Yang Wang*, Yuan Yao1, Baolian Shen1, Yumei Zhang2, Ying Lv3 And Dongying Hao3
Department of Orthodontics and Prosthetics,1Department of Stomatology, North China University of Science and Technology Affiliated Hospital,2Department of Stomatology, Luanzhou People's Hospital, Angshan,3Department of Stomatology, Huanghua People's Hospital, Huanghua, Hebei Province 063000, China
- *Corresponding Author:
- Yang Wang
Department of Orthodontics and Prosthetics,063000, China
E-mail: 769602954@qq.com
| This article was originally published in a special issue, “Recent Progression in Pharmacological and Health Sciences” |
| Indian J Pharm Sci 2024:86(2) Spl Issue “194-201” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Tongue cancer represents a prevalent malignant tumor within the oral and maxillofacial region. The purpose of this study was to clarify the molecular mechanisms underlying Schisandrin A involvement in tongue cancer. The viability and proliferation of tongue cancer cells were evaluated through modulation transfer spectroscopy and clone formation assays. Scratch and Transwell tests were used to investigate migration and invasion capabilities. The Wnt/beta-catenin signaling pathway-related proteins were investigated employing Western blot analysis. The tumorigenic potential of tongue cancer was assessed using a xenograft assay in the presence of Schisandrin A. To further substantiate the impact of Schisandrin A on tongue cancer, lithium chloride treatment was administered, and the effects were validated in tongue cancer xenograft models, researching the Wnt/beta-catenin signaling pathway’s role in particular. In a dose-dependent manner, Schisandrin A exhibited a notable inhibitory effect on the viability, proliferation, migration, and invasive potential of tongue cancer cells (p<0.05). In tongue cancer cells, Schisandrin A efficiently downregulated proteins associated to the Wnt/beta-catenin signaling pathway (p<0.05). Furthermore, in vivo tests demonstrated that Schisandrin A down-regulated the proteins associated with this signaling pathway in the tumor in addition to preventing tongue cancer tumor growth (p<0.05). The inhibitory influence of Schisandrin A on tongue cancer was partially counteracted by lithium chloride. Schisandrin A successfully inhibited the formation of tumors in tongue cancer as well as the spread and multiplication of tongue cancer cells. The Wnt/beta-catenin signaling pathway was downregulated to produce this inhibitory effect. The findings of this investigation shed light on Schisandrin A function and potential mechanism in addressing tongue cancer and provide a novel reference for its treatment.
Keywords
Schisandrin A, Wnt/beta-catenin signaling pathway, tongue cancer, tumor, metastasis
Among oral cancers, tongue cancer stands out as the predominant malignant tumor, which is related to smoking, drinking, and other bad habits, and the prevalence in men is greater than that in women[1]. The most common sites of tongue cancer are the tongue margin, tongue tip, and tongue dorsum, which are characterized by rapid growth, high malignancy, and strong infiltration. The tongue has rich lymphatic and blood circulation and is frequently stimulated by mechanical stimulation due to frequent daily activities such as talking and swallowing, which makes tongue cancer prone to cervical lymphatic and distant metastasis[2]. At present, most of the treatments for tongue cancer are based on surgery, postoperative radiotherapy, and chemotherapy, but the metastasis and mortality rates are still high. The features of traumatic surgery, easy side effects of chemical drugs, and easy recurrence significantly impair patient’s lives of life[3]. Significant importance lies in the quest for natural active compounds capable of impeding the proliferation and metastasis of tongue cancer tumors. Investigating the gene-level mechanisms underlying their actions is crucial in this pursuit, to improve the cure rate and reduce the mortality rate of tongue cancer patients.
Belonging to the Magnoliaceae family, Schisandra chinensis (S. chinensis) is a deciduous vine, whose plant can be admired whose fruit can be used as medicine, and whose main component, lignans, plays important pharmacological roles[4]. Schisandrin A (SchA) is a biologically active lignan extracted from S. chinensis, which possesses multiple pharmacological functions, among them neuroprotection, antioxidant, antiviral, and anticancer[5,6]. SchA has been widely studied as a drug for other purposes, and in past few years SchA has gained popularity as an antitumor drug. Some studies have found that SchA has an obvious restricted effect on cancer cell line HCC827/GR, enhances the sensitivity to gefitinib drug, induces apoptosis of HCC827 cells under the co-stimulation of SchA and gefitinib, thus exerting anti-tumor effects[7].
Additionally, animal experiments have revealed that SchA inhibits tumorigenesis and progression in xenograft mice with breast cancer[8]. At present, several studies have shown that SchA can inhibit tumor growth to play an anticancer role. Nevertheless, it's uncertain how SchA contributes to tongue cancer and how it works.
Belonging to the intercellular signaling cascade, the Wnt signaling pathway actively regulates diverse biological processes, exerting a significant role in cellular activities[9]. Constituting a classical branch of Wnt signaling, this pathway holds a crucial role in both tumorigenesis[10]. Numerous diseases have been correlated with the aberrant expression of proteins linked to this signaling route. Particularly, the key protein in this pathway, Beta (β)-catenin, has been observed to be overexpressed in various conditions, including papillary thyroid carcinoma, glioma, breast cancer, and hepatocellular carcinoma[11]. The Wnt signaling cascade reaction begins with the binding of lipid-modified Wnt to cell surface-specific receptor complexes, and the inactivation of Glycogen Synthase Kinase-3 Beta (GSK-3β) phosphorylation, so that β-catenin avoids being phosphorylated and becomes free and on the other hand, accumulating within the cytoplasm. Subsequently, it orchestrates the expression of genes, including cyclin-D1, thereby triggering cellular proliferation and fostering tumor advancement. On the other hand, β-catenin/E-cadherin complex degradation and decreased E-cadherin mediated adhesion lead to loose tumor cell-to-cell connections, weakened cell-to-cell adhesion, and consequently enhanced migration, which promotes tumor infiltration and metastasis[12]. Numerous investigations have revealed that mucin triggers the proliferation of cholangiocarcinoma’s by triggering this signaling pathway[13]. Conversely, the overexpression of RNF43 hinders cholangiocarcinoma progression by suppressing proteins linked to this pathway[14]. In the case of tongue cancer, Liang et al.[15] uncovered that Metastasis-Associated Lung Adenocarcinoma Transcription 1 (MALAT1) induces Epithelial-Mesenchymal Transition (EMT) and prevent apoptosis via this signaling pathway. This emphasizes the prospect of targeting Wnt/β-catenin pharmacologically for tongue cancer treatment. Nevertheless, the specific involvement of SchA in tongue cancer development through this signaling pathway remains incompletely understood.
This work explores the influence of SchA on the biological features of tongue cancer cells, encompassing cell viability, proliferation, migration, and invasion. The focus is on uncovering SchA’s anti-tumor effects by modulating proteins linked to the Wnt/β-catenin signaling pathway. These insights offer a novel angle that may contribute to refining therapeutic approaches for tongue cancer.
Materials and Methods
Main reagents:
The TSCCA human tongue squamous carcinoma cell line was sourced from Shenzhen Haodi Huatuo Company (Shenzhen, China). SchA was acquired from Nanjing Dasf Biotechnology Co., Ltd., (Nanjing, China). Essential supplies such as Dimethyl Sulfoxide (DMSO), Dulbecco’s Modified Eagle Medium (DMEM) were obtained from Beijing Boosun Biotechnology Co., Ltd., (Beijing, China). Lithium chloride (LiCl) was purchased from Shanghai Titan Technology Co. Antibodies directed against Wnt3a, p-GSK3β, GSK3β, β-catenin, cyclin-D1, Matrix Metalloproteinase (MMP) 3, MMP7, and β-Actin were procured from Wuhan Doctoral Bioengineering Co., Ltd., (Wuhan, China).
Cell culture and grouping:
Culturing the TSCCA cell line, derived from human tongue squamous carcinoma, involved the use of DMEM. Routine incubation was maintained at 37° with 5 % Carbon dioxide (CO2). In investigating the impact of SchA on tongue cancer cells through the Wnt/β-catenin signaling pathway, TSCCA cells received treatment with SchA at concentrations of 10, 20, and 40 μM, along with LiCl (10 mM). The subsequent categorization included control, SchA, and SchA+LiCl groups.
Modulation Transfer Spectroscopy (MTS) experiment:
Inoculate cell suspension into a 96-well plate. After 24 h incubation, replace the original medium with the prepared drug-containing medium. Add 3-(4,5-Dimethyl-2-Thiazolyl)-2,5-Diphenyl-2-H-Tetrazolium Bromide (MTT) solution to each well at 24, 48, and 72 h, and incubate for an additional 4 h. Discard the medium, add 100 μl to each well, and introduce DMSO. Gently shake the plate at 100 rpm for 10 min, and then measure the Optical Density (OD) values at 490 nm using an enzyme labeling instrument.
Clone formation experiment:
Add the cell suspension into the 6-well plate, shake the well, and put it into the incubator at 37°, 5 % CO2 for culture. Refresh the cell culture medium every 3 d. Once the number of cell clones in the wells exceeds 80, dispose of the medium, wash the cells with Phosphate Buffer Solution (PBS) three times, and introduce 800 μl of 4 % paraformaldehyde fixative into each well. 40 min later, suck out the fixative, and rinse it for 3 times. Each well should contain crystal violet. Stain for 5 min, rinse it for three times, observe and count the cells.
Cell scratching experiment:
Cells into 6-well plates and grown until reaching around 90 % confluency. Create a scratch in each well's monolayer of cells with the tip of a pipette gun, followed by gentle rinsing with PBS three times. Introduce the drug-containing medium and continue incubation for an additional 24 h, designated as 0 h. Document photographs at both 0 h and 24 h for record-keeping.
Transwell assay:
Take cells into the upper chamber and allow a 4 h culture for attachment to the chamber wall, followed by aspirating the upper chamber medium. Replace it with a serum-free medium containing drugs, while simultaneously adding a complete medium with serum to the lower chamber. After 24 h, discard the culture solutions. Swab the cells on the upper layer of the filtration membrane in the upper chamber, fix them in 4 % paraformaldehyde, and stain them with crystal violet. Perform three washes with PBS, and then capture images of the cells for counting under the microscope.
Western blot (WB):
Proteins were extracted, and their content was determined. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel electrophoresis was conducted after boiling and denaturation, followed by the transfer of proteins onto a Polyvinylidene Difluoride (PVDF) membrane. Blocking occurred with 5 % skimmed milk at 4° for 2 h. Subsequent steps involved Tris-Buffered Saline with 0.1 % Tween® 20 Detergent (TBST) washing, overnight incubation at 4° with primary antibodies (Wnt3a, p-GSK3β, GSK3β, β-catenin, cyclin-D1, MMP3, MMP7 and β-Actin), TBST washing, and a 2 h incubation at room temperature with Horseradish Peroxidase (HRP)-labeled secondary antibodies. Following TBST washing, Electrochemiluminescence (ECL) luminous solution was applied for chemiluminescence detection. For β-Actin, an additional step included overnight incubation at 4° with secondary antibody, followed by TBST washing. Following the development of chemiluminescence signals, images were taken, and grayscale values were assessed with ImageJ software.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) experiments:
Isolate total Ribonucleic Acid (RNA), conduct reverse transcription to synthesize complementary Deoxyribonucleic Acid (cDNA), and use this cDNA as a template with the specified primers detailed in Table 1. Amplify the samples using a SYBR Real-time PCR kit. Employ the 2-ΔΔCt method for relative quantitative analysis of target gene levels.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Wnt3a | ATGGCGGACGACCTGGAT | TTAGTGAAGATGGCAGTGTTG |
| p-GSK3β | CTTCTCGCGGGAAGATGTCA | GGTGGAGATGATCAGCTTGG |
| GSK3β | TACATGTCGACTTCAACAGC | GTGGTAGCCATGTAAGAGGC |
| β-catenin | CGAGGCGTACAAGGTCTTTAC | GGTGGAGGAGGTGATAGTAG |
| Cyclin-D1 | CTGGCCATGAACTACCTGGA | TCTCCGCCCTCCTTCTGC |
| MMP3 | CTGGAGCAGGCAGATGGAAGA | AGGTGATGATGTAGGAGAGGTG |
| MMP7 | TGTGCCCTCGGAGGATGG | CTGTTGGAAACTCACACGC |
| β-Actin | AGAGCTACGAGCTGCCTGAC | AGCACTGTGTTGGCGTACAG |
Table 1: Primer Sequences Used for qRT-PCR (5'-3')
Nude mice implantation experiment:
Acquire fifteen Balb/c nude mice aged 4 w and accommodate them in a facility that adheres to specific pathogen-free conditions. After a 1 w acclimatization period, initiate the animal experiments. Harvest TSCCA cells in the logarithmic growth phase, subject them to trypsin digestion and dilute the cell concentration to 1×107 cells/ml. Subcutaneously inject 200 μl of the cell suspension into nude mice. After 1 w, randomly assign the mice into the control group, SchA group, and SchA+LiCl group (oral gavage of SchA 80 mg/kg+LiCl 500 mg/kg), with five mice in each group. After a week, distribute the mice randomly among the control, SchA, and SchA+LiCl groups (gavage of SchA 80 mg/kg+LiCl 500 mg/kg), with five mice per group. Measurements of tumor size were conducted every 3 d. Upon reaching the appropriate size, euthanasia was carried out on the nude mice. Subsequently, the tumors were collected to perform WB experiments.
Statistical analysis:
GraphPad Prism 9 was utilized for data processing and analysis, employing a t-test for comparisons between two groups and one-way Analysis of Variance (ANOVA) for comparisons among multiple groups. A significance level of p<0.05 denoted statistical significance.
Results and Discussion
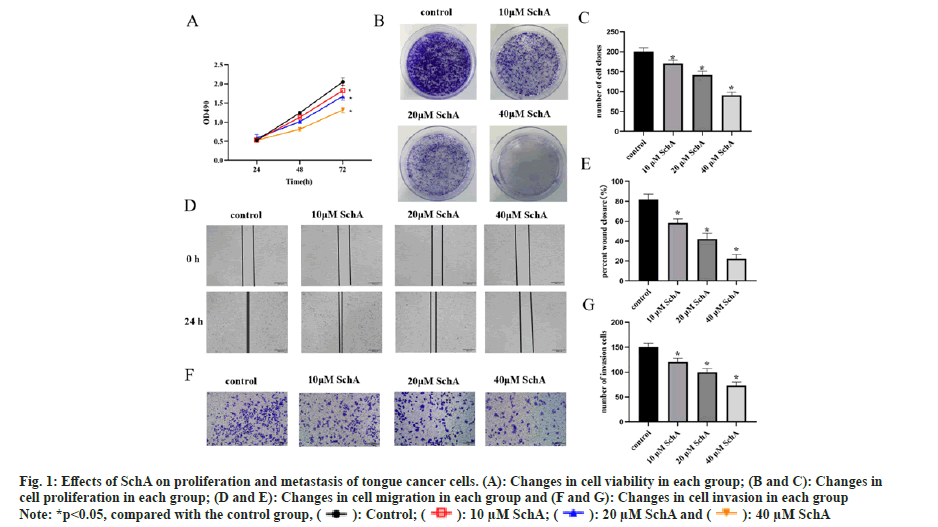
Assessing the impact of SchA on TSCCA cells revealed that, in comparison to the control group, there were alterations in both proliferation and clonogenic capacity, the cell viability was reduced in the SchA group (fig. 1A, p<0.05) and the cloning ability was weakened (fig. 1B and fig. 2C, p<0.05). Detection of the effect of SchA on TSCCA cell metastasis and invasion revealed that SchA had an inhibitory effect on cell migration and invasion (fig. 1D-fig. 1G, p<0.05). These results indicated that SchA inhibited tongue cancer cell proliferation and metastasis.
Fig 1: Effects of SchA on proliferation and metastasis of tongue cancer cells. (A): Changes in cell viability in each group; (B and C): Changes in cell proliferation in each group; (D and E): Changes in cell migration in each group and (F and G): Changes in cell invasion in each group
Note: *p<0.05, compared with the control group, 
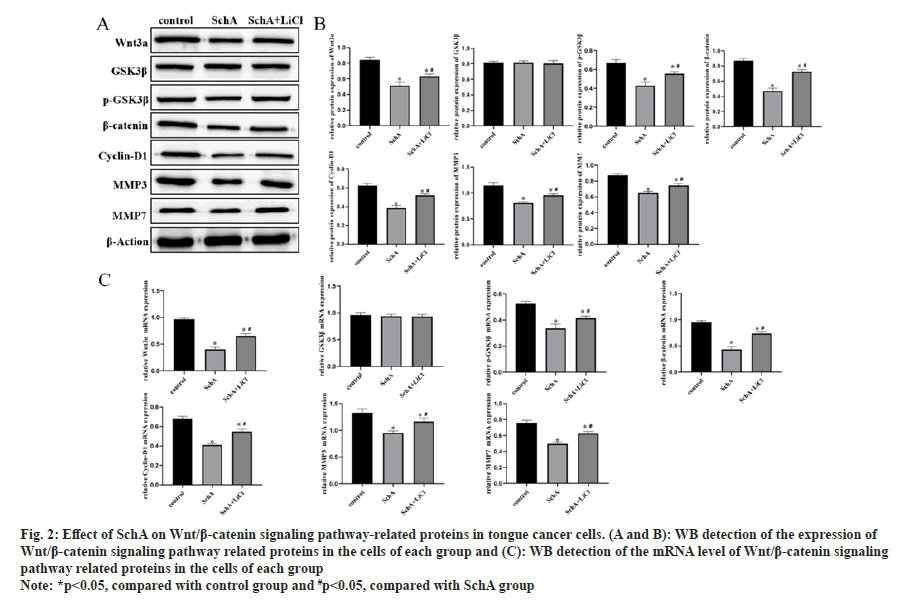
WB and qRT-PCR results showed significant down-regulation of Wnt3a, p-GSK3β, β-catenin, cyclin-D1, MMP3, and MMP7 in SchA induced TSCCA cells (fig. 2A-fig. 2C, p<0.05). After the introduction of LiCl, into TSCCA cells, this pathway and its downstream proteins were significantly upregulated. This suggests that LiCl reversed the effect of SchA on these proteins in tongue cancer cells.
Fig 2: Effect of SchA on Wnt/β-catenin signaling pathway-related proteins in tongue cancer cells. (A and B): WB detection of the expression of Wnt/β-catenin signaling pathway related proteins in the cells of each group and (C): WB detection of the mRNA level of Wnt/β-catenin signaling pathway related proteins in the cells of each group
Note: *p<0.05, compared with control group and #p<0.05, compared with SchA group the SchA+
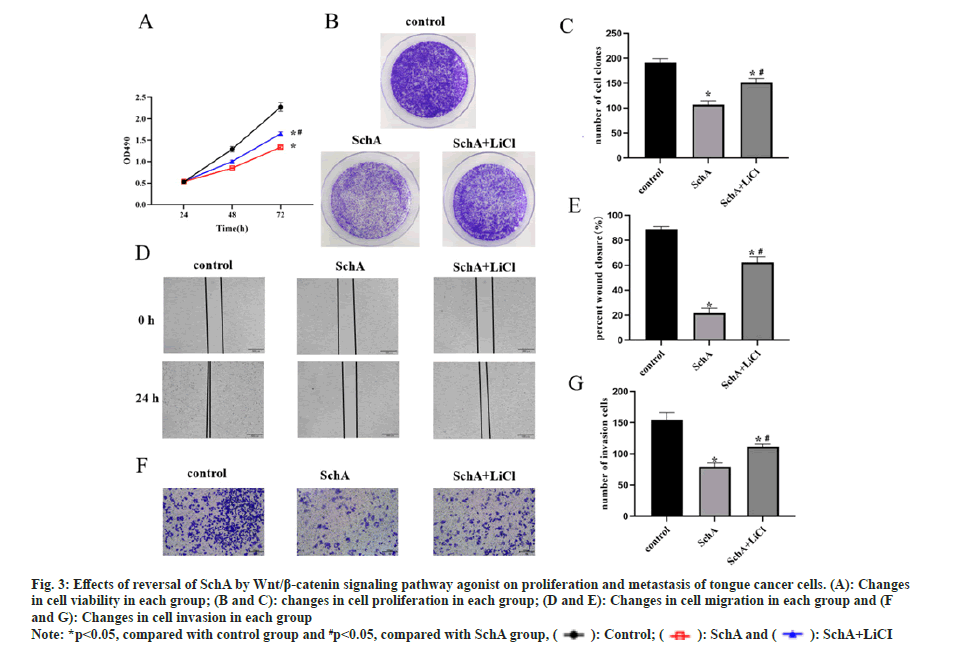
To further elucidate the impact of the Wnt/β-catenin signaling pathway on SchA-treated tongue cancer cells, TSCCA cells were concurrently exposed to SchA and this signaling pathway agonist LiCl. The results revealed that SchA effectively suppressed both the proliferation and metastasis of TSCCA cells in comparison to the control group. However, the co-administration of LiCl with SchA significantly augmented both cell proliferation and metastasis (fig. 3A-fig. 3G, p<0.05). This suggests that the inhibitory effects of SchA on the growth and spread of tongue cancer cells were reversed by this signaling pathway agonist.
Fig 3: Effects of reversal of SchA by Wnt/β-catenin signaling pathway agonist on proliferation and metastasis of tongue cancer cells. (A): Changes in cell viability in each group; (B and C): changes in cell proliferation in each group; (D and E): Changes in cell migration in each group and (F and G): Changes in cell invasion in each group
Note: *p<0.05, compared with control group and #p<0.05, compared with SchA group, 
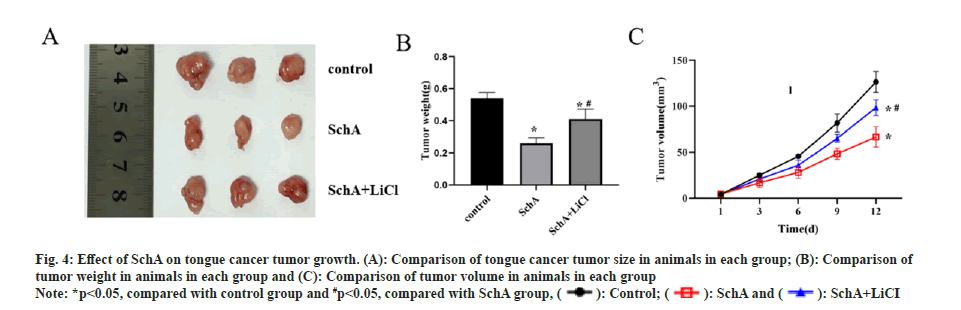
In vivo experiments demonstrated that nude mice treated with SchA exhibited slower tumor growth and smaller volumes compared to the control group. Conversely, when comparing the SchA group with the SchA+LiCl group, the latter showed accelerated tumor growth and larger volumes (fig. 4A-fig. 4C, p<0.05). This suggests that SchA effectively restrains tongue cancer tumor growth, and the addition of LiCl reverses the inhibitory impact of SchA on tumor growth in this context.
Fig 4: Effect of SchA on tongue cancer tumor growth. (A): Comparison of tongue cancer tumor size in animals in each group; (B): Comparison of tumor weight in animals in each group and (C): Comparison of tumor volume in animals in each group
Note: *p<0.05, compared with control group and #p<0.05, compared with SchA group, 
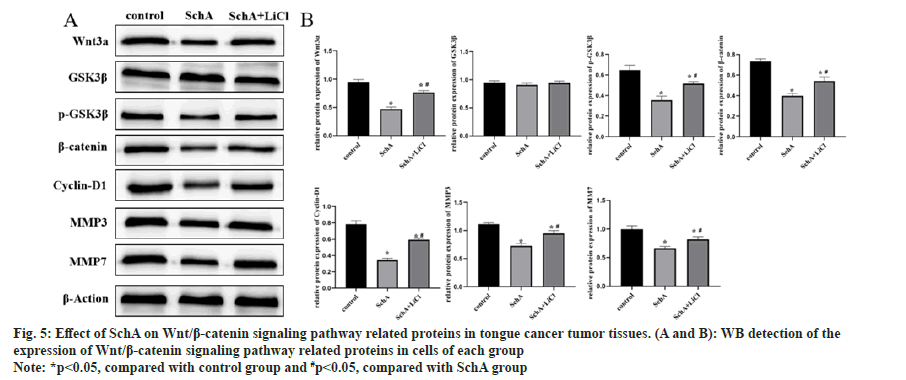
Analysis of proteins in tumor tissues indicated that SchA suppressed Wnt3a, p-GSK3β, β-catenin, cyclin-D1, MMP3 and MMP7. Comparing the SchA, the SchA+LiCl group exhibited an elevation in these protein levels (fig. 5A and fig. 5B, p<0.05). These findings imply that in tongue cancer tumor tissues, SchA down-regulated Wnt/β-catenin signaling pathway, and the addition of LiCl reversed the down-regulation induced by SchA in these proteins.
Fig 5: Effect of SchA on Wnt/β-catenin signaling pathway related proteins in tongue cancer tumor tissues. (A and B): WB detection of the expression of Wnt/β-catenin signaling pathway related proteins in cells of each group
Note: *p<0.05, compared with control group and #p<0.05, compared with SchA group
Oral cancer is among the top ten most common malignancies, and tongue cancer holds the highest incidence rate among all oral cancers[16]. Tongue cancer grows rapidly, is highly malignant and infiltrative, often affecting the tongue muscle and surrounding mucosa, leading to difficulties in speech, feeding, and swallowing, and easily metastasizes throughout the body through lymphatic and blood circulation pathways, with a poor prognosis[17]. While there have been ongoing developments in various therapeutic methods, the persistently low 5 y survival rate for patients presents an imminent danger to individual standards of life and overall survival outcomes. Traditional Chinese medicine has the advantages of holistic regulation and small adverse reactions in tumor treatment, and is clinically effective in various types of malignant tumors, thus becoming a novel choice for the management of those suffering malignant tumors. An escalating body of research highlights the noteworthy and extensive antitumor activity of SchA. It adeptly inhibits tumor cell proliferation, triggers apoptosis, counteracts chemotherapy resistance and restrains the invasion and migration of tumor cells[18].
SchA, a prominent pharmacologically active compound found in S. chinensis, exhibits robust anti-inflammatory and antitumor effects. Its effective antitumor activity has shown evidence of its potent antitumor action, including pancreatic cancer, hepatocellular carcinoma, and breast cancer[19-21]. In a research investigation, it was observed that SchA suppressed cancer cells by reducing microRNA-155[22]. Ding et al.[23] observed that SchA effectively reduced the invasive and migratory capabilities of TPC-1 cells by down-regulating microRNA-429. This down-regulation led to decreased MMP-2, MMP-9, and vimentin. Additionally, SchA demonstrated the ability to inactivate both the Wnt/β-catenin and MEK/ERK signaling pathways. Consequently, these regulatory actions inhibited the growth of TPC-1 cells. Nevertheless, the impact of SchA on tongue cancer remains unexplored in the current literature.
In the course of this study, the application of different concentrations of SchA to TSCCA cells demonstrated a dose-dependent suppression of cell proliferation. Moreover, it exhibited inhibitory effects on invasion and metastasis. To delve into the potential molecular mechanisms governing SchA’s role in tongue cancer development, it was identified that SchA reduced the proteins linked to the Wnt/β-catenin signaling pathway in TSCCA cells. Earlier studies have substantiated the involvement of this signaling pathway in regulating tumor cell proliferation, migration, and invasion by influencing the expression of its downstream proteins[24]. β-catenin functions centrally in the Wnt/β-catenin signaling pathway, mediating signal transmission from the cell membrane to the nucleus. Activation of the Wnt pathway results in the cytoplasmic accumulation of β-catenin, allowing its entry into the nucleus to drive the expression of target genes. Maintaining the stability of cytoplasmic β-catenin involves a degradation complex composed of the tumor suppressor APC, scaffolding protein axin, and kinases CK1α and GSK-3β. GSK-3β phosphorylates the amino-terminal structural domain. Cyclin D1, a cell cycle regulatory protein, oversees the proliferation of different tumor cells. Meanwhile, MMP3 and MMP7 play crucial roles in the EMT process, participating in the EMT of various tumor cells and promoting cell migration and tumor metastasis[25]. In our investigation, SchA showed the ability to reduce the levels of downstream proteins associated with the Wnt/β-catenin signaling pathway, including p-GSK-3β, cyclin-D1, MMP3, and MMP7. For a more comprehensive exploration of SchA's influence on the Wnt/β-catenin signaling pathway in tongue cancer cells, the co-administration of SchA and LiCl, a Wnt/β-catenin agonist, not only reversed SchA’s inhibitory effect on pathway-related proteins but also alleviated SchA’s impact on the proliferation and metastasis of TSCCA cells. In vivo experiments revealed that nude mice treated with SchA displayed sluggish tumor growth, diminished tumor volume, and a reduction in proteins linked to this signaling pathway. The combination with LiCl, however, reversed the effects of SchA on tongue cancer tumors. Consequently, our study suggests that SchA impedes the proliferation and metastasis of tongue cancer cells by inhibiting proteins associated with the Wnt/β-catenin signaling pathway.
In this investigation, SchA displayed inhibitory effects on the in vitro proliferation, migration, and invasion of TSCCA tongue cancer cells. Additionally, it attenuated the proteins linked to the Wnt/β-catenin signaling pathway and hindered tumor growth. Findings from both in vivo and ex vivo experiments revealed that the counteractive effects of Wnt/β-catenin agonists on tongue cancer were evident in the presence of SchA. Nonetheless, further exploration is warranted to discern the specific molecular mechanisms underlying SchA’s regulatory impact on this signaling pathway, whether through direct or indirect means.
By suppressing the formation of tongue cancer tumors and impeding the proliferation and spread of tongue cancer cells, SchA inhibits proteins linked to the Wnt/β-catenin signaling pathway. This study provides a fresh therapeutic approach for the treatment of tongue cancer by revealing the function of SchA in the disease and clarifying its possible mechanism.
Conflict of interests:
The authors declared no conflict of interests.
References
- Amini A. Early stage oral tongue cancer with an ipsilateral nodal recurrence 2 years later: What do you treat. Int J Radiat Oncol Biol Phys 2020;106(5):900-1.
[Crossref] [Google Scholar] [PubMed]
- Dwivedi RC, Chisholm EJ, Khan AS, Harris NJ, Bhide SA, St. Rose S, et al. An exploratory study of the influence of clinico-demographic variables on swallowing and swallowing-related quality of life in a cohort of oral and oropharyngeal cancer patients treated with primary surgery. Eur Arch Otorhinolaryngol 2012;269:1233-9.
[Crossref] [Google Scholar] [PubMed]
- Canis M, Weiss BG, Ihler F, Hummers–Pradier E, Matthias C, Wolff HA. Quality of life in patients after resection of pT3 lateral tongue carcinoma: Microvascular reconstruction vs. primary closure. Head Neck 2016;38(1):89-94.
[Crossref] [Google Scholar] [PubMed]
- Szopa A, Klimek-Szczykutowicz M, Kokotkiewicz A, Ma?lanka A, Król A, Luczkiewicz M, et al. Phytochemical and biotechnological studies on Schisandra chinensis cultivar Sadova No. 1-A high utility medicinal plant. Appl Microbiol Biotechnol 2018;102:5105-20.
- Liu GZ, Liu Y, Sun YP, Li XM, Xu ZP, Jiang P, et al. Lignans and terpenoids from the leaves of Schisandra chinensis. Chem Biodivers 2020;17(4):e2000035.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Wang X, Yao H, Shen C, Geng K, Xie H. A comprehensive review on Schisandrin and its pharmacological features. Naunyn Schmiedebergs Arch Pharmacol 2023;397(2):783-94.
[Crossref] [Google Scholar] [PubMed]
- Xian H, Feng W, Zhang J. Schizandrin A enhances the efficacy of gefitinib by suppressing IKKβ/NF-κB signaling in non-small cell lung cancer. Eur J Pharmacol 2019;855:10-9.
[Crossref] [Google Scholar] [PubMed]
- Xu X, Rajamanicham V, Xu S, Liu Z, Yan T, Liang G, et al. Schisandrin A inhibits triple negative breast cancer cells by regulating Wnt/ER stress signaling pathway. Biomed Pharmacother 2019;115:108922.
[Crossref] [Google Scholar] [PubMed]
- Zhang N, Shen H, Chen B, Hu H, Liu C, Chen Y, et al. The recent progress of peptide regulators for the Wnt/β-catenin signaling pathway. Front Med 2023;10:1164656.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol 2020;13(1):165.
- Wu Y, Chen W, Zhang B, Liu H. ANKRD22 knockdown suppresses papillary thyroid cell carcinoma growth and migration and modulates the Wnt/β-catenin signaling pathway. Tissue Cell 2023;84:102193.
[Crossref] [Google Scholar] [PubMed]
- Ma F, Ye H, He HH, Gerrin SJ, Chen S, Tanenbaum BA, et al. SOX9 drives WNT pathway activation in prostate cancer. J Clin Investig 2016;126(5):1745-58.
[Crossref] [Google Scholar] [PubMed]
- Song F, Chen FY, Wu SY, Hu B, Liang XL, Yang HQ, et al. Mucin 1 promotes tumor progression through activating WNT/β-catenin signaling pathway in intrahepatic cholangiocarcinoma. J Cancer 2021;12(23):6937-47.
[Crossref] [Google Scholar] [PubMed]
- Pangestu NS, Chueakwon P, Talabnin K, Khiaowichit J, Talabnin C. RNF43 overexpression attenuates the Wnt/β-catenin signalling pathway to suppress tumour progression in cholangiocarcinoma. Oncol Lett 2021;22(6):1-9.
[Crossref] [Google Scholar] [PubMed]
- Liang J, Liang L, Ouyang K, Li Z, Yi X. MALAT 1 induces tongue cancer cells' EMT and inhibits apoptosis through Wnt/β-catenin signaling pathway. J Oral Pathol Med 2017;46(2):98-105.
[Crossref] [Google Scholar] [PubMed]
- Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: A period analysis. Oncologist 2010;15(9):994-1001.
[Crossref] [Google Scholar] [PubMed]
- Soudry E, Preis M, Hod R, Hamzany Y, Hadar T, Bahar G, et al. Squamous cell carcinoma of the oral tongue in patients younger than 30 years: Clinicopathologic features and outcome. Clin Otolaryngol 2010;35(4):307-12.
[Crossref] [Google Scholar] [PubMed]
- Kong D, Zhang D, Chu X, Wang J. Schizandrin A enhances chemosensitivity of colon carcinoma cells to 5-fluorouracil through up-regulation of miR-195. Biomed Pharmacother 2018:99:176-183.
[Crossref] [Google Scholar] [PubMed]
- Yamada KM, Collins JW, Cruz Walma DA, Doyle AD, Morales SG, Lu J, et al. Extracellular matrix dynamics in cell migration, invasion and tissue morphogenesis. Int J Exp Pathol 2019;100(3):144-52.
[Crossref] [Google Scholar] [PubMed]
- Hou W, Gao W, Wang D, Liu Q, Zheng S, Wang Y. The protecting effect of deoxyschisandrin and schisandrin B on HaCaT cells against UVB-induced damage. PLoS One 2015;10(5):e0127177.
[Crossref] [Google Scholar] [PubMed]
- Fu K, Zhou H, Wang C, Gong L, Ma C, Zhang Y, et al. A review: Pharmacology and pharmacokinetics of Schisandrin A. Phytother Res 2022;36(6):2375-93.
[Crossref] [Google Scholar] [PubMed]
- Yan H, Guo M. Schizandrin A inhibits cellular phenotypes of breast cancer cells by repressing miR-155. IUBMB Life 2020;72(8):1640-8.
[Crossref] [Google Scholar] [PubMed]
- Ding Q, Li X, Sun Y, Zhang X. Schizandrin A inhibits proliferation, migration and invasion of thyroid cancer cell line TPC-1 by down regulation of microRNA-429. Cancer Biomark 2019;24(4):497-508.
[Crossref] [Google Scholar] [PubMed]
- Wang C, Xu J, Fu H, Zhang Y, Zhang X, Yang D, et al. TRIM 32 promotes cell proliferation and invasion by activating β-catenin signalling in gastric cancer. J Cell Mol Med 2018;22(10):5020-8.
[Crossref] [Google Scholar] [PubMed]
- Cao N, Mu L, Yang W, Liu L, Liang L, Zhang H. MicroRNA-298 represses hepatocellular carcinoma progression by inhibiting CTNND1-mediated Wnt/β-catenin signaling. Biomed Pharmacother 2018;106:483-90.
[Crossref] [Google Scholar] [PubMed]