [PubMed]
- Avice MN, Rubio M, Sergerie M, Delespesse G, Sarfati M. Role of CD47 in the induction of human naive T cell anergy. J Immunol 2001;167(5):2459-68.
[Crossref] [Google Scholar] [PubMed]
- Li Z, He L, Wilson KE, Roberts DD. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J Immunol 2001;166(4):2427-36.
[Crossref] [Google Scholar] [PubMed]
- Germann T, Gately MK, Schoenhaut DS, Lohoff M, Mattner F, Fischer S, et al. Interleukin-12/T cell stimulating factor, a cytokine with multiple effects on T helper type 1 (Th1) but not on Th2 cells. Eur J Immunol 1993;23(8):1762-70.
[Crossref] [Google Scholar] [PubMed]
- Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-β by thrombospondin-1: Mechanisms and physiology. Cytokine Growth Factor Rev 2000;11(1-2):59-69.
[Crossref] [Google Scholar] [PubMed]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, et al. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell 1998;93(7):1159-70.
[Crossref] [Google Scholar] [PubMed]
- Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor β1–An intimate relationship. Eur J Cell Biol 2008;87(8-9):601-15.
[Crossref] [Google Scholar] [PubMed]
- Young GD, Murphy-Ullrich JE. Molecular interactions that confer latency to transforming growth factor-β. J Biol Chem 2004;279(36):38032-9.
[Crossref] [Google Scholar] [PubMed]
- Xing T, Wang Y, Ding WJ, Li YL, Hu XD, Wang C, et al. Thrombospondin-1 production regulates the inflammatory cytokine secretion in THP-1 cells through NF-κB signaling pathway. Inflammation 2017;40(5):1606-21.
[Crossref] [Google Scholar] [PubMed]
- Fang LL, Yu HQ, Wu RJ, He C, Li M, Yan H, et al. Thrombospondin 1 modulates monocyte properties to suppress intestinal mucosal inflammation. J Innate Immun 2015;7(6):601-11.
[Crossref] [Google Scholar] [PubMed]
- Grimbert P, Bouguermouh S, Baba N, Nakajima T, Allakhverdi Z, Braun D, et al. Thrombospondin/CD47 interaction: A pathway to generate regulatory T cells from human CD4+ CD25-T cells in response to inflammation. J Immunol 2006;177(6):3534-41.
[Crossref] [Google Scholar] [PubMed]
- Kaur S, Soto-Pantoja DR, Stein EV, Liu C, Elkahloun AG, Pendrak ML, et al. Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci Rep 2013;3(1):1673.
[Crossref] [Google Scholar] [PubMed]
- O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 2010;327(5969):1098-102.
[Crossref] [Google Scholar] [PubMed]
- Baumgartner JM, Palmer BE, Banerjee A, McCarter MD. Role of melanoma secreted thrombospondin-1 on induction of immunosuppressive regulatory T cells through CD47. J Cancer Mol 2008;4(5):145-52.
- Wan X, Pei W, Shahzad KA, Zhang L, Song S, Jin X, et al. A tolerogenic artificial APC durably ameliorates experimental autoimmune encephalomyelitis by directly and selectively modulating myelin peptide–autoreactive CD4+ and CD8+ t cells. J Immunol 2018;201(4):1194-210.
[Crossref] [Google Scholar] [PubMed]
- Ferrari D, Gorini S, Callegari G, la Sala A. Shaping immune responses through the activation of dendritic cells–P2 receptors. Purinergic Signal 2007;3(1):99-107.