- *Corresponding Author:
- Zhiqin Wu
Department of General Internal Medicine, Daqing Hospital Combining Chinese Traditional and Western Medicine, Daqing, Heilongjiang 163515, China
E-mail: wuzhiqin2021lili@163.com
| This article was originally published in a special issue, “New Advancements in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(2)Spl Issue “252-261” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To analyze the mechanism of dexmedetomidine through mitochondrial and endoplasmic reticulum oxidative stress pathway in neonatal rat cardiomyocytes is the main objective of the study. Sprague Dawley neonatal rats were selected as the research object and then control group (group A), hydrogen peroxide group (group B), dexmedetomidine group (Group C) and dexmedetomidine+hydrogen peroxide group were constructed (Group D), four groups of neonatal rat cardiomyocyte oxidative injury models. After drug treatment, the morphology, lactate dehydrogenase, reduced glutathione, reactive oxygen species, caspase-3, 8, 9 and 12 activities of neonatal rat cardiomyocytes were detected in turn. The mitochondrial membrane potential and apoptosis rate were detected by flow cytometry and glucose-regulated protein 78 and inositol-requiring enzyme 1 alpha were detected by Western blot, expression of B-cell lymphoma 2 and Bcl-2-associated X proteins. 500 μmol/l was selected as the best reaction concentration of hydrogen peroxide. Compared with group A, the activity levels of lactate dehydrogenase and reactive oxygen species in cardiomyocytes in group B increased significantly and the activity level of reduced glutathione decreased significantly. Compared with group B, the activity of reduced glutathione in group D was significantly increased, while the activity of lactate dehydrogenase and reactive oxygen species decreased significantly. The apoptosis rate of cardiomyocytes in group A was 18.36 %±5.68 % and that in group D was 39.64 %±9.36 %. Compared with group A, the apoptosis rate in group B increased significantly; there was no significant change in the apoptosis rate in group C. Compared with group A, glucose-regulated protein 78 and inositol-requiring enzyme 1 alpha significantly improved; compared with group B, GRP78 and IRE1α in group D significantly reduced. Dexmedetomidine can inhibit the oxidative stress response and apoptosis induced by hydrogen peroxide in neonatal rat cardiomyocytes.
Keywords
Dexmedetomidine, mitochondria, endoplasmic reticulum, oxidative stress, apoptosis, myocardial ischemia-reperfusion
Global cardiovascular diseases have become the biggest disease threatening human health. The incidence rate and mortality rate of coronary heart disease are the highest. The most commonly used treatment for patients with myocardial infarction is drug and surgical thrombolysis. However, ischemic myocardium is easy to cause myocardial Ischemia- Reperfusion (IR) injury after reperfusion. At present, the effective myocardial protection measures are to complete ischemic preconditioning and post-treatment through physics and drugs. Oxidative stress refers to the functional damage and internal environment disorder caused by oxygen free radical production, calcium overload and inflammatory reaction when IR injury occurs in myocardium. Generally, human Reactive Oxygen Species (ROS) will increase or the function of antioxidant defense system will decrease, which will also destroy the biological substances and functions of mitochondria and Endoplasmic Reticulum (ER)[1-3]. A large number of studies have shown that the main way of IR injury in cardiomyocytes is apoptosis, which is directly related to cell types and mitochondria and ER are related to apoptosis. Dexmedetomidine is a highly selective alpha 2 (α2) adrenoceptor agonist. Its main function is inhibition. It is widely used in intensive care unit and surgery. Studies have confirmed that the drugs can protect important organs under stress through α2 adrenoceptor[4-6]. Some scholars also pointed out that dexmedetomidine reduce myocardial IR injury, which may be related to upstream mitochondrial Adenosine Triphosphate (ATP) sensitive potassium channel. At present, there is no formal report on the apoptosis signalling pathway of mitochondrial and ER oxidative stress transformation in the medical field. In view of this, we studied the mechanism of dexmedetomidine on reducing cardiomyocyte IR injury, in order to provide a scientific basis for the recovery of cardiomyocyte IR injury.
Materials and Methods
General information:
Experimental animals: 65 male Sprague Dawley (SD) rats, weighing 200±10 g, were used in the experiment. The source was Beijing Weitonglihua Laboratory Animal Technology Co., Ltd. The study was reviewed by the ethics committee of the People’s Liberation Army (PLA) General Hospital.
Experimental equipment and reagents: The model of enzyme labeling instrument is Tecan and Infinite M200 in Switzerland and the fluorescence microscope is from Tokyo, Japan.
Fetal bovine serum is from Gibco Company of the United States, low sugar medium is from Luzhou Shengxin biological Co., Ltd., and type II collagenase is from Sigma Company of the United States. Lactate Dehydrogenase (LDH) from Nanjing Jiancheng Bioengineering Institute was used, reduced Glutathione (GSH), ROS activity test kit; China’s Bi Yun Tian company’s pancreatic cell digestive juice, Western blot gel preparation reagent, caspase-3, 8, 9 and 12 activity detection kit; BD membrane potential detection kit and apoptosis detection kit; China Boster company’s mouse anti α-actin; Hermo of American Hermo company, protein loading buffer and protein pre-staining marker; Hydrogen Peroxide (H2O2) of sigma; Dexmedetomidine from Jiangsu Hengrui company, China; Donkey antimouse fluorescent secondary antibody of British Abcam company; F-actin fluorescent probe of cytoskeleton company; Abcam Rabbit anti-mouse Glucose- Regulated Protein 78 (GRP78) antibody; Rabbit antimouse B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax) and Inositol-Requiring Enzyme 1 alpha (IRE1α) primary antibodies from cell signaling company of the United States. The liquid required for the experiment is phosphate buffer solution and 5× running buffer, 10× rotating membrane buffer, 5× Tris- Buffered Saline (TBS), Western blot blocking solution, 10 % separating gel and 5 % concentrated gel.
Research methods:
Animal model preparation: The rats were anesthetized with 50 mg/kg pentobarbital sodium, the suckling rats were fixed in the supine position, the heart was quickly removed and placed in the cold phosphate buffer solution, the excess parts such as fat were removed by ophthalmic scissors, transferred to the new phosphate buffer solution, the heart was cut into 1-3 mm tissue blocks and the red blood cells were removed, placed in a sterile culture bottle and added with 0.125 % trypsin cell digestive solution until immersion. After digesting at 4o for 1 d and removing the excess digestive solution, add fetal bovine serum to terminate trypsin digestion, then add type II collagenase digestive solution for multiple digestion, centrifuge and transfer the cell suspension to a 10 cm petri dish for culture for 2 h and remove more fibroblasts and endothelial cells through differential adhesion. Then, the medium containing 10 % fetal bovine serum was diluted and inoculated into different culture plates and dishes, and then the inhibitory fibroblasts were added to ensure that the medium concentration was 10 μmol/l and cultured for 48 h. Four groups of animal models were set up for research. There were 15 rats in each group, control group (group A), H2O2 group (group B), dexmedetomidine group (Group C) and dexmedetomidine+H2O2 group (Group D). Group A was added with normal medium, group B was added with H2O2 to ensure the concentration of 500 μmol/l, group C was added with dexmedetomidine to ensure the concentration of 5 μmol/l and group D was added with dexmedetomidine to ensure the concentration of 5 μmol/l. After 2 h, H2O2 was added to ensure the concentration of 500 μmol/l.
Cell immunofluorescence assay: Fluorescein isothiocyanate (FITC) and F-actin were used to label α-actin and cytoskeleton to judge the morphological changes of cardiomyocytes. Cardiomyocytes were placed on glass slides, washed three times with phosphate buffer solution after 48 h, fixed with 4 % paraformaldehyde at room temperature for 20 min, perforated and sealed at 5 % Bovine Serum Albumin (BSA) at room temperature for 1 h and then incubated with mouse anti α-actin primary antibody at 4o overnight. The α-actin labeled by FITC was incubated in the dark for 1 h at room temperature, then incubated with F-actin fluorescent probe in the dark for 30 min and labeled the cytoskeleton and then passed through 4’,6-Diamidino-2-Phenylindole (DAPI) fluorescent agent was incubated at room temperature in the dark for 10 min and the nuclei were labeled, and finally detected under fluorescence microscope. The excitation wavelengths of DAPI, F-actin and α-actin fluorescent probes were 405 nm, 550 nm and 488 nm respectively.
LDH, GSH, ROS, caspase-3, 8, 9 and 12 activity detection: Determine the absorbance value from the microplate reader and obtain the LDH activity in the reaction system. As mentioned above detection kit for the determination process was used. The absorbance value of the reaction product of GSH and 2-nitrobenzoic acid at 412 nm is obtained by enzyme labeling instrument. Sodium dihydrofluorescein diacetate was diluted with low glucose medium without fetal bovine serum. The concentration of Dichloro-Dihydro-Fluorescein Diacetate (DCFH-DA) was 10 μM. The cells were collected by flow cytometry. 1 ml of diluted DCFHDA was added to each group of cells and incubated in the dark at 37o for 30 min. After washing twice with phosphate buffer salt solution, it was resuspended with 600 μl phosphate buffer salt solution and finally tested by flow cytometry. The positive control reagent ROS reagent (ROSUP) in the positive control group was added 30 min before the start of the test and ROSUP after 30 min. It can significantly increase the level of ROS in cells. The activity of caspase-3, 8, 9 and 12 can be measured by the absorbance value of Peptide Nucleic Acid (PNA) formed by catalysis of different substrates. The wavelength corresponding to the absorption peak is 405 nm. The PNA of the control group was set to 1 and the other groups were standardized according to the control group.
Western blot detection: Firstly, cardiomyocytes were lysed, centrifuged and protein was quantified to obtain total protein, 200 μl cracking liquid is used (Radio Immunoprecipitation Assay (RIPA)), the whole cracking operation was completed in ice, centrifugation was carried out at 14 000 r/min under the condition of 4o and the total protein concentration was 1 μg/μl after dilution by the cracking solution and loading buffer solution. After the determination was completed by Bicinchoninic Acid (BCA) protein determination kit, it was boiled in a 100o dry bath for 10 min and placed at -20o for standby. Then, Western blot detection was carried out including glue filling, sample loading, protein electrophoresis, membrane transfer, blocking, antibody incubation and development. Mix 5 at a volume ratio of 1:4. The running buffer and samples are boiled at 99o for 15 min and cooled. The sampling sequence is marker sample. The environment of electrophoresis experiment is Bio-Rad’s vertical electrophoresis tank, the voltage of concentrated gel is 60 V and then 100 V is used after the sample runs to the separation gel until all dyes reach the bottom of the gel. According to the distribution of the marker strip and the molecular weight of the target protein, the gel layer is placed on the negative plate of the mould clamp according to the arrangement of the 3 layer filter paper sponge of the 3 filter paper Polyvinylidene Fluoride (PVDF) film. After the mold transfer, it is necessary to determine whether to transfer the marker strip to the moving mold and make corresponding marks. Glucose regulatory protein 78 (GRP78), IRE1α, the rabbit anti-mouse monoclonal antibodies against Bcl-2, Bax and Glyceraldehyde-3- Phosphate Dehydrogenase (GAPDH) proteins were diluted in the ratio of 1:1000 through the diluent and the incubation temperature was 4o. In the incubation stage of primary antibody and secondary antibody, special attention should be paid to the incubation time of secondary antibody as it should not be too long. The developer is Database Administrator (DBA) and the image analysis is completed by Image Lab image analysis software.
Apoptosis and mitochondrial membrane potential: Apoptosis was detected by Propidium Iodide (PI) and annexin V-FITC double labeling method. Annexin V can label apoptotic cells by binding with phosphatidylserine. As a nucleic acid fuel, PI can dye the red nucleus. This detection method can identify the apoptotic period of cells. In the experiment, the mitochondrial membrane potential was detected by the fluorescence color generated by Tetraethylbenzimidazolylcarbocyanine iodide (JC-1) fluorescent probe and the existing form of JC-1 is related to that of mitochondria. The function is related to the emitted fluorescence. The positive control group was added with oxidative phosphorylation uncoupling agent. When the mitochondrial membrane potential is high, JC-1 mostly exists in the mitochondrial matrix in the form of polymer and produces red fluorescence, when the mitochondrial membrane potential is low, most of JC-1 exists as monomers and the fluorescence color is green.
Statistical methods:
The data analysis software was Statistical Package for the Social Sciences (SPSS) 23.0 software, the measurement data are expressed in the form of mean±standard deviation and the comparison between groups is analyzed by one-way analysis of variance, where p<0.05, there was significant statistical difference between the two groups.
Results and Discussion
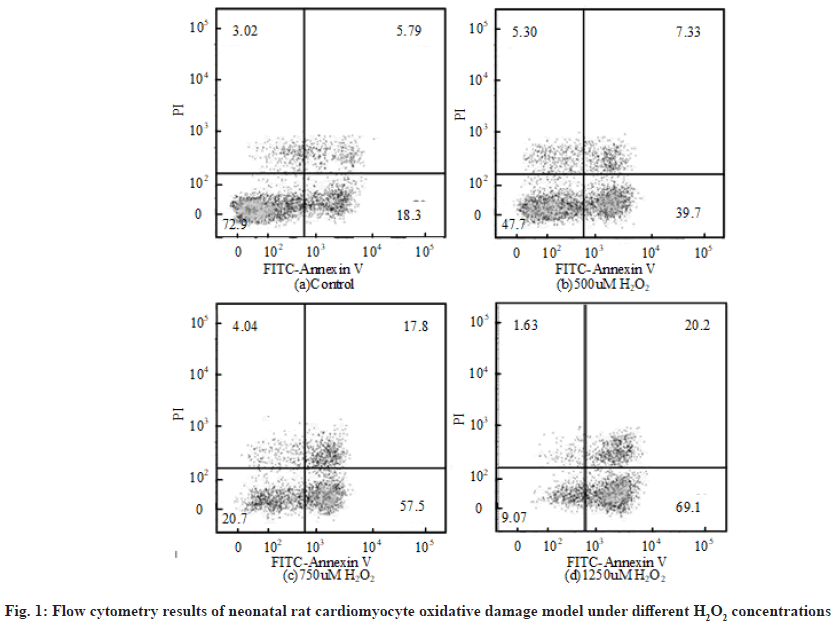
The flow cytometry results of neonatal rat cardiomyocyte oxidative damage model under different H2O2 concentrations are shown in fig. 1. The first quadrant, the second quadrant, the third quadrant and the fourth quadrant represent completely apoptotic cells, allowable error cells, normal living cells and early apoptotic cells respectively. Relevant literatures pointed out that the value range of H2O2 concentration in cell modeling was 100 μmol/l to 1 mmol/l. The apoptosis rate of neonatal rat cardiomyocytes treated with different H2O2 concentrations for 6 h was obtained by flow cytometry. The apoptosis rate refers to the sum of the first quadrant and the fourth quadrant. Overall, when the concentration of H2O2 was 500 μmol/l, the apoptosis rate of neonatal rat cardiomyocyte oxidative injury model was about 50 %. Therefore, 500 μmol/l was selected as the concentration of H2O2.
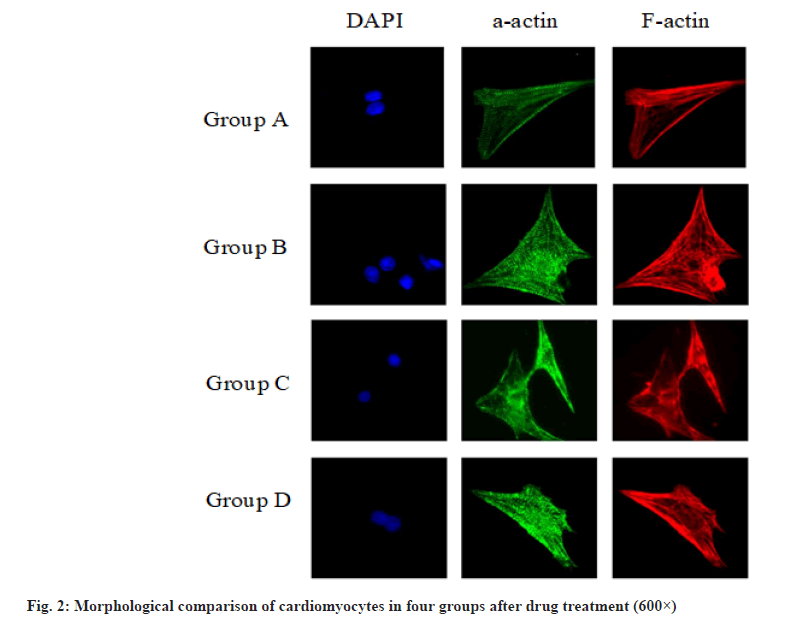
The morphological comparison of cardiomyocytes of the four groups after drug treatment is shown in fig. 2, showing the F-actin labeled cytoskeleton, α-actin labeled actin and DAPI stained nucleus. It can be seen that H2O2 will cause the disorder of cytoskeleton sequencing and the disappearance of myocardial striations. Dexmedetomidine will reduce the damage of H2O2 to cardiomyocytes and protect the cell structure.
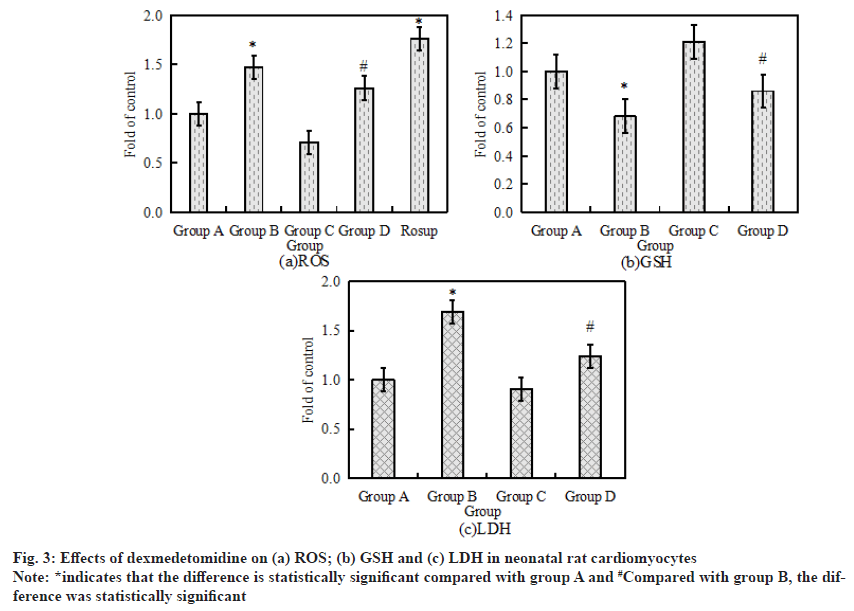
The activity levels of LDH, GSH and ROS are the key indicators of oxidative stress response. The activity levels of LDH, GSH and ROS in neonatal rat cardiomyocytes after drug treatment were measured by Enzyme-Linked Immunosorbent Assay (ELISA) and flow cytometry. The specific results are shown in fig. 3. Fig. 3a-fig. 3c shows the activity of LDH, GSH and ROS in neonatal rat cardiomyocytes respectively. Compared with the control group, the activity levels of LDH and ROS in cardiomyocytes increased significantly after 6 h of treatment with 500 μmol/l H2O2, while the activity level of GSH decreased significantly (p<0.05). Compared with H2O2 alone, the activity of GSH in cardiomyocytes increased significantly after the combined application of H2O2+ dexmedetomidine, while the activity of LDH and ROS decreased significantly (p<0.05).
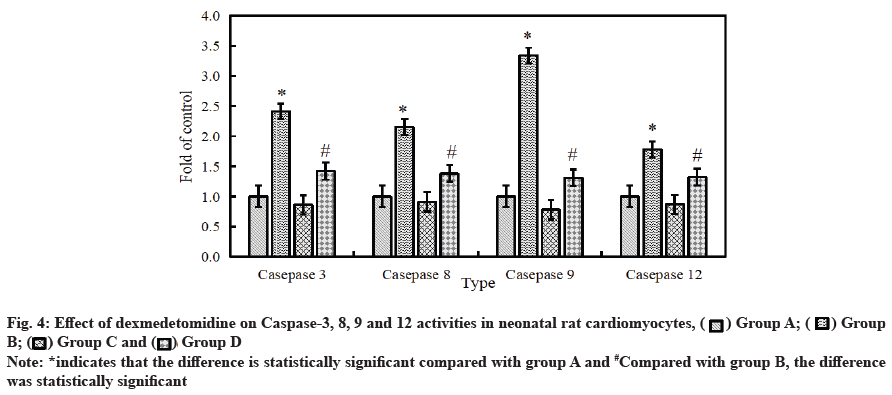
Effect of dexmedetomidine on caspase-3, 8, 9 and 12 activity in neonatal rat cardiomyocytes after H2O2 injury is as follows. The activity of intracellular apoptotic protease was detected by ELISA. The results are shown in fig. 4. Compared with the control group, the activity of caspase-3, 8, 9 and 12 in cardiomyocytes was significantly improved after 6 h of treatment with 500 μmol/l H2O2. After the combined application of H2O2+dexmedetomidine, the activity of caspase-3, 8, 9 and 12 in cardiomyocytes decreased significantly (p<0.05).
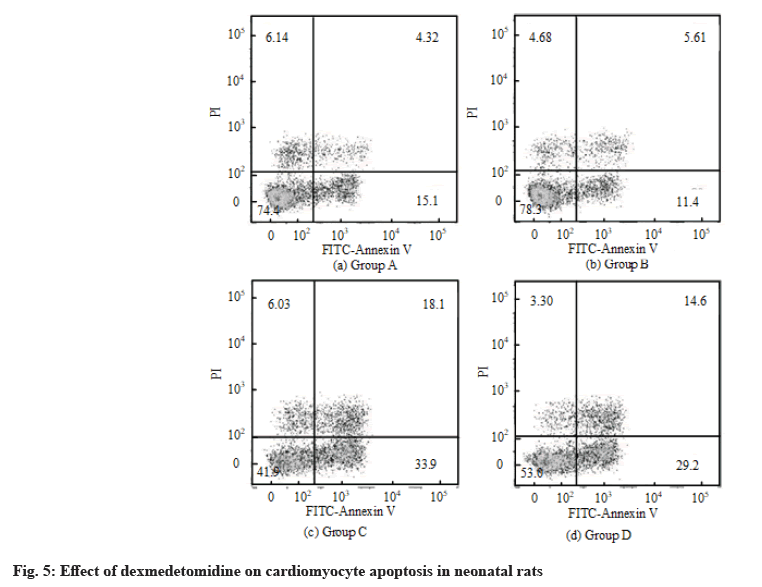
The effect of dexmedetomidine on cardiomyocyte apoptosis in neonatal rats was detected by flow cytometry. The results of flow cytometry are shown in fig. 5. The apoptosis rate of the control group was 18.36 %±5.68 %. After H2O2 treatment, the apoptosis rate increased and the value was 57.69 %±10.56 %. After H2O2+dexmedetomidine combined application, the apoptosis rate decreased to a certain extent and the values were 39.64 %±9.36 %.
Mitochondrial function can be reflected by mitochondrial membrane potential. The study was detected by flow cytometry. The results are shown in Table 1. Compared with the control group, the mitochondrial membrane potential of cardiomyocytes decreased significantly after 6 h of treatment with 500 μmol/l H2O2 and the difference was statistically significant (p<0.05). After the combined application of H2O2+dexmedetomidine, the mitochondrial membrane potential in cardiomyocytes further showed a significant downward trend and the difference was statistically significant (p<0.05).
| 1h | 2h | 4h | 6h | |
|---|---|---|---|---|
| Group A | 29 | 27 | 26 | 26 |
| Group B | 82* | 75* | 68* | 61* |
| Group C | 36 | 31 | 28 | 25 |
| Group D | 48# | 42# | 38# | 34# |
| Positive control | 89* | 87* | 86* | 86* |
Note: *indicates that the difference is statistically significant compared with group A and #Compared with group B, the difference was statistically significant
Table 1: Effect of Dexmedetomidine on Mitochondrial Membrane Potential of Neonatal Rat Cardiomyocytes (Jc-1 Monomer/%)
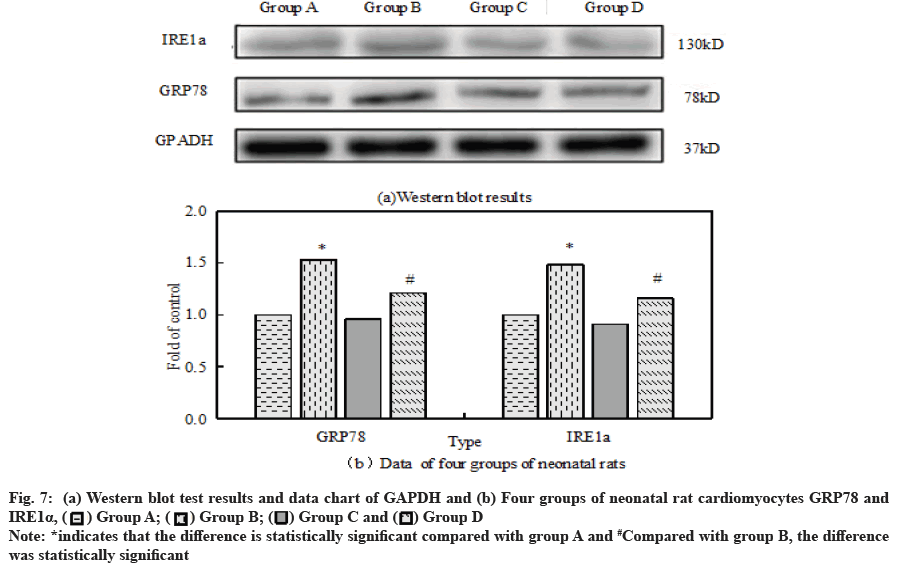
The ER stress response related proteins GRP78 and IRE1α were analyzed by Western blot assay, antiapoptotic protein Bcl-2 and pro apoptotic protein Bax.
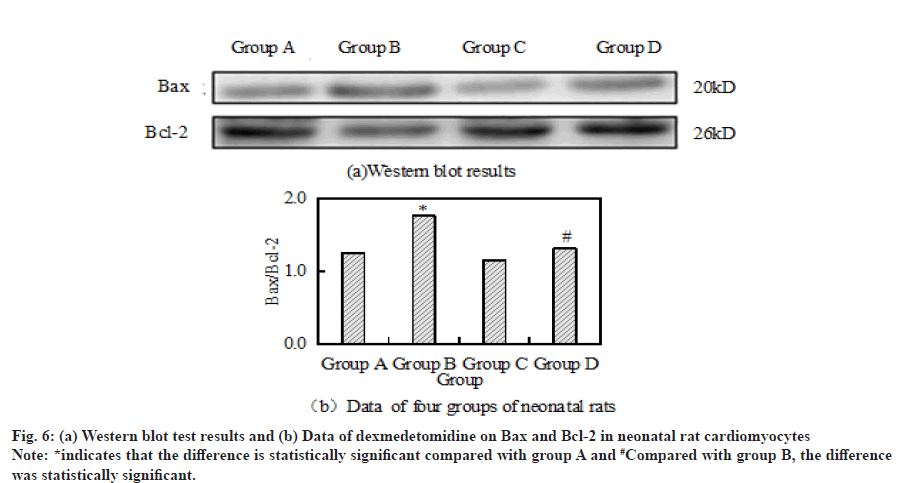
Compared with the control group, the ratio of Bax to Bcl- 2 in group B was significantly increased. However, after the combined application of H2O2+dexmedetomidine, the ratio of Bax to Bcl-2 decreased significantly compared with group B. Fig. 6a and fig. 6b respectively show the results and data of Western blot detection of Bax and Bcl-2 in cardiomyocytes of four groups of neonatal rats.
Fig. 7a and fig. 7b show GRP78 and IRE1α of neonatal rat cardiomyocytes in four groups respectively, Western blot test results and data map of GAPDH. Compared with the control group, GRP78 and IRE1α in group B significantly improved. Dexmedetomidine did not change GRP78 and IRE1α but it can reduce the expression of GRP78 and IRE1α caused by H2O2 overexpression.
Cardiovascular disease has become the highest mortality disease in China, which has an important impact on individuals and families. The oxidative stress model of neonatal rat cardiomyocytes was established by H2O2 to analyze the mechanism of dexmedetomidine on neonatal rat cardiomyocytes with oxidative stress response[7-9]. At present, a large number of studies believe that dexmedetomidine can promote the balance of myocardial oxygen supply and demand, reduce myocardial oxygen consumption, reduce cardiac output and so on. Dexmedetomidine attenuates myocardial IR injury by activating cell protection related signaling pathways.
Relatively mature studies are mainly reflected in the following. Combined with α2 receptor and dexmedetomidine on cardiomyocytes, Mitogen-activated protein kinase kinase (MEK) 1/2-Extracellular Signal-Regulated Kinase (ERK) 1/2 and Phosphatidylinositol 3-Kinase (PI3K)/Protein kinase B (Akt) signaling pathways can improve myocardial capacity and ensure cell survival, so as to reduce infarct area. A large number of researchers believe that dexmedetomidine preconditioning can avoid myocardial IR injury by inhibiting signal pathway[10-12]. In vitro cell experiment, the concentration of dexmedetomidine was 0.01 nmol/l to 600 μmol/l but no study confirmed the optimal action time and concentration of myocardial protection.
Dexmedetomidine protects cardiomyocytes through the apoptosis signal pathway transmitted by mitochondrial oxidative stress. Mitochondria will produce a large amount of ROS in the production stage. Under physiological conditions, ROS can repair tissues and protect the physiological function of cells. ROS overproduction will produce a very strong antioxidant capacity, which will promote the oxidative stress response of cardiomyocytes and eventually apoptosis. The main mechanism of myocardial IR injury is cardiomyocyte apoptosis and the most important pathway is mitochondrial apoptosis. Compared with group B, the activity of GSH in group D was significantly increased, while the activity of LDH and ROS decreased significantly. In group D, the activity of caspase-3, 8, 9 and 12 in cardiomyocytes decreased significantly. Authoritative medical journals point out that the index of mitochondrial apoptosis pathway is due to the increase of caspase-3,8,9, expression and activity. Caspase-9 is the relatively upstream protease in the signal transduction stage of apoptosis. The protease can be activated not only by upstream caspase-8, but also by the complex of Apoptotic protease activating factor-1 (Apaf-1) and cytochrome C released by mitochondria. After caspase-9 is activated, caspase-3, a key enzyme in the process of apoptosis signal transduction, can be activated to start the apoptosis program[13-15]. Some studies have also pointed out that caspase-3 is the downstream regulatory protein of Bcl-2 anti-apoptotic protein. Living fossil protease can activate the enzymatic hydrolysis process of anti-apoptotic protein. The cleavage product of this reaction exists in mitochondria. The cleavage product will promote cytochrome C in mitochondrial structure to enter the cytoplasm and then cascade activate the family of apoptotic proteases to cause apoptosis[16-18].
Compared with group A, the mitochondrial membrane potential in group B decreased significantly. Compared with group B, the ratio of Bax to Bcl-2 in group D decreased significantly.
A large number of scholars believe that apoptosis is closely related to ER stress. In the process of myocardial IR, inflammatory reaction, calcium overload and oxidative stress can cause ER stress. GRP78 is the most important molecular chaperone in the ER. When the ER is stressed, GRP78 will alleviate the internal unfolded protein response[19,20]. Some studies have pointed out that GRP78 can bind to caspase-12 on the ER membrane after dissociation and then inhibit the occurrence of apoptosis[21,22]. The markers of stress response in ER were GRP78 and IRE1α. The results showed that GRP78 and IRE1α in group D were significantly higher than those in group B. Compared with group B, the activity of caspase-12 in cardiomyocytes in group D decreased significantly.
In conclusion, dexmedetomidine can alleviate the increase of caspase-3, 8, 9 and 12 activity, ROS, LDH, GRP78 and IRE1α in neonatal rat cardiomyocytes caused by H2O2. The expression increased, GSH activity decreased and mitochondrial membrane potential decreased. Dexmedetomidine can inhibit apoptosis through apoptosis signals transmitted by mitochondrial and ER oxidative stress and then produce the function of protecting cardiomyocytes.
Conflict of interests:
The authors declared no conflict of interest.
References
- Bunte S, Behmenburg F, Majewski N, Stroethoff M, Raupach A, Mathes A, et al. Characteristics of dexmedetomidine postconditioning in the field of myocardial ischemia-reperfusion injury. Anesth Analg 2020;130(1):90-8.
[Crossref] [Google scholar] [PubMed]
- Huang Y, Sun X, Juan Z, Zhang R, Wang R, Meng S, et al. Dexmedetomidine attenuates myocardial ischemia-reperfusion injury in vitro by inhibiting NLRP3 Inflammasome activation. BMC Anesthesiol 2021;21(1):1-2.
[Crossref] [Google scholar] [PubMed]
- Ma J, Chen Q, Li J, Zhao H, Mi E, Chen Y, et al. Dexmedetomidine-mediated prevention of renal ischemia-reperfusion injury depends in part on cholinergic anti-inflammatory mechanisms. Anesth Analg 2020;130(4):1054-62.
[Crossref] [Google scholar] [PubMed]
- Zheng X, Cai X, Ye F, Li Y, Wang Q, Zuo Z, et al. Perioperative dexmedetomidine attenuates brain ischemia reperfusion injury possibly via up-regulation of astrocyte Connexin 43. BMC Anesthesiol 2020;20(1):1-2.
[Crossref] [Google scholar] [PubMed]
- Du J, Xu Z, Zhen J, Liu J, Yang D, Zheng EL, et al. Dexmedetomidine attenuates myocardial ischemia/reperfusion injury through regulating lactate signaling cascade in mice. Eur Rev Med Pharmacol Sci 2019;23(8):3527-32.
[Crossref] [Google scholar] [PubMed]
- Zhang J, Jiang H, Liu DH, Wang GN. Effects of dexmedetomidine on myocardial ischemia-reperfusion injury through PI3K-Akt-mTOR signaling pathway. Eur Rev Med Pharmacol Sci 2019;23(15):6736-43.
- Lim H, Kim TY, Kim SY, Ro SJ, Koh SR, Ryu S, et al. The protective effects of dexmedetomidine preconditioning on hepatic ischemia/reperfusion injury in rats. Transplant Proc 2020;53(1):427-35.
[Crossref] [Google scholar] [PubMed]
- Yang J, Wu Y, Xu Y, Jia J, Xi W, Deng H, et al. Dexmedetomidine resists intestinal ischemia-reperfusion injury by inhibiting TLR4/MyD88/NF-κB signaling. J Surg Res 2021;260:350-8.
[Crossref] [Google scholar] [PubMed]
- Zhang L, Yang W, Xue F, Riaz A, Zhu Z. Intraoperative low-dose dexmedetomidine attenuates hepatic ischemia-reperfusion injury in pediatric living donor liver transplantation. Transplantation 2020;104(3):116-8.
[Crossref] [Google scholar] [PubMed]
- Zhai M, Liu C, Li Y, Zhang P, Yu Z, Zhu H, et al. Dexmedetomidine inhibits neuronal apoptosis by inducing Sigma-1 receptor signaling in cerebral ischemia-reperfusion injury. Aging 2019;11(21):9556-68.
[Crossref] [Google scholar] [PubMed]
- Ji Z, Wang L, Wang S, Liang G. Dexmedetomidine hydrochloride up-regulates expression of hypoxia inducible factor-1α to alleviate renal ischemia reperfusion injury in diabetic rats. Nan Fang Yi Ke Da Xue Xue Bao 2019;39(8):944-9.
[Crossref] [Google scholar] [PubMed]
- Echavarría R, Garcia D, Figueroa F, Franco-Acevedo A, Palomino J, Portilla-Debuen E, et al. Anesthetic preconditioning increases sirtuin 2 gene expression in a renal ischemia reperfusion injury model. Minerva Urol Nefrol 2019;72(2):243-9.
[Crossref] [Google scholar] [PubMed]
- Yoshikawa Y, Hirata N, Terada H, Sawashita Y, Yamakage M. Identification of candidate genes and pathways in dexmedetomidine-induced cardioprotection in the rat heart by bioinformatics analysis. Int J Mol Sci 2019;20(7):1614.
[Crossref] [Google scholar] [PubMed]
- Taman HI, Elhefnawy E. Hepatic protective effect of dexmedetomidine after partial hepatectomy surgery: A prospective controlled study. Anesth Essays Res 2019;13(1):132-7.
[Crossref] [Google scholar] [PubMed]
- Castillo RL, Ibacache M, Cortínez I, Carrasco-Pozo C, Farías JG, Carrasco RA, et al. Dexmedetomidine improves cardiovascular and ventilatory outcomes in critically ill patients: Basic and clinical approaches. Front Pharmacol 2020;10:1641-58.
[Crossref] [Google scholar] [PubMed]
- Zhao Y, Kong GY, Pei WM, Zhou B, Zhang QQ, Pan BB. Dexmedetomidine alleviates hepatic injury via the inhibition of oxidative stress and activation of the Nrf2/HO-1 signaling pathway. Eur Cytokine Netw 2019;30(3):88-97.
[Crossref] [Google scholar] [PubMed]
- Ahmed S, Ahmed N, Rungatscher A, Linardi D, Kulsoom B, Innamorati G, et al. Cocoa flavonoids reduce inflammation and oxidative stress in a myocardial ischemia-reperfusion experimental model. Antioxidants 2020;9(2):167-79.
[Crossref] [Google scholar] [PubMed]
- Wang R, Wang M, Zhou J, Ye T, Xie X, Ni D, et al. Shuxuening injection protects against myocardial ischemia-reperfusion injury through reducing oxidative stress, inflammation and thrombosis. Ann Transl Med 2019;7(20):562.
[Crossref] [Google scholar] [PubMed]
- Tao L, Huang K, Wang J, Xue Y, Zhou Y, He F, et al. Retinol palmitate protects against myocardial ischemia/reperfusion injury via reducing oxidative stress and inhibiting apoptosis. Am J Transl Res 2019;11(3):1510-20.
- Liu S, He Y, Shi J, Liu L, Ma H, He L, et al. Allicin attenuates myocardial ischemia reperfusion injury in rats by inhibition of inflammation and oxidative stress. Transplant Proc 2019;51(6):2060-5.
[Crossref] [Google scholar] [PubMed]
- Shi Y, Qiu X, Dai M, Zhang X, Jin G. Hyperoside attenuates hepatic ischemia-reperfusion injury by suppressing oxidative stress and inhibiting apoptosis in rats. Transplant Proc 2019;51(6):2051-9.
[Crossref] [Google scholar] [PubMed]
- Jiang L, Yin X, Chen YH, Chen Y, Jiang W, Zheng H, et al. Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics 2021;11(4):1703-20.
[Crossref] [Google scholar] [PubMed]