- *Corresponding Author:
- Ying Liu
Department of Hematology, The First Affiliated Hospital, Guangxi Medical University, Nanning, Guangxi 530021, China
E-mail: liuying_xiaoyu@163.com
| This article was originally published in a special issue, “Exploring the Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(1) Spl Issue “146-153” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A meta-analysis was conducted to figure out the long-term effectiveness of bortezomib in managing patients with multiple myeloma. To retrieve relevant literature, the Chinese National Knowledge Infrastructure and Wanfang databases were searched using the keywords bortezomib, multiple myeloma, and long-term efficacy. Additionally, Embase, the Web of Science, PubMed, and Cochrane Library databases were queried using the terms bortezomib, multiple myeloma, and long-term effect. Two assessors utilized Cochrane scoring to evaluate the quality of the articles. Retrieve pertinent data from the included research articles and analyze it using RevMan 5.4 software to evaluate the effectiveness of bortezomib through meta-analysis. Ten publications were incorporated into the meta-analysis. According to the findings of the meta-analysis, patients undergoing consolidation therapy for multiple myeloma exhibited significantly superior progression-free survival and overall survival outcomes when treated with bortezomib compared to those who were not. In terms of overall survival during consolidation therapy, there was no notable distinction observed between the two groups of patients. Additionally, the funnel plot analysis indicated the absence of any significant publication bias. This meta-analysis unequivocally establishes the notable advantages of utilizing bortezomib as an initial medication choice for consolidation and maintenance therapy in multiple myeloma. Significant enhancements in both progression-free survival and overall survival were observed, underscoring its efficacy.
Keywords
Bortezomib, multiple myeloma, long-term effect, meta-analysis
Significant morbidity and mortality are commonly associated with Multiple Myeloma (MM). This hematologic malignancy is the 2nd most frequently diagnosed cancer, accounting for approximately 1 % of cases[1]. Advances in understanding the pathophysiology of the disease have led to recent advancements in treatment and significant improvement in patient outcomes. For individuals newly diagnosed with MM who meet the criteria for transplantation, the first-line therapeutic strategy involves the use of induction chemotherapy, followed by high-dose therapy and subsequent transplantation of their own stem cells. In cases where transplantation is not a viable option, induction therapy for eligible individuals often involves the prescription of combinations of two or three drugs[2]. Despite the progress in treatment, disease relapse after initial therapy is inevitable for most patients. Hence, consolidation or maintenance therapy is frequently administered to extend both Progression-Free Survival (PFS) and Overall Survival (OS). During the course of consolidation therapy, the primary focus centers on amplifying the efficacy of the initial treatment, while the primary aim of maintenance therapy is to protract the disease-free interval by employing extended low-intensity treatment protocols[3].
Maintenance therapy involving the administration of lenalidomide is frequently incorporated into the treatment regimens of MM patients undergoing autologous stem cell transplantation. Despite being generally well-tolerated, it is important to note the heightened risks of neutropenia, infections, anemia, thromboembolism, thrombocytopenia and second primary malignancies associated with its use[4,5]. As an induction therapy for MM, bortezomib has garnered significant utilization due to its first-in-class nature as a proteasome inhibitor, effectively inducing cell cycle arrest and apoptosis[6-9]. Bortezomib has been employed in a clinical capacity for off-label use in MM patients, specifically as consolidation or maintenance therapy subsequent to initial treatment, especially in cases where high-risk disease is present[10]. The extent of bortezomib’s long-term efficacy in consolidation or maintenance therapy is not as well-established as that of lenalidomide. To address this gap, we undertook a meta-analysis to assess the effectiveness of bortezomib in treating MM, with a particular emphasis on long-term outcomes.
Materials and Methods
Study selection:
In accordance with the 2010 PRISMA guidelines[11], this study was conducted and the methodological quality of systematic reviews was evaluated employing the AMSTAR guidelines. Systematic literature searches were conducted on Cochrane Library, Embase, Web of Science, PubMed, Chinese National Knowledge Infrastructure (CNKI), and Wanfang database up to June 30, 2023. Chinese search terms included bortezomib, MM, and long-term efficacy. English search terms included bortezomib, MM and long-term effect. In addition, we meticulously examined the reference lists of all relevant articles to uncover any additional literature that may be pertinent to our study. Two assessors independently screened and selected studies based on eligibility criteria, and trial data regarding predefined endpoints were subsequently extracted.
Inclusion and exclusion criteria:
Inclusion criteria: Randomized Controlled Trials (RCTs) comparing the administration of bortezomib-containing consolidation/maintenance regimens in the intervention group with regimens without bortezomib or no consolidation/ maintenance therapy in the control group; a comprehensive corpus, which encompasses essential data for statistical analysis, as well as one or more of the clinical endpoints including PFS, OS, and adverse events, is imperative and publications from the same author or research center reporting two or more publications, including recent publications, larger-scale publications, or highquality publications.
Exclusion criteria: Letters, animal experimental studies, commentaries, reviews, conference reports, case reports, and clinical trial registrations and articles lacking necessary data for statistical analysis.
Assessment of methodological quality:
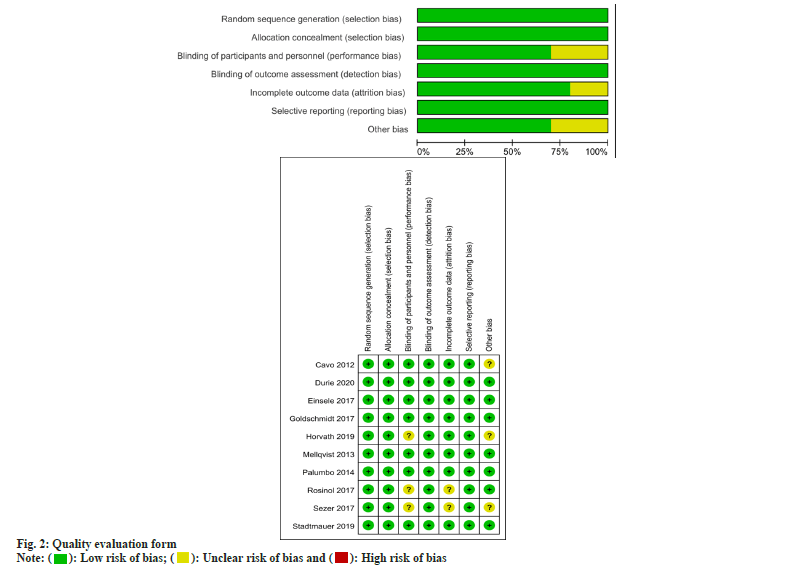
Two assessors conducted the quality assessment of RCTs via the Cochrane risk-of-bias assessment tool, which covers different aspects including selective reporting, blinding, random sequence generation, allocation concealment, incomplete outcome data, and potential sources of biases.
Statistical analysis:
Review Manager 5.4 was utilized to perform the meta-analyses. For survival outcomes, the pooled Hazard Ratio (HR) with a 95 % Confidence Interval (CI) was calculated using the inverse variance method, while the pooled Risk Ratio (RR) with a 95 % CI for safety data was calculated using either the Mantel-Haenszel or DerSimonian-Laird methods. Utilizing the I2 statistic, heterogeneity was examined and classified as low if I2 was below 25 %, moderate if I2 ranged from 25 % to 50 %, and high if I2 surpassed 50 %. When high heterogeneity was present (I2>50 % or p<0.05), a random-effects model was utilized; otherwise, a fixed-effects model was employed. Significance was determined at a significance level of p<0.05.
Results and Discussion
By performing a literature search with predefined inclusion criteria, and referring to the selection process diagram (fig. 1) as well as the quality assessment table (fig. 2), eight studies were enrolled in this meta-analysis. An overview of the general characteristics of these included publications was shown in Table 1[12-21].
| Study | Country | Research span | Sample size | Age | Sex (Male/female) | |||
|---|---|---|---|---|---|---|---|---|
| OG | CG | OG | CG | OG | CG | |||
| Cavo et al.[12] | Italy | 2006.05-2008.08 | 160 | 161 | 55.6±7.3 | 55.5±7.6 | 96/64 | 95/66 |
| Durie et al.[13] | USA | 2008.04-2012.02 | 235 | 225 | NA | NA | 149/86 | 120/105 |
| Einsele et al.[14] | Germany | 2006.10-2013.10 | 186 | 185 | 59 (35-73) | 59 (36-76) | 112/74 | 118/67 |
| Goldschmidt et al.[15] | Germany | 2005.07-2008.07 | 413 | 414 | 57 (31-65) | 57 (25-65) | 253/160 | 247/167 |
| Horvath et al.[16] | Australia | 2012.01-2016.01 | 103 | 100 | 58 (34-71) | 58 (32-71) | 53/50 | 57/43 |
| Mellqvist et al.[17] | Sweden | 2005.10-2009.04 | 187 | 183 | 59.1±9.90 | 58.7±8.80 | 111/76 | 109/74 |
| Palumbo et al.[18] | Italy | 2006.05-2009.01 | 254 | 257 | 71 (68-75) | 71 (68-76) | 130/124 | 122/135 |
| Rosinol et al.[19] | Spain | 2006.04-2009.08 | 91 | 88 | 56 | 59 | 54/37 | 51/37 |
| Sezer et al.[20] | Germany | 2009.07-2012.05 | 51 | 53 | 58 (27-75) | 57 (35-73) | 33/25 | 31/22 |
| Stadtmauer et al.[21] | USA | 2010.06-2013.11 | 254 | 257 | 57 (20-70) | 56 (30-70) | 146/108 | 161/96 |
Note: (OG): Observation Group and (CG): Control Group
Table 1: General characteristics of the inclusion study
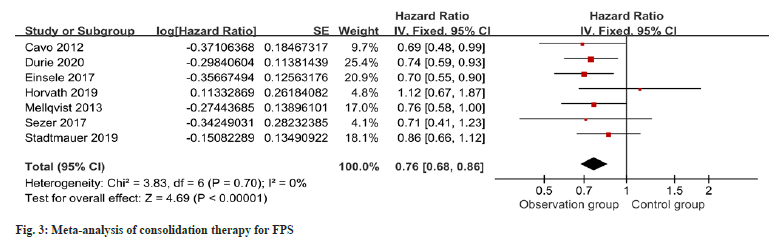
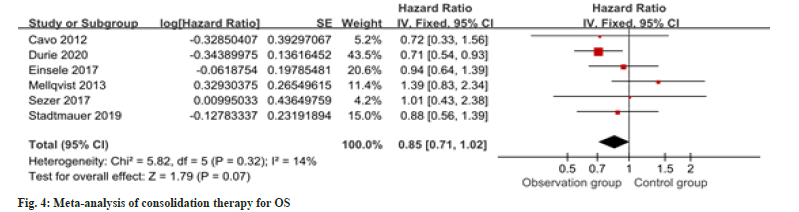
Seven studies provided information regarding PFS in the context of consolidation therapy. The results revealed a notable enhancement in PFS among the intervention group as opposed to the control group as shown in fig. 3. For OS in consolidation therapy, a meta-analysis of six studies revealed no remarkable difference between groups as shown in fig. 4.
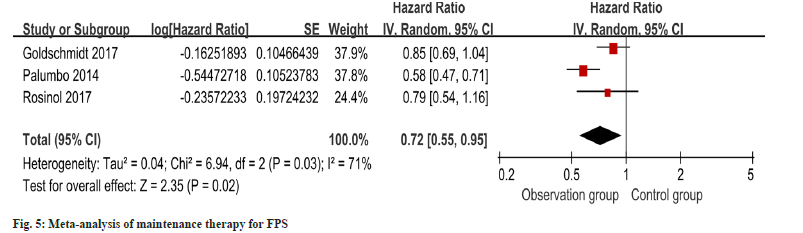
PFS outcomes for maintenance therapy patients were reported in three studies, and heterogeneity was identified (I2=71 %, p=0.03). Employing a random-effects model, the meta-analysis exhibited a notable enhancement in PFS within the intervention group as opposed to the control group as shown in fig. 5.
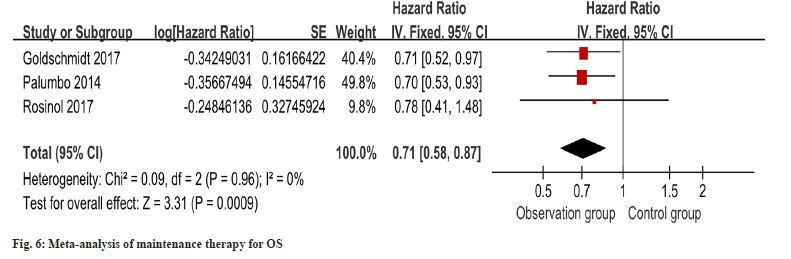
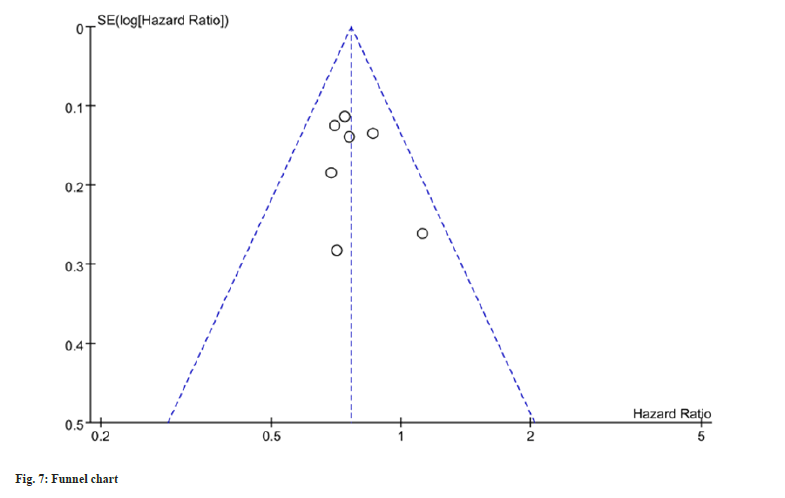
OS outcomes for maintenance therapy patients were reported in three studies, and minimal heterogeneity was detected (I2=0 %). The metaanalysis, utilizing a fixed-effects model, exhibited a notable improvement in OS within the intervention group as opposed to the control group as shown in fig. 6. In order to detect publication bias, the implementation of funnel plots was undertaken, revealing no notable indications or evidence indicating the presence of such bias as shown in fig. 7.
An unmet need persists in MM, as maintaining remission after induction therapy continues to pose challenges, despite the introduction of various novel therapeutics and amalgamation protocols in recent times[22]. The fundamental objective underlying consolidation and maintenance therapeutic approaches is to prolong the period of remission and optimize OS within this patient cohort. Within our meta-analysis, it became evident that the implementation of bortezomibbased maintenance therapy following induction therapy, irrespective of autologous stem cell transplantation, leads to a notable extension in both PFS and OS for MM individuals. Through the compilation of data from multiple studies, our research provides an elevated level of evidentiary support concerning the utilization of bortezomib in consolidation and maintenance therapy for MM, even though dissenting findings remain scarce within the majority of these studies.
Individuals with MM, particularly those who undergo autologous stem cell transplantation, receive short-term chemotherapy as part of consolidation therapy following the initial treatment[23]. By conducting a meta-analysis, we juxtaposed the influence of consolidation regimens based on bortezomib against regimens that do not involve bortezomib. The outcomes derived from this analysis unveiled a notable postponement in the progression of the disease linked to consolidation therapy incorporating bortezomib. Nevertheless, the findings derived from published RCTs examining the efficacy of bortezomib-based consolidation regimens on OS have demonstrated incongruous outcomes. In spite of the absence of statistical significance, an evident tendency towards improved OS is discernible in relation to the utilization of regimens incorporating bortezomib. Within the scope of maintenance therapy, individuals with MM who have received autologous stem cell transplantation are subjected to the ongoing administration of lowdose chemotherapy over an extended duration[24]. As a standard practice, maintenance therapy is administered for duration of 2 y-3 y, terminating upon disease progression, relapse, or the emergence of intolerable toxicity. Through the undertaking of three RCTs, the efficacy of maintenance therapy involving bortezomib in MM was investigated. Based on our meta-analysis findings, maintenance therapy utilizing regimens containing bortezomib demonstrates potential in enhancing OS. The precise explanation for the discrepancy in OS outcomes between bortezomib-based regimens in the maintenance and consolidation settings is still not fully comprehended. One could posit the hypothesis that sustained inhibition of myeloma cells through the implementation of regimens containing bortezomib is necessary to achieve a favorable effect on OS. Conversely, greater utilization of bortezomib may potentially result in an elevated prevalence of adverse reactions. In view of the absence of discernible OS benefits attributable to consolidation therapy, the unresolved query revolves around the precedence determination between bortezomib-based maintenance therapy and bortezomib-based consolidation therapy in the context of high-risk individuals. In the realm of clinical practice, bortezomib-based maintenance therapy is frequently administered for a minimum of 2 y or until disease advancement, taking into account factors such as toxicity, tolerability, and cost when determining the treatment duration. Further research is warranted to investigate the issues of consolidation and maintenance in MM.
Consolidation and maintenance approach for MM have seen extensive research focused on the utilization of immunomodulatory drugs. The Food and Drug Administration (FDA) has approved lenalidomide as the sole drug for posttransplant maintenance therapy, leading to its widespread use in this context. The investigation undertaken by McCarthy et al.[25] unveiled that the implementation of lenalidomide as postautologous stem cell transplantation maintenance therapy yielded enhanced PFS and OS outcomes. However, the utilization of lenalidomide is impeded by adverse events, encompassing the development of secondary primary neoplasms, diminished effectiveness in high-risk pathology, and significant financial burdens on afflicted individuals. In reality, the high cost of oral lenalidomide as maintenance therapy leads some patients to decide against its use and instead opt for non-oral alternatives. The findings of this study emphasize the potency of maintenance therapy utilizing bortezomib in bolstering both PFS and OS. Additionally, the distinct advantage of bortezomib resides in its capability to be administered in patients with renal impairment without the need for dosage modification, thereby establishing it as a feasible alternative option for specific patient cohorts.
Despite certain studies proposing adverse prognostic implications associated with bortezomib[26], a significant study conducted by Avet-Loiseau et al.[27] involving 507 patients did not observe such implications. Our endeavor to carry out a subgroup analysis to assess high-risk individuals could not be realized due to inadequate data from the published studies, thereby impeding a comprehensive meta-analysis. Nevertheless, to substantiate these recommendations, it is imperative to obtain high-quality evidence, and additional research endeavors may uncover the patient subgroups that can achieve the most significant advantages from consolidation and maintenance therapy, whether it is bortezomibbased or lenalidomide-based.
Our study is subject to various limitations. Firstly, the availability of clinical trials, especially those focusing on maintenance therapy and nontransplant settings, is relatively scarce. Secondly, the meta-analysis was performed utilizing data extracted from published RCTs, employing a group-level analysis rather than relying on individual patient-level data. Therefore, subgroup analysis for high-risk patients was not possible. Thirdly, there was significant heterogeneity among the RCTs in terms of patient populations, study designs, induction therapy regimens, bortezomib dosages, timing schedules, and other factors. Hence, further clinical trials are indispensable to substantiate our findings in this area.
Funding:
This work was supported by the Youth Scientific Research Foundation of Guangxi Natural Science Foundation of China (No. 2020GXNSFBA297061).
Conflict of interests:
The authors declared no conflict of interests.
References
- Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin oncol 2016;43(6):676-81.
[Crossref] [Google Scholar] [PubMed]
- Kumar SK, Rajkumar V, Kyle RA. Multiple myeloma. Nat Rev Dis Primers 2017;3:17046.
- Lipe B, Vukas R, Mikhael J. The role of maintenance therapy in multiple myeloma. Blood Cancer J 2016;6(10):e485.
- McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med 2012;366(19):1770-81.
[Crossref] [Google Scholar] [PubMed]
- Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med 2012;366(19):1782-91.
[Crossref] [Google Scholar] [PubMed]
- Richardson PG, Hideshima T, Anderson KC. Bortezomib (PS-341): A novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers. Cancer Control 2003;10(5):361-9.
[Crossref] [Google Scholar] [PubMed]
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008;359(9):906-17.
[Crossref] [Google Scholar] [PubMed]
- Durie BG, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone vs. lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017;389(10068):519-27.
[Crossref] [Google Scholar] [PubMed]
- Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 2017;376(14):1311-20.
[Crossref] [Google Scholar] [PubMed]
- Kumar SK, Mikhael JR, Buadi FK, Dingli D, Dispenzieri A, Fonseca R, et al. Management of newly diagnosed symptomatic multiple myeloma: Updated mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc 2009;84(12):1095-110.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021;372:n71.
[Crossref] [Google Scholar] [PubMed]
- Cavo M, Pantani L, Petrucci MT, Patriarca F, Zamagni E, Donnarumma D, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood J Am Soc Hematol 2012;120(1):9-19.
[Crossref] [Google Scholar] [PubMed]
- Durie BG, Hoering A, Sexton R, Abidi MH, Epstein J, Rajkumar SV et al. Longer term follow-up of the randomized phase III trial SWOG S0777: Bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J 2020;10(5):53.
[Crossref] [Google Scholar] [PubMed]
- Einsele H, Knop S, Vogel M, Müller J, Kropff M, Metzner B, et al. Response-adapted consolidation with bortezomib after ASCT improves progression-free survival in newly diagnosed multiple myeloma. Leukemia 2017;31(6):1463-6.
[Crossref] [Google Scholar] [PubMed]
- Goldschmidt H, Lokhorst HM, Mai EK, vander Holt B, Blau IW, Zweegman S, et al. Bortezomib before and after high-dose therapy in myeloma: Long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia 2018;32(2):383-90.
[Crossref] [Google Scholar] [PubMed]
- Horvath N, Spencer A, Kenealy M, Joshua D, Campbell PJ, Lee JJ, et al. Phase 3 study of subcutaneous bortezomib, thalidomide, and prednisolone consolidation after subcutaneous bortezomib-based induction and autologous stem cell transplantation in patients with previously untreated multiple myeloma: The VCAT study. Leuk Lymphoma 2019;60(9):2122-33.
[Crossref] [Google Scholar] [PubMed]
- Mellqvist UH, Gimsing P, Hjertner O, Lenhoff S, Laane E, Remes K, et al. Bortezomib consolidation after autologous stem cell transplantation in multiple myeloma: A Nordic myeloma study group randomized phase 3 trial. Blood J Am Soc Hematol 2013;121(23):4647-54.
[Crossref] [Google Scholar] [PubMed]
- Palumbo A, Bringhen S, Larocca A, Rossi D, di Raimondo F, Magarotto V, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: Updated follow-up and improved survival. J Clin Oncol 2014;32(7):634-40.
[Crossref] [Google Scholar] [PubMed]
- Rosiñol L, Oriol A, Teruel AI, de La Guía AL, Blanchard M, de La Rubia J, et al. Bortezomib and thalidomide maintenance after stem cell transplantation for multiple myeloma: A PETHEMA/GEM trial. Leukemia 2017;31(9):1922-7.
[Crossref] [Google Scholar] [PubMed]
- Sezer O, Beksac M, Hajek R, Sucak G, Cagirgan S, Linkesch W, et al. Effects of single-agent bortezomib as post-transplant consolidation therapy on multiple myeloma-related bone disease: A randomized phase II study. Br J Haematol 2017;178(1):61-71.
[Crossref] [Google Scholar] [PubMed]
- Stadtmauer EA, Pasquini MC, Blackwell B, Hari P, Bashey A, Devine S et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: Results of the BMT CTN 0702 trial. J Clin Oncol 2019;37(7):589.
[Crossref] [Google Scholar] [PubMed]
- Ji J, Zhao W. Progress in immunotherapy of multiple myeloma. J Clin Hematol 2023;36(9):680-7.
- Sun X. Clinical research progress in diagnosis and treatment of multiple myeloma. Electron J Clin Med Literat 2020;7(18):193-4.
- Lin Y. Observation on the efficacy of maintenance therapy after autologous hematopoietic stem cell transplantation for multiple myeloma. Fujian Med Univ 2021.
- McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: A meta-analysis. J Clin Oncol 2017;35(29):3279.
[Crossref] [Google Scholar] [PubMed]
- Neben K, Lokhorst HM, Jauch A, Bertsch U, Hielscher T, vander Holt B, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood J Am Soc Hematol 2012;119(4):940-8.
[Crossref] [Google Scholar] [PubMed]
- Avet-Loiseau H, Leleu X, Roussel M, Moreau P, Guerin-Charbonnel C, Caillot D, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t (4; 14) myeloma but not outcome of patients with del (17p). J Clin Oncol 2010;28(30):4630-4.
[Crossref] [Google Scholar] [PubMed]