- *Corresponding Author:
- Govand SH Tawfeeq
Department of Clinical Analysis,
College of Pharmacy,
Hawler Medical University,
Erbil,
Kurdistan Region,
Iraq
E-mail: govand.tawfeeq@hmu.edu.krd
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3) Spl Issue “64-70” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this study, the antioxidative effect of melatonin was investigated in aging retinal pigment epithelial cells in vitro. The objective of this study is to explore the administration of melatonin pharmacological doses for the prevention of aging symptoms.Specific concentrations of melatonin (20 µM, 40 µM and 80 µM) were used to treat hydrogen peroxide-induced aging retinal pigment epithelial cells and flow cytometry was employed to examine retinal pigment epithelial cell apoptosis. A specific probe was utilized to detect the intracellular reactive oxygen species concentration and apoptosis-associated proteins were detected in real-time polymerase chain reaction. Commercially available assay kits detected the expression of oxidative biomarkers, superoxide dismutase, malondialdehyde and glutathione.In comparison to normal cells, the aging retinal pigment epithelial cell model had lower cell viability, higher apoptosis rates and a more severe oxidation status. Melatonin increased cell viability while lowering apoptosis and oxidative stress. It influenced the expression of apoptosis-related proteins as well as oxidative stress indicators. Finally, treatment with melatonin was able to regulate proliferation, oxidative stress and apoptosis in aging retinal pigment epithelial cells.The application of melatonin may be a novel strategy to protect against age-related changes in age-related macular degeneration. Melatonin has protective effects against hydrogen peroxide-induced retinal cell death. It inhibits hydrogen peroxide-induced retinal pigment epithelial cell damage and decreases the apoptosis rate.
Keywords
Melatonin, retinal pigment epithelial, oxidative biomarkers, real-time polymerase chain reaction
Aging is a normal multi-factorial phenomenon characterized by the accumulation of degenerative processes underpinned by molecular pathways, resulting in multiple alterations and damage; as a result, the functions of cells, tissues and organs are endangered[1]. Deoxyribonucleic Acid (DNA) injury, changes in gene and non-coding Ribonucleic Acid (RNA) expression, genotoxicity, oxidative stress and the incidence of shorter telomeres are some of the suggested mechanisms that lead to the aging process and the development of chronic age-related disorders[2].
Age-related Macular Degeneration (AMD) is one of the most prevalent and significant vision issues among the elderly and oxidative stress complicates its etiology. A prominent risk factor for AMD is oxidative stress in Retinal Pigment Epithelial (RPE) cells[3] and the disease’s treatments are still limited. Patients with neovascular AMD can benefit from photodynamic therapy and anti-vascular endothelial growth factor agents, but there are currently no cures available[4]. As a result, the only option is to monitor and prevent progression from early to late AMD. Clinical trials focusing on the treatment of early AMD have yielded no conclusive results based on previous research. As a result, elucidating the pathogenesis of AMD is critical. AMD is thought to be linked to both advancing age and tobacco smoking[5]. Naturally, it is possible that age-related structural changes in the retina play a role in AMD pathogenesis. RPE cells are found in the outer layer of the retina[6] and are involved in photoreceptor cell regeneration and repair. In the normal state, these cells have unique characteristics and their loss may result in retinal degeneration and possibly blindness[7]. Degenerative and progressive conditions of the RPE cells are also recognized as important pathogenetic mechanisms in AMD. Aside from RPE modification, clinically, one of the most important markers for AMD diagnosis was RPE modification[8]. Protection of the RPE cells from age-related changes could be crucial in preventing progression from early AMD to neovascular AMD. The aging of retinal tissues has been associated with oxidative stress, which has been demonstrated to have a substantial impact on a number of ocular illnesses. A reactive or pathogenic process marked by the formation of Reactive Oxygen Species (ROS) or a decline in antioxidant capacity is referred to as oxidative stress. Superoxide anions (O2), hydroxyl radicals (–OH–) and Hydrogen Peroxide (H2O2) are examples of ROS. The uncontrolled production of ROS can result in serious cellular damage. Since abnormal proteins and lipids accumulate with age, oxidative stress results in less cell repair and regeneration. Furthermore, given its high metabolic activity, the retinal pigment epithelium has a large number of mitochondria to generate a sufficient amount of Adenosine Triphosphate (ATP) to carry out all of its physiological functions[9]. Age-related mitochondrial dysfunction can thus lead to an increase in oxidative stress in the RPE, resulting in AMD[10]. RPE cells are extremely important to photoreceptor function, as they are not only responsible for the recycling of the visual pigments but also key in the phagocytosis of photoreceptor outer segments. The retinal pigment epithelium is vulnerable to oxidative harm because of its placement in a highly oxygenated and illuminated environment, which can lead to cellular malfunction, inflammation and finally cell death[11]. Melatonin (N-acetyl-5-methoxy tryptamine) is a free radical scavenger and the primary antioxidant defender against reactive hydroxyl radicals. Melatonin has several beneficial effects on physiological functions associated with age, including metabolic sensing, mitochondrial modulation and proliferation, anti-oxidative protection of biomolecules and anti-inflammatory actions[12]. Nocturnal production of melatonin decreases as age progresses in animals of different species, including humans[13]. The grafting of the pineal gland by young donors either into the thymus of old syngeneous mice or in situ into pinealectomized old mice prolongs the life of the recipients[14]. Melatonin’s possibilities to produce life-span extensions are a popular subject for discussion; numerous studies show melatonin’s anticarcinogenic and anticancer capabilities[14].
Materials and Methods
Materials:
Melatonin was purchased from Glenham Life Sciences Ltd. (Corsham, United Kingdom). Dulbecco’s Modified Eagle’s Medium (DMEM)/Ham’s F-12 (1:1), supplemented with 5 mM glucose, 10 % fetal bovine serum and 1 % penicillin/streptomycin, was obtained from Gibco (Carlsbad, California, United States of America (USA)) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) dye was sourced from Sigma Aldrich. Total RNA was extracted from the different groups using a Pars Tous Kit (Pars Tous Co., Iran) according to the manufacturer’s recommendations. Moreover, Superoxide Dismutase (SOD), Malondialdehyde (MDA) and Glutathione (GSH) test kits were obtained from ZellBio GmbH (Germany). Finally, the Annexin V-fluorescein isothiocyanate apoptosis detection kit was purchased from eBioscience (USA) and the ROS detection kit was procured from Bioquochem (Spain).
Cell culture:
The Aging Retinal Pigment Epithelial (ARPE-19) cells were sourced from the cells obtained from the National Cell Bank of Iran (Pasteur Institute, Iran). DMEM/Ham’s F-12 (1:1), supplemented with 5 mM glucose, 10 % fetal bovine serum and 1 % penicillin/streptomycin, was used to grow and passage the cells. For each experiment, ARPE-19 cells were employed at 80 % confluence and passaged every 3 d after being placed into culture medium. The ARPE-19 cells employed were in the 3rd to 6th passages. The cells were cultured at 37° in a humidified incubator containing 5 % Carbon Dioxide (CO2).
Aging cell model:
The method described by Zhuge et al. was used to create a pulsed H2O2 exposure aging cell model[15]. In general, passaged ARPE-19 cells were transplanted to a 10 cm culture dish with a 50 % cell density control. On the 1st d, the cultivated cells were incubated for 2 h at 37° in full medium containing 800 M H2O2. The cells were then rinsed three times with phosphate buffered saline buffer after the growth medium was removed. For cell culture, fresher medium was introduced. The first H2O2 exposures were done on d 2-5 and the cell culture medium was changed to complete medium on d 6. On d 8, the treated RPE cells were passaged and replated on 10 cm cell culture dishes after being digested with trypsin-ethylenediaminetetraacetic acid.
Treatment of cells:
Normal and aging ARPE-19 cells were given different treatment concentrations of melatonin (10, 20, 40, 60, 80 and 100 µM) for 24 h.
MTT assay:
The MTT assay was performed to test the vitality of the cells. The 96-well plate was filled with MTT dye (Sigma Aldrich) and incubated overnight at 37°. The 96-cell plates were extensively shocked for 10 min after adding 150 l of dimethyl sulfoxide to each well, until the crystals disintegrated completely. The BioTek ELx808 microplate reader was used to measure the absorbance of the wells at 540 nm.
Flow cytometry:
Apoptosis was measured using the Annexin V-fluorescein isothiocyanate apoptosis detection kit (eBioscience). All of the cultivated ARPE-19 cells in each group were trypsinized and centrifuged. The cells were treated with ice-cold 70 % ethanol after two washings with phosphate buffered saline. The cells were then transferred to a new Eppendorf tube after being resuspended in 1 ml of phosphate buffered saline. The Propidium Iodide (PI) solution and Annexin V-fluorescein isothiocyanate were added and the cells were grown in the dark for 20 min. Flow cytometry was used to examine the cells. All of the detections were carried out in duplicate.
Measurement of ROS:
The 2’,7’-Dichlorofluorescin Diacetate (DCFH-DA) used in the ROS assay kit (Bioquochem- Spain) can be produced by stress circumstances such as exposure to oxidants or medicines. At a density of 1106 cells/ml, the cultivated ARPE-19 cells were transferred to a 12-well plate. DCFH-DA, at a working concentration of 50 µM, was added to each well. The cells were then cultured in the dark at 37° for 15-30 min before being washed twice in phosphate buffered saline and used for advanced flow cytometry analysis.
Detection of oxidative biomarkers:
The levels of oxidative stress in different groups were measured using three typical oxidative biomarkers: SOD, MDA and GSH. We obtained aging RPE cells that had been treated with low (20 µM), medium (40 µM) or high (80 µM) doses of curcumin for 24 h. Thereafter, oxidative biomarkers in these three therapy groups were assessed. In general, commercially available assay kits were used to determine SOD, MDA and GSH activity levels. All procedures were carried out according to the manufacturer’s instructions and each sample was tested three times.
Quantitative analysis of gene expression:
Total RNA was extracted from the different groups using a Pars Tous Kit (Pars Tous Co., Iran) according to the manufacturer’s recommendations. Concentrations of RNA were determined by Ultraviolet (UV) spectrophotometry (Eppendorff, Germany). Complementary DNAs (cDNAs) were synthesized from 500 ng Deoxyribonuclease (DNase)-treated RNA samples with a QuantiTect reverse transcription kit using oligo (deoxythymine (dT)) primers. The speci?c primers used for Polymerase Chain Reaction (PCR) reactions are listed in Table 1. These primers were synthesized by Pishgam Company (Tehran, Iran). PCRs were performed using Master Mix and EvaGreen in an Applied Biosystems StepOne™ thermal cycler (Applied Biosystems, USA). The PCR program commenced with an initial melting cycle for 5 min at 95° to activate the polymerase, followed by 40 cycles of melting (30 s at 95°), annealing (30 s at 58°) and extension (30 s at 72°). Melting curve analyses were used to verify the PCR reactions quality. A standard curve was used to determine how efficient each gene was (logarithmic dilution series of cDNA from the testes). For each sample, the reference gene ((Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH)) and target gene were amplified in the same run. Reference genes were approximately equal. The target genes were normalized to a reference gene and expressed relative to a calibrator.
| Primer | Sequence | Probes |
|---|---|---|
| Caspase 3 F | AAGCGAATCAATGGACTCTGG | 62 |
| Caspase 3 R | CAAGTTTCTGAATGTTTCCCTGAG | |
| GAPDH F | ACCCAGAAGACTGTGGATGG | 60 |
| GAPDH R | TCTAGACGGCAGGTCAGGTC | |
| Bax F | CGAGAGGTCTTTTTCCGAGTG | 60 |
| Bax R | GTGGGCGTCCCAAAGTAGG | |
| Bcl2 F | AGCATCACGGAGGAGGTAGAC | 62 |
| Bcl2 R | CTGGATGAGGGGGTGTCTTC |
Note: F represents Forward primer and R represents Reverse primer
Table 1: Sequences of the Pars Tous Primers and Probes
Statistical analysis:
All the data are presented as the mean±standard deviation of three independent experiments. GraphPad Prism was used to conduct all statistical analyses. The one-way Analysis of Variance (ANOVA) test was employed to observe differences and p<0.01 was considered to be statistically significant.
Results and Discussion
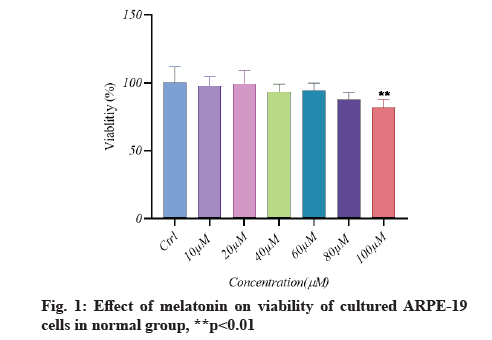
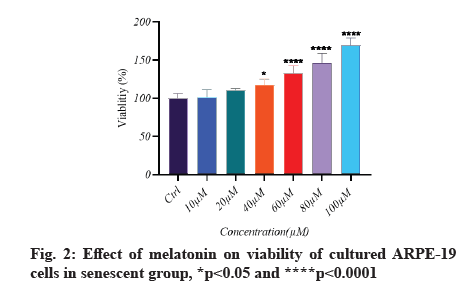
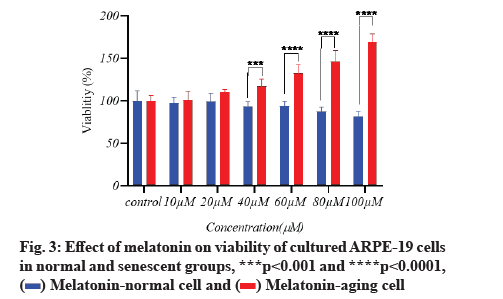
Melatonin’s effect on cell viability is described below.In vitro, the effect of different doses of melatonin on ARPE-19 cell viability in both normal and senescent cells was assessed using the MTT assay. Melatonin exhibited a significant difference; a substantial decrease in cell viability under normal conditions only at 100 µM (fig. 1). In senescent cells, melatonin at 10 µM and 20 µM concentrations showed a non-significant difference in cell viability when compared to the control group (there was a protective effect), whereas melatonin at 40 µM, 60 µM and 80 µM concentrations showed significantly increased cell viability (fig. 2). However, in the aging cells, when compared to the control group, melatonin dramatically improved cell viability (fig. 3). Melatonin’s protective effect on aging RPE cells was thus dose-dependent.
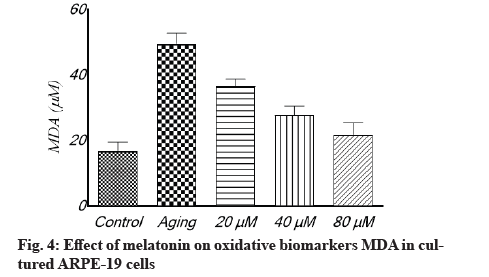
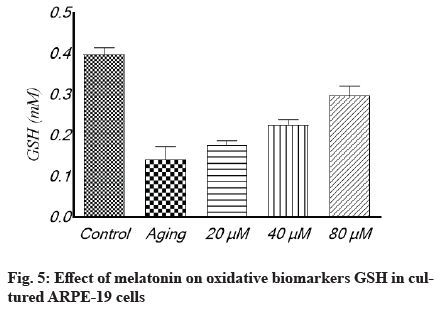
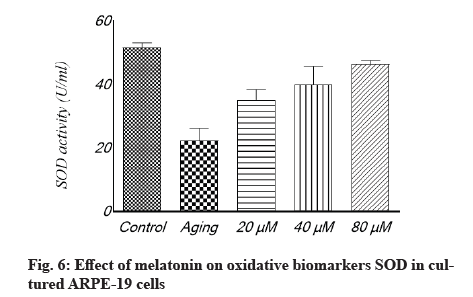
Antioxidant effect of melatonin in aging RPE cells is shown below. In cultivated ARPE-19 cells treated with different concentrations of melatonin, the levels of oxidative stress indicators MDA, SOD and GSH were assessed. The results revealed that the non-treated aging ARPE-19 cells had greater MDA levels and reduced SOD and GSH levels (p<0.001). In the aged ARPE-19 cells, treatment with melatonin resulted in significantly higher levels of anti-apoptotic proteins SOD and GSH and much lower levels of MDA when compared to the non-treated group. In this study, the MDA level was downregulated as melatonin concentrations increased, while SOD and GSH levels were elevated (fig. 4-fig. 6).
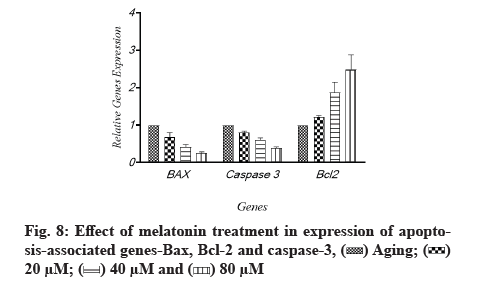
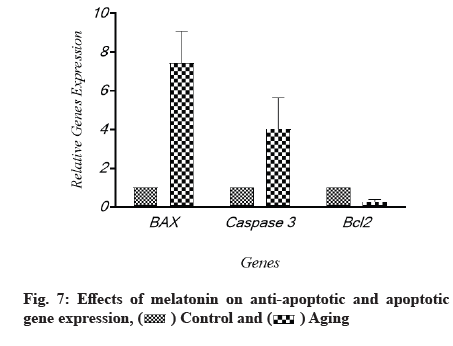
The effects of melatonin on the expression of apoptosis associated genes, B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax) and caspase-3 in aging-induced apoptosis in cultured ARPE-19 cells were measured using quantitative gene expression analysis. The aged cells had decreased levels of anti-apoptotic Bcl-2 and higher amounts of apoptotic Bax and caspase-3, according to the flow cytometry results (fig. 7). When cells were treated with melatonin at different concentrations (20 µM, 40 µM and 60 µM), the expression of Bax and caspase-3 was reduced compared to non-treated cells. The level of Bcl-2, an anti-apoptotic protein, was also elevated as the quantity of melatonin was increased; Bcl-2 was enhanced by different amounts of melatonin, while Bax and caspase-3 were lowered. The same tendency was seen when oxidative-associated proteins were treated with varying melatonin doses. The levels of Bcl-2 and caspase-3 in the 20 µM and 40 µM melatonin-treated groups were similar in general. However, as compared to the 20 µM and 40 µM groups, the increase in Bcl-2 in the melatonin 80 µM groups was not significant. Each protein’s tendency to increase in melatonin-treated groups was substantial, as illustrated in fig. 8.
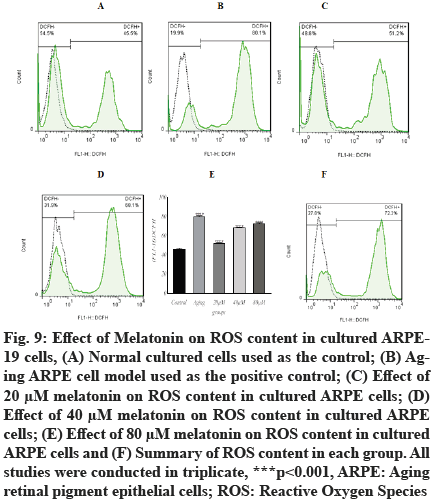
In the current study, the intracellular ROS concentrations were significantly increased (p<0.001, 80.07±1.05) in aging ARPE-19 cells as detected by the specific fluorescent probe, compared with normally cultured RPE cells (46.27±0.86). The intracellular ROS concentrations of aging ARPE-19 cells treated with 20 µM, 40 µM and 80 µM of melatonin were increased (51.93±0.66, 68.27±0.66 and 72.37±1.05, respectively), as illustrated in fig. 9. The content of ROS decreased as melatonin concentrations increased. These findings suggest that the administration of 20 µM, 40 µM and 80 µM concentrations of melatonin can effectively inhibit age-related ROS generation.
Fig. 9: Effect of Melatonin on ROS content in cultured ARPE- 19 cells, (A) Normal cultured cells used as the control; (B) Aging ARPE cell model used as the positive control; (C) Effect of 20 μM melatonin on ROS content in cultured ARPE cells; (D) Effect of 40 μM melatonin on ROS content in cultured ARPE> cells; (E) Effect of 80 μM melatonin on ROS content in cultured ARPE cells and (F) Summary of ROS content in each group. All studies were conducted in triplicate, ***p<0.001, ARPE: Aging retinal pigment epithelial cells; ROS: Reactive Oxygen Species.
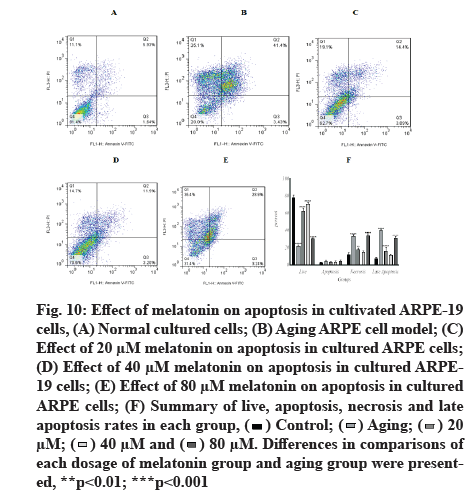
Melatonin has an inhibitory effect on aging-induced apoptosis in ARPE-19 cells. When ARPE-19 cells were exposed to 800 µM H2O2 in pulsed-wave mode, the percentage of aging live cells decreased significantly (21.51±1.58). Low-dose treatments of 20 µM and 40 µM of melatonin increased the percentage of aging live cells (62.66±3.08 and 70.82±2.163, respectively), whereas the high dose of 80 µM cause increase by 30.32±1.01, in comparison to a control group (78.48±2.74).
In the apoptosis groups, the results revealed a non-significant difference in apoptotic cell percentages. In the necrosis group, there was a significant increase in aging cells (33.63±1.76), with the highest increase in the 80 µM group (33.99±2.14), when compared with the control group (11.88±2.21), while there was no significant change in the 40 µM group (14.59±1.867). In late apoptosis and at 20 µM and 80 µM doses of melatonin, there was a significant increase in aging (40.42±0.93, 15.99±4.32 and 31.11±2.24, respectively), when compared with the control group (7.39±1.792, p<0.001). Fig. 10 depicts representative flow cytometry data and detailed results.
Fig. 10: Effect of melatonin on apoptosis in cultivated ARPE-19 cells, (A) Normal cultured cells; (B) Aging ARPE cell model; (C) Effect of 20 μM melatonin on apoptosis in cultured ARPE cells; (D) Effect of 40 μM melatonin on apoptosis in cultured ARPE- 19 cells; (E) Effect of 80 μM melatonin on apoptosis in cultured ARPE cells; (F) Summary of live, apoptosis, necrosis and late apoptosis rates in each group,
 Differences in comparisons of each dosage of melatonin group and aging group were presented, **p<0.01; ***p<0.001.
Differences in comparisons of each dosage of melatonin group and aging group were presented, **p<0.01; ***p<0.001.
According to growing evidence, the oxidative stress of RPE cells is a critical aspect of the pathophysiology of AMD[16]. As a therapy option for AMD, research has concentrated on finding techniques to protect RPE cells from oxidative stress. In this regard, H2O2 exposure is a common model for conveying RPE cell oxidative stress susceptibility and antioxidant activity[17-19].
As previously mentioned the circulatory melatonin level has been lowered with aging, in patients with AMD[20]. Melatonin has been shown to protect RPE cells against blue light and H2O2 damage in vitro as well as against oxidative stress[17,18,21]. It is an antioxidant and endogenous ROS scavenger with a higher antioxidant capacity than other antioxidants such as vitamin E[22,23]. Different types of retinal cells, such as RPE cells and photoreceptors, may be protected by melatonin[23]. According to a diabetes mellitus study, endogenous and exogenous melatonin may influence metabolic abnormalities not only by modulating insulin secretion but also by providing protection against ROS[24]. According to the findings of the present study, melatonin has numerous anti-oxidative properties in RPE cells. Its administration resulted in lower cell viability, higher apoptosis rates and a more severe oxidative state in aging RPE cell cultures.
Melatonin has strong antioxidative properties, making it a promising candidate for the prevention of oxidative stress-related diseases such as premature aging and degenerative disease in humans. It could thus play a role in the pathogenesis of AMD, a disease that affects photoreceptors and the retinal pigment epithelium and has been linked to oxidative stress. A study has shown that melatonin can protect RPE cells from damage caused by ROS. Melatonin behaves similarly to synthetic mitochondria-targeted antioxidants, which concentrate at relatively high levels in mitochondria; thus, it may prevent mitochondrial damage in AMD[25]. A number of blinding retinal diseases, such as diabetic retinopathy, retinitis pigmentosa and AMD, are linked to increased oxidative stress. Antioxidant vitamins and minerals have been demonstrated to lower the risk of AMD. The apoptotic death of RPE cells, followed by photoreceptor cell death, is a major factor contributing to the pathogenesis of the dry form of AMD[26]. However, MDA reflects oxidative damage in RPE cells. Therefore, to slow the progress and development of early AMD resulting from oxidative damage, it is critical to protect RPE cells by reducing ROS and MDA formation[27]. The current study indicated that the exposure of ARPE-19 cells to H2O2 resulted in increase in ROS and MDA generation, but these effects were significantly ameliorated by treatment with melatonin.
In this study, we used a pulsed H2O2 exposure paradigm as an in vitro aging model. The feasibility of this cellular model was higher. Furthermore, the results from this in vitro model would be more accurate than a single H2O2 exposure.
According to this study, melatonin has antioxidant and anti-apoptotic properties in aged RPE cells that have been exposed to oxidative stress and apoptosis. The findings also provide evidence for melatonin’s use in the treatment of AMD. These results suggest that melatonin may play a role in protecting RPE cells from oxidative stress. In conclusion, melatonin has protective effects against H2O2-induced retinal cell death; it inhibits H2O2-induced RPE cell damage, decreases the apoptosis rate, increase mitochondrial membrane potential and decreases caspase activation.
Acknowledgements:
The authors would like to thank the management and staff at the College of Pharmacy, Hawler Medical University for their assistance.
Conflict of interests:
The authors declared no conflict of interest.
References
- Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest 2013;123(3):951-7.
[Crossref] [Google Scholar] [PubMed]
- Franzke B, Neubauer O, Wagner KH. Super DNAging-new insights into DNA integrity, genome stability and telomeres in the oldest old. Mutat Res Rev Mutat Res 2015;766:48-57.
[Crossref] [Google Scholar] [PubMed]
- Bardak H, U?uz AC, Bardak Y. Protective effects of melatonin and memantine in human retinal pigment epithelium (ARPE-19) cells against 2-ethylpyridine-induced oxidative stress: Implications for age-related macular degeneration. Cutan Ocul Toxicol 2018;37(2):112-20.
[Crossref] [Google Scholar] [PubMed]
- Wolf S. Current status of anti-vascular endothelial growth factor therapy in Europe. Jpn J Ophthalmol 2008;52(6):433-9.
[Crossref] [Google Scholar] [PubMed]
- Zanon-Moreno V, Garcia-Medina JJ, Zanon-Viguer V, Moreno-Nadal MA, Pinazo-Duran MD. Smoking, an additional risk factor in elder women with primary open-angle glaucoma. Mol Vis 2009;15:2953-9.
[Google Scholar] [PubMed]
- Zhang X, Hughes BA. KCNQ and KCNE potassium channel subunit expression in bovine retinal pigment epithelium. Exp Eye Res 2013;116:424-32.
[Google Scholar] [PubMed]
- Majji AB. Retinal pigment epithelial autotransplantation: Morphological changes in retina and choroid. Graefes Arch Clin Exp Ophthalmol 2000;238(9):779-91.
[Crossref] [Google Scholar] [PubMed]
- Subhi Y, Henningsen GØ, Larsen CT, Sørensen MS, Sørensen TL. Foveal morphology affects self-perceived visual function and treatment response in neovascular age-related macular degeneration: A cohort study. PLoS One 2014;9(3):e91227.
[Crossref] [Google Scholar] [PubMed]
- Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res 2017;60:201-18.
[Crossref] [Google Scholar] [PubMed]
- Golestaneh N, Chu Y, Cheng SK, Cao H, Poliakov E, Berinstein DM. Repressed SIRT1/PGC-1α pathway and mitochondrial disintegration in iPSC-derived RPE disease model of age-related macular degeneration. J Transl Med 2016;14(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Tian B, Maidana DE, Dib B, Miller JB, Bouzika P, Miller JW, et al. miR-17-3p exacerbates oxidative damage in human retinal pigment epithelial cells. PLoS One 2016;11(8):e0160887.
- Al-babily EO, Tawfeeq FK. Effect of melatonin on some biochemical parameters in D-galactose induced aging in rats. Rafidain J Sci 2020;29(1):39-47.
- Korkushko OV, Khavinson VK, Shatilo VB, Magdich LV. Effect of peptide preparation epithalamin on circadian rhythm of epiphyseal melatonin-producing function in elderly people. Bull Exp Biol Med 2004;137(4):389-91.
[Crossref] [Google Scholar] [PubMed]
- Anisimov VN, Popovich IG, Zabezhinski MA, Anisimov SV, Vesnushkin GM, Vinogradova IA. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim Biophys Acta Bioenerg 2006;1757(5-6):573-89.
[Crossref] [Google Scholar] [PubMed]
- Zhuge CC, Xu JY, Zhang J, Li W, Li P, Li Z, et al. Fullerenol protects retinal pigment epithelial cells from oxidative stress-induced premature senescence via activating SIRT1. Invest Ophthalmol Vis Sci 2014;55(7):4628-38.
[Crossref] [Google Scholar] [PubMed]
- Yildirim Z, Ucgun NI, Yildirim F. The role of oxidative stress and antioxidants in the pathogenesis of age-related macular degeneration. Clinics 2011;66(5):743-6.
[Google Scholar] [PubMed]
- Zhu C, Dong Y, Liu H, Ren H, Cui Z. Hesperetin protects against H2O2-triggered oxidative damage viaupregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed Pharmacother 2017;88:124-33.
[Crossref] [Google Scholar] [PubMed]
- Liang FQ, Green L, Wang C, Alssadi R, Godley BF. Melatonin protects human retinal pigment epithelial (RPE) cells against oxidative stress. Exp Eye Res 2004;78(6):1069-75.
[Crossref] [Google Scholar] [PubMed]
- Rosen RB, Hu DN, Chen M, McCormick SA, Walsh J, Roberts JE. Effects of melatonin and its receptor antagonist on retinal pigment epithelial cells against hydrogen peroxide damage. Mol Vis 2012;18:1640-8.
[Google Scholar] [PubMed]
- Rosen R, Hu DN, Perez V, Tai K, Yu GP, Chen M, et al. Urinary 6-sulfatoxymelatonin level in age-related macular degeneration patients. Mol Vis 2009;15:1673-9.
[Google Scholar] [PubMed]
- Argun M, Tök L, U?uz AC, Çelik Ö, Tök ÖY, Naziro?lu M. Melatonin and amfenac modulate calcium entry, apoptosis and oxidative stress in ARPE-19 cell culture exposed to blue light irradiation (405 nm). Eye 2014;28(6):752-60.
[Crossref] [Google Scholar] [PubMed]
- Brennan R, Jan JE, Lyons CJ. Light, dark and melatonin: Emerging evidence for the importance of melatonin in ocular physiology. Eye 2007;21(7):901-8.
[Crossref] [Google Scholar] [PubMed]
- Tosini G, Baba K, Hwang CK, Iuvone PM. Melatonin: An underappreciated player in retinal physiology and pathophysiology. Exp Eye Res 2012;103:82-9.
[Crossref] [Google Scholar] [PubMed]
- Espino J, Rodríguez AB, Pariente JA. Melatonin and oxidative stress in the diabetic state: Clinical implications and potential therapeutic applications. Curr Med Chem 2019;26(22):4178-90.
[Crossref] [Google Scholar] [PubMed]
- Blasiak J, Reiter RJ, Kaarniranta K. Melatonin in retinal physiology and pathology: The case of age-related macular degeneration. Oxid Med Cell Longev 2016;2016:1-12.
[Crossref] [Google Scholar] [PubMed]
- Hollborn M, Chen R, Wiedemann P, Reichenbach A, Bringmann A, Kohen L. Cytotoxic effects of curcumin in human retinal pigment epithelial cells. PLoS One 2013;8(3):e59603.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Sun Z, Shen J, Wu D, Liu F, Yang R, et al. Octreotide protects the mouse retina against ischemic reperfusion injury through regulation of antioxidation and activation of NF-κB. Oxid Med Cell Longev 2015;2015:1-11.
[Crossref] [Google Scholar] [PubMed]
 .
.
 .
.