- *Corresponding Author:
- Mingyue Chen
Department of Critical Care Medicine, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310052, China
E-mail: cmy20160909@stu.xjtu.edu.cn
| This article was originally published in a special issue, “New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “152-160” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This meta-analysis aims to assess the antihypertensive effectiveness, safety, and cost-effectiveness of allisartan to support its clinical use and promote rational antihypertensive drug usage. A meta-analysis was registered on International Prospective Register of Systematic Reviews (CRD42022370300) and collected allisartan research on hypertensive patients from sources like PubMed, Cochrane library, China National Knowledge Infrastructure, Wanfang and Vipshop databases from the establishment of the databases to September 2022. Review manager and Stata software evaluated the literature quality, analyzed the outcome indicators, assessed publication bias via Egger's test, and conducted pharmacoeconomic evaluation. Inclusion criteria covered 14 studies with 2125 patients, including 734 allisartan-treated patients. When compared with other angiotensin receptor blocker antihypertensives, allisartan showed reduced systolic blood pressure (5.46 mmHg, 95 % confidence interval: 1.99~8.93, p<0.001) and diastolic blood pressure (2.90 mmHg, 95 % confidence interval: -0.28~6.08, p<0.001). However, this was clinically within the normal blood pressure fluctuations and did not reach the threshold for adverse reactions (relative risk=0.74, 95 % confidence interval: 0.53~1.04, p=0.953). Safety and efficacy of allisartan were compared with other angiotensin receptor blocking drugs. Cost-minimization analysis indicated favorable economics for allisartan. Allisartan exhibits antihypertensive safety and efficacy with other angiotensin receptor blocker antihypertensive drugs, while also displaying costeffectiveness. This supports its clinical use and rationalizes antihypertensive drug choices.
Keywords
Allisartan, blood uric acid, meta-analysis, cost-minimization analysis
Activation of the Renin-Angiotensin System (RAS) plays a key role in the development of hypertension and cardiovascular disease[1-3]. Angiotensin II, a key effector of the RAS, mediates cardiovascular disease by binding to the Angiotensin II Type 1 receptor (AT1) and also exerts cardioprotective effects by binding to the Angiotensin II Type 2 receptor (AT2)[4,5]. A large body of evidence suggests that Angiotensin Receptor Blockers (ARBs) as first-line antihypertensive agents having significant antihypertensive effects and they exert target organ protection during the development of hypertension.
Allisartan is a newly developed ARB class drug in China. Allisartan ester is a prodrug of the active metabolite EXP-3174 of losartan, which produces 41 times more EXP-3174 than losartan potassium.
However, unlike losartan, allisartan ester can be hydrolyzed by esterases and can be directly converted to the active substance EXP-3174. After absorption through the gastrointestinal tract, it does not require the metabolic catalysis of Cytochrome P450 (CYP450); therefore, the interaction between different drugs and the incidence of adverse drug reactions are low, and it has obvious advantages in terms of safety and tolerability.
Previous studies have shown that allisartan has efficient effects in lowering blood pressure and organ protection with low toxicity in animal models[6]. However, till date there is only little evidence from clinical studies. Thus, in this study we studied the clinical efficacy of allisartan in the treatment of hypertensive patients, quantitatively evaluated the antihypertensive effect of allisartan by two indicators, systolic and diastolic blood pressure, measured the safety of allisartan according to the occurrence of adverse reactions, and evaluated the economics of allisartan using appropriate pharmacoeconomic methods, so as to provide a reference for further securing the rational use of antihypertensive drugs.
Materials and Methods
Literature search strategy:
Literature search was performed using PubMed, Cochrane Database of Systematic Reviews (CDSR), China National Knowledge Infrastructure (CNKI) (https://www.cnki.net/), Wanfang (https://www.wanfangdata.com.cn/) and Wipu databases (https://www.cqvip.com/). The search terms used were “allisartan”, “hypertension” and the search period was considered from the establishment of the databases to September 2022. According to the previously published literature reviews, meta-analyses and studies, a manual search was also conducted for references included in this meta-analysis. This literature search process was performed independently by two researchers respectively.
Inclusion criteria:
It included studies with appropriate clinical efficacy outcomes including systolic and diastolic blood pressure; Randomized Controlled Trials (RCTs); healthy adults in the study population and interventions in which allisartan were given to the experimental group and other ARB antihypertensive drugs to the control group.
Exclusion criteria:
Literature that did not meet the above inclusion criteria; literature for which the full text was not available; review literature, conference literature and animal experiments; literature with missing data and results not using mean±standard deviation; literature with poor quality assessment and literature with duplicate publications was excluded.
Data extraction:
After the data was extracted according to the above inclusion and exclusion criteria, both the researcher’s extracted relevant indicators from the screened literature, including first author, sample size, age, dose administered, time of administration, control measures, primary outcome indicators and adverse events. In case of disagreement during the data extraction, a third investigator joined the discussion to make the final decision regarding data extraction. Since some of the included studies in the literature did not directly give the mean and standard deviation of systolic and diastolic blood pressure changes in the control and experimental groups, the difference between pre- and post- dosing for each group was used to represent the changes in systolic and diastolic blood pressure using the below mentioned formula[7].
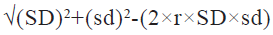
Here, SD is the standard deviation of the pre- dosing index; sd is the standard deviation of the post-dosing index, and r is the correlation coefficient between pre-dosing and post-dosing measurements, r=0.5.
Literature quality evaluation:
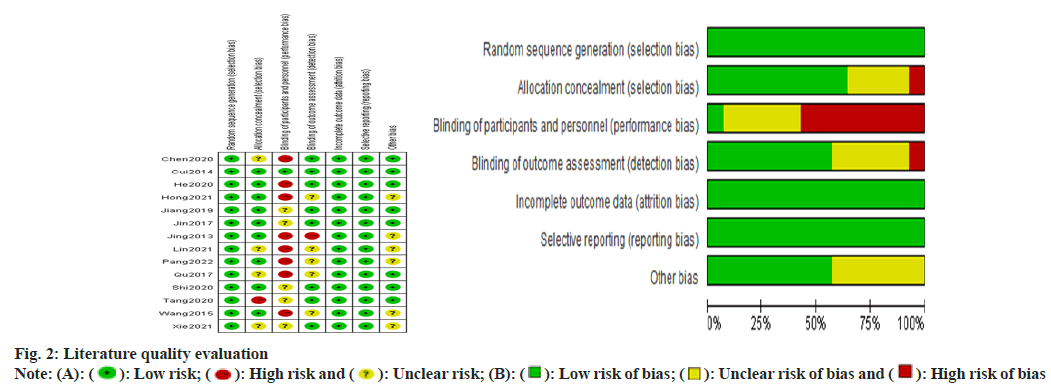
The study was evaluated for literature quality using Review Manager (RevMan) 5.4 software, which uses a modified version of the Cochrane risk bias assessment tool. It included random sequence generation; allocation concealment; double- blinding of performers and participants; blinding in outcome assessment; incomplete outcome data; selective publication and other bias criteria, each of which was evaluated to include three scenarios like high risk, low risk and unclear risk.
Statistical analysis:
The data was extracted and statistically analyzed using Stata 15. The data processing included forest plot, funnel plot drawing, sensitivity analysis, subgroup analysis and publication bias test. I2 was used as a measure of heterogeneity; when I2<25 %, heterogeneity was low; when 25≤I2<50 %, moderate heterogeneity existed; in both cases, a fixed effects model was selected; when I2≥50 %, it indicated high heterogeneity among studies, and a random effects model was used for analysis. Egger's test was used to detect publication bias in the included literature, and when p>0.05, it indicated that the study had no publication bias. Normally distributed measures were expressed as mean±standard deviation and p<0.05 was considered statistically significant.
Cost analysis:
Based on the results of the meta-analysis, an appropriate economic evaluation method was selected. If the efficacy and safety of allisartan and other ARB antihypertensive drugs did not differ significantly, “cost minimization analysis” was used to analyze the pharmacoeconomics of two regimens while if the efficacy or safety of the two drugs differed significantly, “cost- effectiveness analysis” was selected to compare the pharmacoeconomics of the two regimens.
This study focused only on direct medical costs. It is assumed that patients have similar baseline characteristics such as source, basic information and major symptoms. The cost of tests and registration incurred during the treatment are same, so only drug costs are included in this study. Drug price data was obtained from the average winning prices of domestic drugs published in the 2022 DrugWise Data-Drug Winning Bids Database (https://db.yaozh.com/yaopinzhongbiao). The average drug prices in China were used as estimated costs to compare the cost differences between the two groups.
Results and Discussion
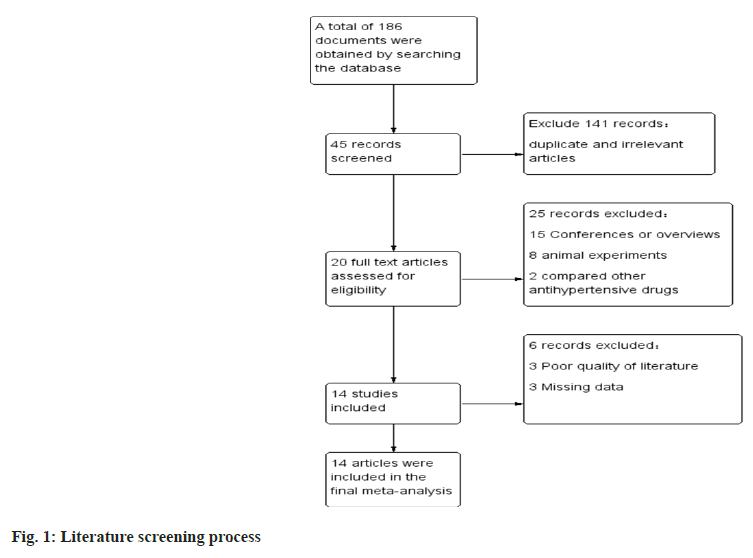
Literature screening results were carried out where we searched the database to obtain a total of 186 publications, and after screening a total of 14 RCTs which met our inclusion and exclusion criteria were considered in the final meta-analysis (fig. 1).
General information and quality evaluation of the included literature was carried out. A total of 2125 patients were included in the 14 publications, 734 of whom were treated with allisartan. 14 publications which were included were assessed using a modified version 2 of the Cochrane Risk of Bias (RoB 2) tool for randomized trials, and those consisting of more than two high-risk conditions were considered to be of poor quality. The general profile of the literature is specified in Table 1 and the risk assessment is shown in fig. 2.
| Sample size | Age | Dose | Time of administration | Control group | Control group dose | References |
|---|---|---|---|---|---|---|
| 80 | 60.33±5.05 | 80 mg | 12 mo | Losartan | 80 mg | [8] |
| 35 | 70.56±3.47 | 240 mg | 2 mo | Valsartan | 80 mg | [9] |
| 50 | 56.3±7.2 | 80 mg | 2 mo | Telmisartan | 50 mg | [10] |
| 30 | 68.33±6.05 | 240 mg | 3 mo | Valsartan | 80 mg | [11] |
| 55 | 70.29±5.69 | 240 mg | 6 mo | Irbesartan | 150 mg | [12] |
| 68 | 57.82 | 240 mg | 1 mo | Irbesartan | 150 mg | [13] |
| 69 | 54±9.7 | 80 mg | 2 mo | Losartan | 50 mg | [14] |
| 20 | / | 80 mg | 3 mo | Irbesartan | 150 mg | [15] |
| 59 | 52.89±5.13 | 240 mg | 2 mo | Irbesartan | 150 mg | [16] |
| 40 | 63.14±5.82 | 240 mg | 3 mo | Valsartan | 80 mg | [17] |
| 50 | 51.3±6.2 | 240 mg | 0.5 mo | Losartan | 100 mg | [18] |
| 30 | 63.7±7.3 | 240 mg | 2 mo | Valsartan | 80 mg | [19] |
| 43 | 70.36±3.62 | 80 mg | 3 mo | Irbesartan | 150 mg | [20] |
| 105 | 67.64±6.76 | 240 mg | 3 mo | Losartan | 100 mg | [21] |
Note: (/): Not available
Table 1: General Situation of the Included Literature
The effect of allisartan on systolic blood pressure was evaluated using 14 papers[8-21]. When compared with other ARB antihypertensive agents, the results showed that allisartan was able to reduce systolic blood pressure in patients to a greater extent (5.46 mmHg, 95 Confidence Interval (CI) %-1.99~8.93, p<0.001) with an I2>50 %. Therefore random effects model was chosen and the difference was statistically significant (fig. 3). Although there is statistical difference, the continuous variable was different and the difference was clinically normal. Fluctuations in blood pressure did not reach the clinical threshold, and therefore, it was not considered that allisartan could provide more meaningful antihypertensive benefit compared to other ARBs. Therefore, it is considered that allisartan has a role in lowering systolic blood pressure that is not weaker than other ARBs.
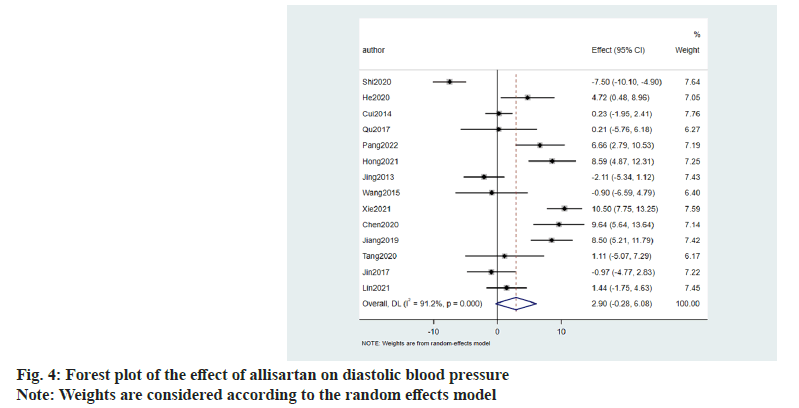
Similarly the effect of allisartan on diastolic blood pressure was also evaluated using 14 papers[8-21]. When compared with other ARB antihypertensive agents, the results denoted that allisartan reduced diastolic blood pressure in patients to a greater extent (2.90 mmHg, 95 CI %-0.28~6.08, p<0.001) with I2>50 %. So random effects model was chosen and the difference was statistically significant (fig. 4).
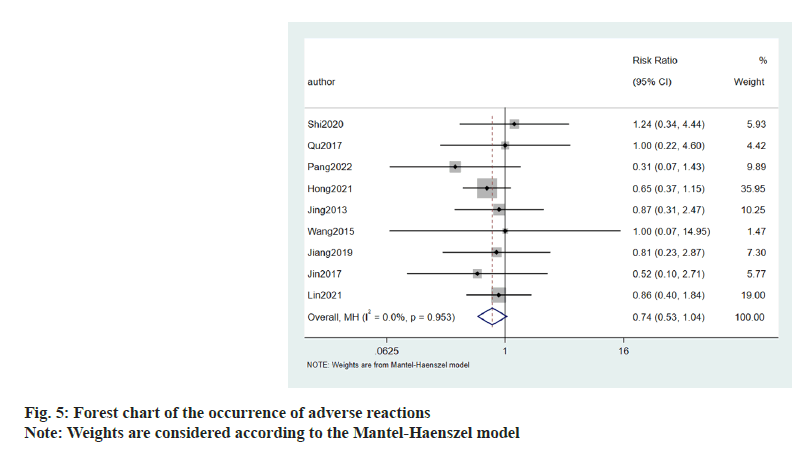
Occurrence of adverse reactions to allisartan treatment was evaluated using 9 publications. Patients suffering with hypertension were treated with allisartan. I2<50 %, and meta-analysis was performed using a fixed effects model. The results showed that the occurrence of adverse reactions (Relative Risk (RR)=0.74, 95 CI %-0.53~1.04, p=0.953) compared to the control group, was not statistically significant (fig. 5).
Further, 3 articles evaluated the effect of allisartan on uric acid, and found that allisartan significantly reduced uric acid levels in patients, compared with other ARB antihypertensive agents.
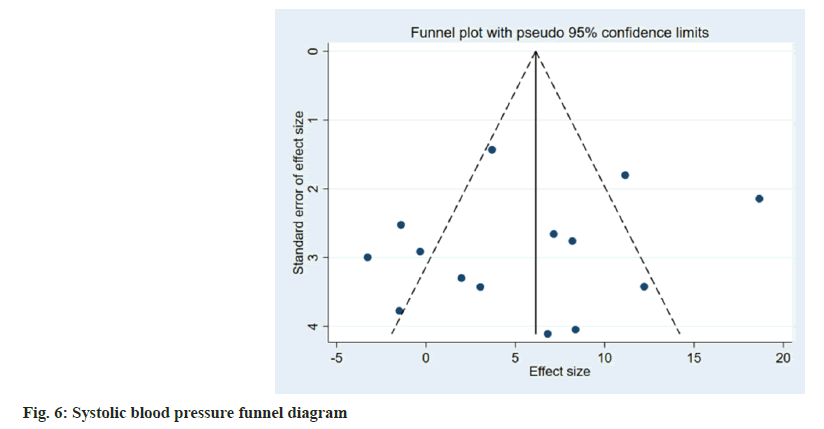
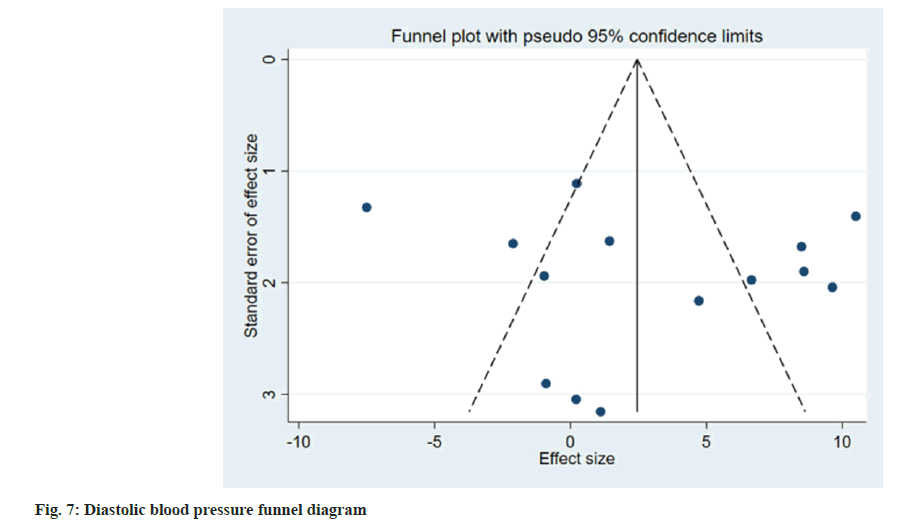
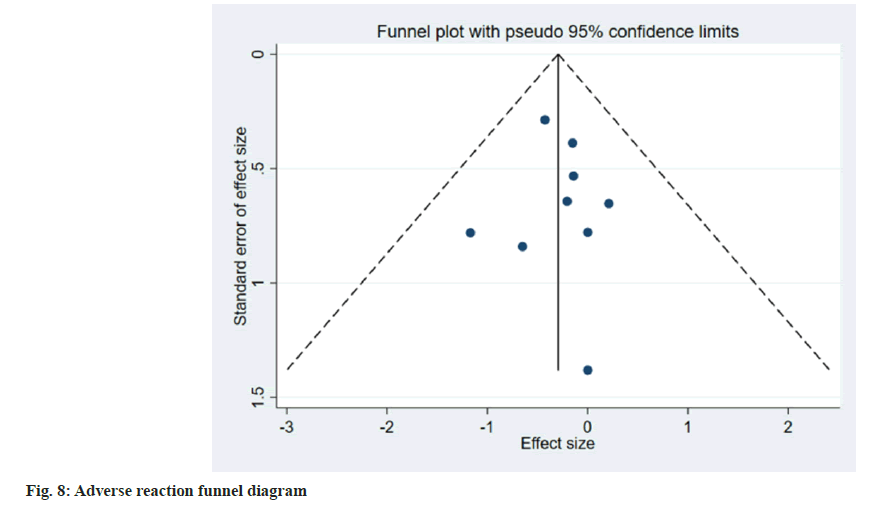
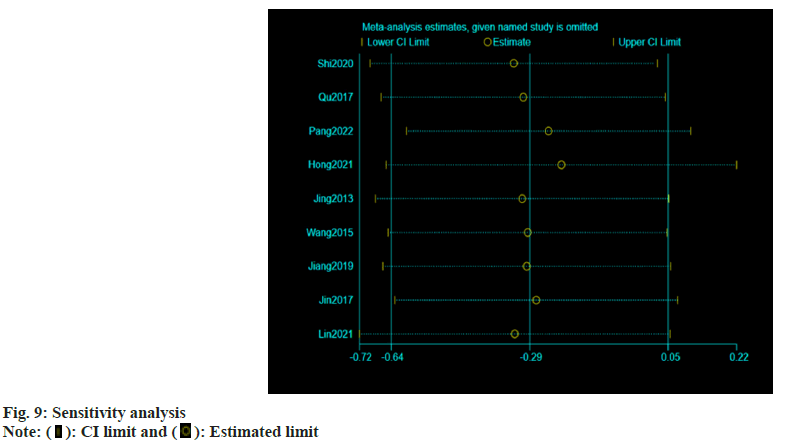
Publication bias evaluation and sensitivity analysis evaluation was conducted using funnel plot analysis, where each of the 3 indicators of systolic blood pressure, diastolic blood pressure and adverse reactions, showed inverted symmetric funnel plots (fig. 6-fig. 8), indicating no significant publication bias. However, the funnel plot was highly subjective, and Egger's test was performed to evaluate the publication bias of the included literature. The p values obtained were >0.05, indicating no significant publication bias. Sensitivity analysis was performed for each result (fig. 9). 14 studies were included in the study and by excluding one study; the combined results of the remaining 13 studies were not statistically significant and were consistent with the originally combined results of 14 studies, indicating stable results.
Cost analysis was carried out according to the results of the above meta-analysis. The antihypertensive effect and safety of allisartan were compared to other ARB antihypertensive drugs, and allisartan had good economics using the least-cost analysis.
Due to the different treatment doses of each sartan, the average daily dosage of the drug with the same efficacy is calibrated, where 9 sartan treatments include telmisartan tablets (80 mg), valsartan capsules (160 mg), irbesartan (300 mg), losartan potassium (100 mg), candesartan medoxomil tablets (32 mg), olmesartan medoxomil tablets (40 mg), alisartan medoxomil tablets (240 mg), mexartan potassium tablets (80 mg), eprosartan tablets (1200 mg). According to DrugWise Data-Drug Winning Bids Database, the value is calculated by two decimal places, telmisartan tablet is Renminbi (RMB) 4.79/d, valsartan capsule is RMB 7.01/d, irbesartan is RMB 6.35/d, losartan potassium is RMB 6.42/d, candesartanate tablet is RMB 12.24/d, olmesartanate tablet is RMB 11.38/d, allisartan tablet is RMB 4.30/d, azilsartan medoxomil potassium tablet is RMB 12.80/d and eprosartan tablet is RMB 23.14/d.
To our knowledge, this is a meta-study comparing the efficacy and safety of allisartan and the first meta-analysis to compare the efficacy and safety of allisartan with other ARB class antihypertensive agents in patients with hypertension. In this meta- analysis, we included 14 RCTs involving a total of 2125 patients, and we combined all relevant RCTs of allisartan wherever possible and compared their efficacy using systolic and diastolic blood pressure as the primary outcomes, as they are considered an important alternate endpoint for cardiovascular disease. The results of the analysis showed that allisartan has an antihypertensive efficacy and safety that is not weak than other ARB class antihypertensive agents.
With this evidence, we can use the least-cost analysis method to evaluate the economics of allisartan vs. other ARB class antihypertensive drugs. The least-cost analysis is one of the four main methods of pharmacoeconomic evaluation, and it is applicable when the benefits or outcomes of the alternative options are the same. In such case, the pharmacoeconomic study compares only cost differences. In this meta-analysis, allisartan had good economics compared with other ARB class antihypertensive drugs.
ARB class antihypertensive drugs are widely used among hypertensive patients with combined atherosclerosis, diabetes mellitus, and heart failure because of their target organ protective effects[22-24]. Studies by Chen et al.[17] and Hong et al.[13] showed that, compared with valsartan and irbesartan, allisartan tablets were safer and more effective in reducing patients’ blood pressure in the treatment of essential hypertension and in relieving their heart failure symptoms. Additionally, these tablets reasonably improve cardiac functioning in patients with combined ejection fraction preserved heart failure. Studies by Tang et al.[19], Jiang et al.[18] and Shi et al.[8] showed that the clinical efficacy of allisartan tablets for hypertension combined with hyperuricemia was definite and could significantly reduce uric acid compared with olmesartan and valsartan in case of comparable blood pressure lowering. Studies by Xie et al.[16] showed that the clinical efficacy of allisartan tablets was superior to that of irbesartan tablets in the treatment of patients with hypertension combined with coronary artery disease, and the safety profile was higher comparatively. Studies by He et al.[9] showed that allisartan reduced blood pressure levels and improved vascular endothelial function in elderly hypertensive patients. Studies by Pang et al.[12] showed that the clinical efficacy of allisartan compared with irbesartan in the treatment of geriatric hypertension is definite, and it can effectively improve the vascular endothelial function, reduce blood pressure and heart rate in patients with a higher safety profile. This is because allisartan has a strong renoprotective effect, reducing renal hyperfiltration pressure, relieving and improving proteinuria, reducing uric acid and also has some cardiovascular protection. The adverse effects of allisartan are mild, transient and usually causes dizziness, headache while most of them can be relieved by themselves or after symptomatic treatment. Allisartan is metabolized by gastrointestinal esterases after oral administration, producing the same active metabolite EXP-3174 as that of losartan potassium after hepatic metabolism. Allisartan is not metabolized by hepatic enzymes, so it has fewer drug interactions than losartan and is safer to co- administer. A pharmacokinetic study by Deng et al.[25] showed that the area under the drug-time curve was higher in subjects who generated EXP- 3174 after a single oral dose of allisartan 240 mg than in the group who administered 100 mg losartan potassium. This indicates that allisartan has a better clinical safety profile.
For the ARB class of antihypertensive drugs, lowering blood pressure is not the main objective, but they are more valued for their extra-hypertensive benefits such as delaying carotid intima-media thickness, improving insulin resistance, reducing cardiac remodeling, and reducing the occurrence and recurrence of atrial fibrillation. However, in the present meta-analysis, the above-mentioned outcomes were not observed, which brings up a limitation of the present meta-analysis. Allisartan is firstly, a new drug marketed in China and secondly, not marketed abroad, so there are not many studies that meet the criteria and even fewer studies with statistically significant results. Although the above results mentioned extra-hypertensive benefits, there were few relevant studies with homogeneity and no statistical significance, so they were not selected for inclusion in the analysis. However, the extra-hypertensive benefits are also reported above. Another limitation of this study is the large heterogeneity of the results of this meta-analysis, which may not be due to the fact that the antihypertensive effect of allisartan is related to the type of other ARB drugs, duration of administration, period of time and the health status of the subjects. It is difficult to perform subgroup analysis and some bias is inevitable. Therefore, it is suggested that future studies should focus on exploring the antihypertensive effect and safety of allisartan in a wider population with respect to dose and duration of administration, as well as conducting similar clinical trials in foreign populations, so as to provide adequate theoretical guidance for the application of allisartan. The allisartan group has antihypertensive safety and efficacy that are not inferior to other ARB antihypertensive drugs, and has good economy.
Funding:
This study is supported by The Construction Fund of Medical Key Disciplines of Hangzhou.
Conflict of interests:
The authors declared no conflict of interest.
References
- Lu Z, Chen Y, Li L, Wang G, Xue H, Tang W. Combination therapy of renin-angiotensin system inhibitors plus calcium channel blockers vs. other two-drug combinations for hypertension: A systematic review and meta-analysis. J Hum Hypertens 2017;31(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Uijl E, Danser AH. Renin-angiotensin-aldosterone system parameters as biomarker in heart failure patients with preserved ejection fraction: Focus on angiotensinogen. Am J Hypertens 2018;31(2):175-7.
[Crossref] [Google Scholar] [PubMed]
- Park H, Kim HK, Jeong MH, Cho JY, Lee KH, Sim DS, et al. Clinical impacts of inhibition of renin-angiotensin system in patients with acute ST-segment elevation myocardial infarction who underwent successful late percutaneous coronary intervention. J Cardiol 2017;69(1):216-21.
[Crossref] [Google Scholar] [PubMed]
- de Dios Ruiz-Rosado J, Lee YU, Mahler N, Yi T, Robledo-Avila F, Martinez-Saucedo D, et al. Angiotensin II receptor I blockade prevents stenosis of tissue engineered vascular grafts. FASEB J 2018;32(12):6822-32.
[Crossref] [Google Scholar] [PubMed]
- Eguchi S, Kawai T, Scalia R, Rizzo V. Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension 2018;71(5):804-10.
[Crossref] [Google Scholar] [PubMed]
- Ling QS, Zhang SL, Tian JS, Cheng MH, Liu AJ, Fu FH, et al. Allisartan isoproxil reduces mortality of stroke-prone rats and protects against cerebrovascular, cardiac, and aortic damage. Acta Pharmacol Sin 2021;42(6):871-84.
[Crossref] [Google Scholar] [PubMed]
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Cochrane Collaboration; 2011.
- Shi T. Efficacy of aliskiren in the treatment of hypertension combined with hyperuricemia. Int J Lab Med 2020;41(2):76-9.
- He YH. Effect of aliskiren on blood pressure and serum ET, NO and HGD levels in elderly patients with hypertension. Electron J Health Res 2020;4(21):51-2.
- Cui X. Application effect observation of different doses of allisartan isoproxil in patients with mild to moderate hypertension. Chin Community Doctor 2014;30(19):20-3.
- Qu WJ, Zhou J, Li F, Gao F. Efficacy and safety of allisartan in elderly patients with mild to moderate essential hypertension. South China J Cardiovasc Dis 2017;23(2):186-90.
- Pang Q. Clinical efficacy of allisartan in the treatment of geriatric hypertension and its effect on vascular endothelial function in patients. J Clin Ration Drug Use 2022;15(6):44-6.
- Hong RC, Yao JP, Liu YC, Ou JM. Study on the effect of aliskiren in the treatment of patients with essential hypertension combined with heart failure with preserved ejection fraction. Famous Med 2021;11(1):172-4.
- Jing S, Sun NL, Zhang LY, Ke YN, Chen YD, Wei M, et al. The efficacy and safety of allisartan in patients with mild to moderate essential hypertension. Chin J Clin Pharmacol 2013;29(10):728-31.
- Wang J, Tong C, Tang HQ. The efficacy of allisartan in old hypertensive patients. Chin J Clin Health Care 2015;18(3):247-9.
- Xie LQ, Hu XQ. Clinical trial of allisartan axetil tablets and irbesartan tablets in the treatment of patients with hypertension and coronary heart disease. Chin J Clin Pharmacol 2021;37(19):2562-8.
- Chen C, Cai L. Study on the efficacy of allisartan in patients with essential hypertension and heart failure with preserved ejection fraction. J Qiqihar Med Coll 2020;41(21):2680-2.
- Jiang L, Du GS, Chen RJ, He JM. Observation on curative effect of allisartan in treating mild to moderate hypertension complicated with hyperuricemia. Lingnan J Emer Med 2019;24(5):485-6.
- Tang Y, Yang D, Yi D. Comparison of the effects of allisartan isoproxil and valsartan in the treatment of hypertension combined with hyperuricemia. J Hunan Normal Univ 2020;17(1):40-2.
- Jin Z. Observation on the clinical efficacy of allisartan medoxomil in the treatment of hypertension in the elderly. Chin Health Nutr 2017;27(11):142-5.
- Lin C, Cai Y. Comparison of the efficacy and safety of allisartan medoxomil and losartan potassium in the treatment of hypertension. J Clin Ration Drug Use 2021;14(3):37-8.
- Zheng H, Li N, Ding Y, Miao P. Losartan alleviates hyperuricemia-induced atherosclerosis in a rabbit model. Int J Clin Exp Pathol 2015;8(9):10428-35.
[Google Scholar] [PubMed]
- Jin H, Zhang HN, Hou XL, Zhang B, Wu J, Zhang HB. Clinical study of double dose of valsartan combined with tacrolimus in treatment of diabetic nephropathy. Eur Rev Med Pharmacol Sci 2016;20(1):174-9.
[Google Scholar] [PubMed]
- Marinšek M, Sinkovič A. Ramipril and losartan exert a similar long-term effect upon markers of heart failure, endogenous fibrinolysis, and platelet aggregation in survivors of ST-elevation myocardial infarction: A single centre randomized trial. Biomed Res Int 2016:2016;1-7.
[Crossref] [Google Scholar] [PubMed]
- Deng C, Yang G, Feng Z. Pharmacokinetic study of allisartan and losartan potassium in healthy subjects. Chin J Clin Pharmacol 2021;37(24):3358-62.
 High risk of bias
High risk of bias 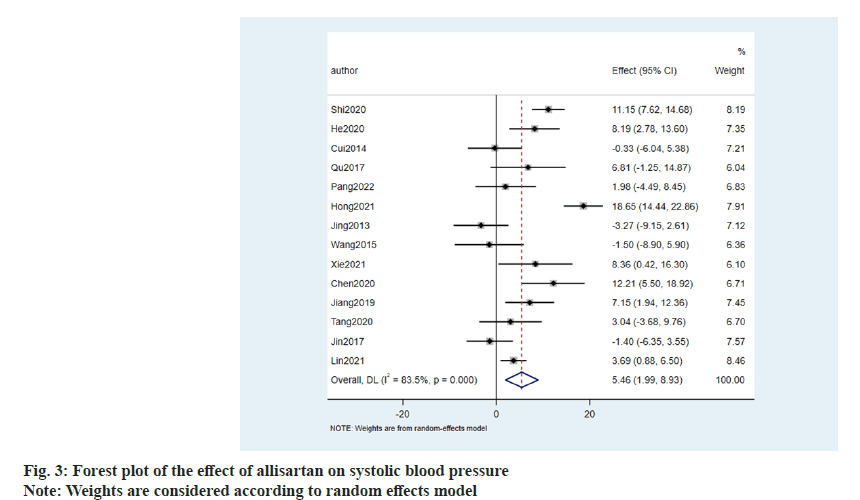
 Estimated limit
Estimated limit



