- *Corresponding Author:
- Hui Ma
Department of Neurosurgery, The General Hospital of Ningxia Medical University, Ningxia Key Laboratory of Cerebrocranial Diseases, Incubation Base of National Key Laboratory, Ningxia Medical University 804 Shengli Street, Yinchuan, Ningxia 750004, China
E-mail: meitun181@163.com - He Chun Xia
Department of Neurosurgery, The General Hospital of Ningxia Medical University, Ningxia Key Laboratory of Cerebrocranial Diseases, Incubation Base of National Key Laboratory, Ningxia Medical University 804 Shengli Street, Yinchuan, Ningxia 750004, China
E-mail: meitun181@163.com
| This article was originally published in a special issue, “Biomedical Research in Clinical and Preclinical Pharmaceutics” |
| Indian J Pharm Sci 2020:82(3)Spl issue7;59-66 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Glioma was one of the most common malignant brain tumors. The effect of routine clinical treatment of glioma was not ideal, and the molecular mechanism of the occurrence and development of the disease was still unclear. In this paper, the mechanism of MicroRNA-16-5p in the occurrence and development of glioma was studied by the cell experiment in vitro. Through the analysis of the The Cancer Genome Atlas database and clinical tissue samples, the expression of MicroRNA-16-5p in glioma was detected. The inhibitory effect of MicroRNA-16-5p on the proliferation, invasion, cell cycle and apoptosis of glioma cells was evaluated by the cell proliferation, invasion and flow cytometry. The relationship between the expression of p53, Bcl-2 Associated X-protein and MicroRNA-16-5p was studied by dual-luciferase gene assay, Quantitative Reverse transcription- polymerase chain reaction, and Western blotting. Compared with the normal tissues, the expression of MicroRNA-16-5precursor was low in glioma tissues, which was consistent with that in the Uppsala87 and Uppsala251 cell lines. The over expression of MicroRNA-16-5p can inhibit the growth and invasion of the glioma cell line, promote the apoptosis and delay the cell cycle. The results of Quantitative Reverse transcription- polymerase chain reaction and Western blotting showed that the target gene of MicroRNA-16-5p was p53, and the over expression of MicroRNA-16-5p could promote the expression of p53 and Bcl-2 Associated X-protein, which was positively correlated. MicroRNA-16-5p was a tumor suppressor in the progression of glioma. We have demonstrated the MicroRNA-873-5p-p53/ Bcl-2 Associated X-protein regulatory pathway in the development of glioma, which provides a theoretical basis for the development of the alternative strategies for the clinical treatment of glioma.
Keywords
Alzheimer’s disease, extended nursing, behavioral and psychological symptoms, cognitive function, ability of daily life activities
Glioma (GM) was the most common primary brain tumor. In the past 40 y, GM has the highest mortality among all brain malignant tumors [1]. There were about 20 000 new cases of GM in the United States every y [2]. Despite surgery, chemotherapy and/or radiotherapy, the median survival time for the patients with GM was only 12 to 14 mo [3]. Therefore, it is necessary to explore the mechanism of the development and progress of GM and to find a more effective treatment.
MicroRNA was a non-coding small molecule RNA with a length of 22-25 nt, which exists in eukaryotes. A large number of studies have shown that the miRNA can regulate not only the biological effects such as proliferation, apoptosis, senescence and differentiation of GM cells [4-7], but also autophagy and drug resistance of GM [8,9]. Therefore, it has been proposed as a new target for the anticancer therapy in recent y [7,8]. More than 50 % of the miRNA was known to be involved in the human tumorigenesis by directly targeting the oncogenes or tumor suppressor genes [9]. Recent studies have shown that the miR-16 can inhibit the proliferation, migration, invasion and other tumor suppressor genes of GM cells, which may be related to the low expression of Sal-like protein 4 (SALL4), zyxin, Cyclin D1 (CCND1), Cyclin E1 (CCNE1), B lymphoma Mo-MLV insertion region 1 homolog (BMI1), Wip1 and other signal proteins [8-12]. Therefore, miR-16 can be used as a potential target for the treatment of GM.
p53 [13] is the tumor suppressor gene at present. Nearly 5000 studies have been published on the inhibitory role of p53 in cancer progression, including its significance in the GM mechanism. p53 was initially mistaken for p53 oncogene [14] because of the high expression of its mutant Complementary DNA (cDNA) in various cancers. In fact, the inhibition of p53 can activate the occurrence of cancer, which has a significant effect on the cancer cell cycle and apoptosis [15,16]. Cyclindependent kinase inhibitor p21 plays an important role in regulating cell cycle progression, and the apoptosis promoter Bcl-2 Associated X-protein (BAX), which was essential for apoptosis, was also induced by p53 [17].
In this study, the clinical samples were used to determine the abnormal expression of miRNA-16-5p in GM, and the database was used to analyze and predict the GM-related target genes that miRNA-16-5p may regulate, and to identify the binding sites between GM- 5p and target genes. Then Uppsala87 and Uppsala251 cell models were used for further comprehensive and in-depth analysis of the molecular mechanism of miRNA-16-5p in the treatment of GM, to provide a valuable reference for the same kind of research and development of miRNAs as the target for the treatment of GM.
Materials and Methods
Tissue slice:
A total of 30 human tissue samples were collected from the pathology department of our hospital, including 10 normal brain samples and 20 GM samples. The samples were immediately frozen in the liquid nitrogen for the following Ribonucleic acid (RNA) analysis, or fixed in the formaldehyde solution for hematoxylineosin staining (H&E staining). Liquid nitrogen as a part of cryopreservation is widely used for banking cells and tissues which suggests Liquid nitrogen is more advantageous in freezing the tissue samples. The patient understands the study and signs the informed consent form. H&E staining was carried out according to the above-mentioned scheme.
Cell lines and cell cultures:
Normal human brain astrocytes (HA) and human brain U87 and U251 cell lines were purchased from China typical Culture Preservation Center (Wuhan). Each cell line was divided into two groups: the experimental group with miR-16-5p agonist (miR-16-5p group) and the negative control group (NC group). Each group of cells was cultured in the Dulbecco’s Modified Eagle Medium (DMEM) medium supplemented with 10 % fetal bovine serum and 100x penicillin/streptomycin (37º, 5 % CO2).
Cell transfection and cloning:
Cells in the good growth condition (1×105) were selected for transfection. The miR-16-5p simulator and negative control simulator were purchased from Gene Pharma Co., Ltd. (Shanghai). According to the operating instructions of the manufacturer, the miR-16-5p simulants and the negative control simulants with the concentration of 62.5 nmol/l were transferred into the cells by Lipofectamine2000TM (Invitrogen) and cultured for 48 h. The sequence of the simulants were as follows, miR-16-5p stimulants (GCAGGAACUUGUGAGUCUCCU), anti-miR-16- 5p stimulants (AGGAGACTCTGTGTTCCTGC). The cells of each group were treated with trypsin and centrifuged to make single cell suspension, and the cells were counted, and the mean value of three times was taken as the result of cell count. The cells were diluted in proportion and inoculated in a 6-well plate according to 200 cells per well. The culture was terminated and stained after 10-12 d of culture until the visible clone ball appeared on the culture plate. Under the microscope, the number of clones of more than 50 cells was counted. Clone formation rate (%) = number of clones/the inoculated cells*100 %.
MTT detection:
MTT assay was used to evaluate the cell proliferation and viability. A total of 5mg MTT reagent (Cell Proliferation Kit I, USA) was dissolved in 1ml Phosphate-buffered saline (PBS) and filtered by a 0.22 μm filter membrane to prepare the reserve solution. The cells were cultured in 96-well plates for 48 h. Then 20 μl reserve solution (5 mg/ml, Sigma-Aldrich, USA) was added to each well and incubated for 4 h. After the supernatant was removed, 150 μl Dimethyl sulfoxide (DMSO) (Sigma-Aldrich) was added to the plate to dissolve the methanone crystals, and the absorbance of 490 nm wavelength was measured using an enzyme labeling instrument (Molecular Devices, California, USA).
Determination of transwell migration and invasion:
Transwell was used to detect the degree of cell migration and invasion. The matrigel coated (invasion assay) or matrigel uncoated (migration assay) membrane was placed in the upper chamber overnight at 37º. After cleavage with trypsin, a serum-free medium of about 1×105 cells was used to prepare the diluent. The cells were transferred to the upper chamber, and the medium supplemented with 5 mg/l fibronectin and 10 % Fetal bovine serum (FBS) was added to the lower chamber and cultured for 24 h (5 % CO2, 37º). Cells entering the lower chamber through the membrane were fixed with methanol for about 20 min, followed by staining with crystal violet (0.1 %) for 10 min. Finally, the cells were examined and counted by a light microscope (Nikon, Japan).
Cell Cycle:
Apoptosis was detected according to the instructions of FITC Annexin V apoptosis detection kit I. The specific steps were as follows, 1×106 cells were collected and re-suspended in 100 μl binding buffer. 5 μl FITCAnnexin V stain and 5 μl Propidium iodide (PI) stain were added to each test tube, and 400 μl binding buffer was added after incubation in the dark for 15 min. Then the apoptosis was evaluated by the flow cytometer within 1 h.
Luciferase reporter gene assays:
According to the manufacturer’s instructions, Phusion Site-Directed Mutagenesis Kit was used to induce the mutagenesis in p53 3’UTR. The wild type and mutant p53 3’UTR were inserted into the psiCHECK (Promega, Madison, WI, USA), and the recombinant psiCHECK TM-2 vector was cotransfected with miR-16-5p into GM cells. The overexpression of miR-16-5p, miR- 16-5p inhibitor or psiCHECK empty vector. Finally, the relative luciferase activity was determined by the luciferase assay reagent II at 48 h after transfection.
qRT-PCR analyis:
In this study, RNA was extracted by TRIzol reagent (Invitrogen, USA). According to the instructions of the reagent, the Reverse Transcription Kit (Takara Bio,Inc., Japan) was used to the reverse transcription of the equal portion of RNA into cDNA. The amplification of p53 was carried out on the Biorad Realtime PCR platform. The amplification conditions were as follows, pre-denaturing at 94º for 2 min, 30 cycles including denaturing at 94º for 30 sec, annealing at 56º for 30 sec, and extending at 72º for 1 min, and final extending at 72º for 10 min. GAPDH was used as the endogenous control. Primer sequences were as follows, p53 F: 5’-CCTCAGCATCTTATCCGAGTGG-3’, p53 R: 5’-CCTCAGCATCTTATCCGAGTGG-3’, BAX F: 5’-CAATTGGCCTTGCTACT-3’, BAX R: 5’-TCTTTCGCTGTGAGGTTGTG-3’, GAPDH F: 5’-GCA-ACT-AGG-ATG-GTG-TGG-CT-3’, GAPDH R: 5’-TCC-CAT-TCC-CCA-GCT-CTC-ATA-3’. The expression level of hsa-mir-127-5p was detected by Taq Man miRNA assay kit. TaqMan MicroRNA Reverse Transcription Kit (Taq Man miRNA RT kit) with miRNA specific RT primers was used to purify and reverse transcribe miRNA. The Reverse transcription polymerase chain reaction (RT-PCR) experiment was carried out on the Step One Plus real-time PCR platform. The reaction mix including 0.2 μm Taq Man probe, 2 μl RT products, 10 μm forward and reverse primers, and 5 μlTaq Man Universal PCR Master Mix.
Western blotting:
The total protein in the lysate buffer was determined by the radioimmunoprecipitation and separated by SDSPAGE (Bio-Rad, USA) method. Transfer the protein to the polyvinylidene fluoride (PVDF) membrane and blocked it with 5 % skim milk for 1 h. Then the membrane was incubated with the first antibody of p53 and BAX at 4º overnight. Next, after incubation with anti-APS IgG-HRP (BOSTER) secondary antibody, the immune complex was revealed by the enhanced chemiluminescence. The relative protein expression was determined by the optical density method using Image J software.
Statistical analysis:
All the experiments were repeated three times independently. Quantitative data were expressed as mean±SD, GraphPad Prism 8.0.1 (GraphPad Software, La Jolla, CA) was used for the comparing and evaluating data between groups. The differences between the two groups were analyzed by t-test. Kruskal-Wallis test was used for comparison among the three groups. #p<0.05 means the difference was considered significant.
Results and Discussion
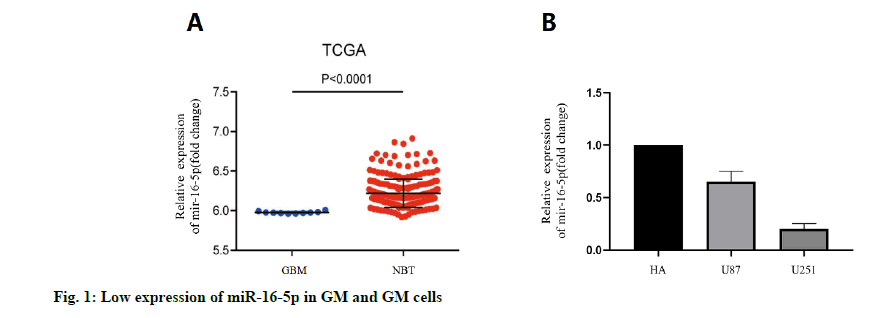
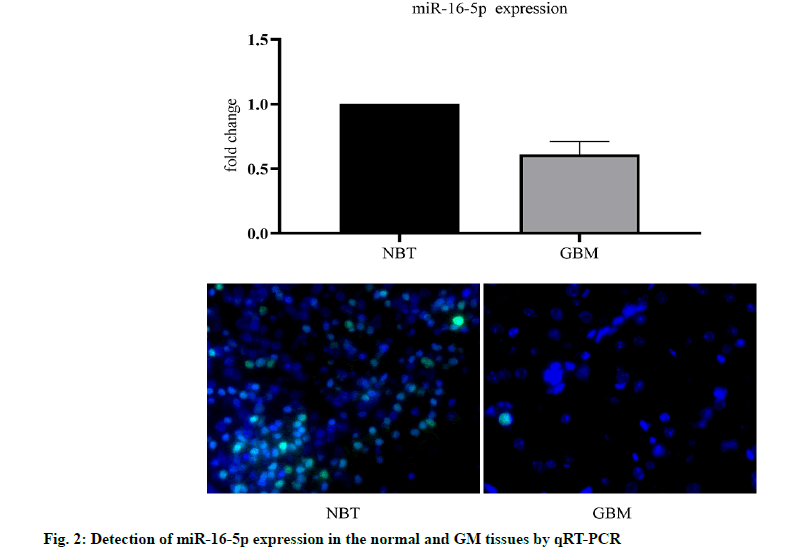
We analyzed the expression level of the miR-16- 3p in 255 non-cancerous brain GM specimens and 158 different grades of GM tissues from the TCGA public database. The results showed that the expression of miR-16-5p in GM tissues was lower than that in normal tissues (fig. 1A). In addition, the expression level of miR-16-5p in U87 and U251 cell lines was detected. The results showed that the expression of miR-16-5p in the GM cell line was lower than that in a normal brain cell lines (fig. 1B). The expression level of miR-16-5p in the collected tissue slices was analyzed. The results also showed that the miR-16-5p was significantly down-regulated in the GM tissues (fig. 2). These results suggest that the miR-16-5p was lowly expressed in the GM and may play an important role in its occurrence and development.
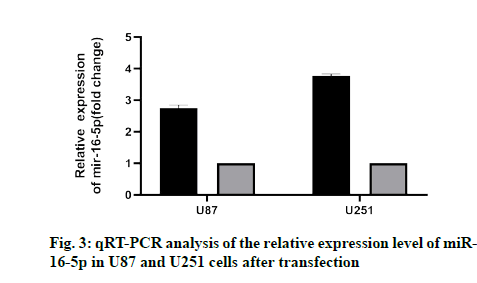
To study the effect of miR-16-5p on GM cells, U87 and U251 cells were transfected with the miR-16-5p simulator and miR-16-5p inhibitor simulator. The results showed that the miR-16-5p simulator significantly increased the expression of miR-16-5p (fig. 3), while miR-16-5p inhibitor decreased the expression of miR- 16-5p, which indicated that the transfection experiment of miR-16-5p was very successful.
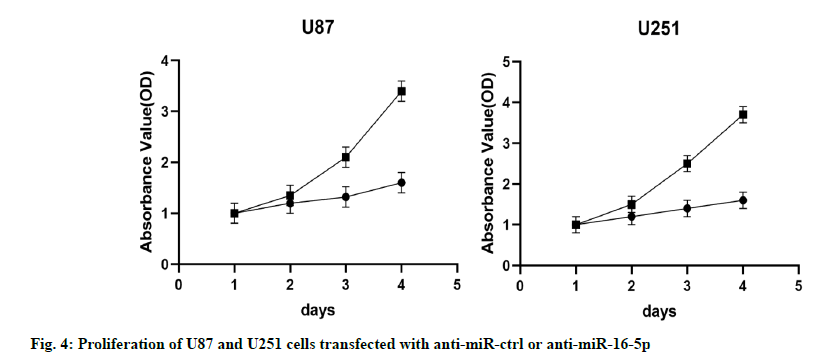
The results of MTT assay showed that compared with the control group (NC group), the proliferation ability of U87 and U251 cells in the miR-16-5p overexpression group decreased significantly, indicating that the overexpression of miR-16-5p inhibited the proliferation of U87 and U251 cells (fig. 4).
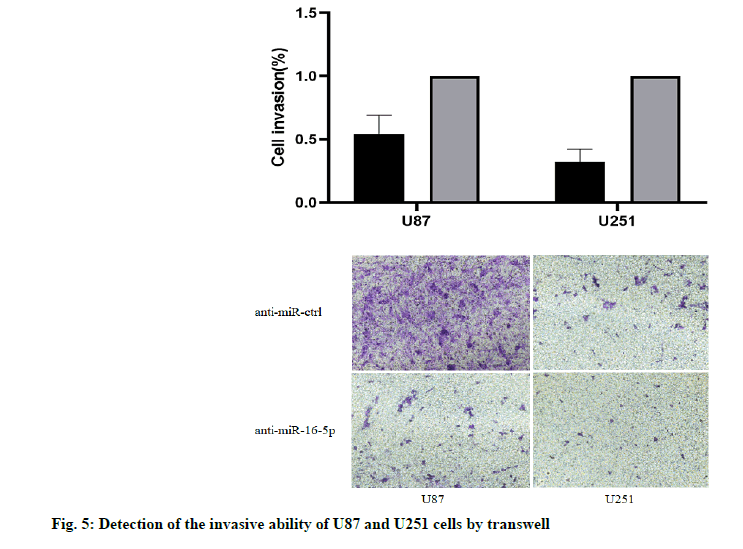
The results of Transwell chamber showed that, the number of U87 and U251 cells passing through the chamber decreased significantly when overexpressing miR-16-5p, and the difference was statistically significant (p<0.05). It was suggested that the overexpression of miR-16-5p can inhibit the invasive ability of U87 and U251 cells (fig. 5).
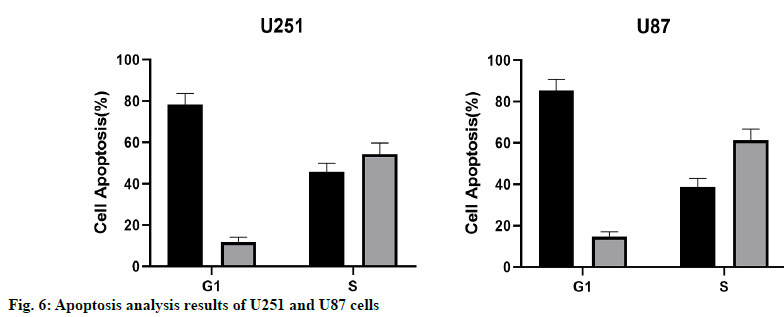
The results of apoptosis analysis showed that the early apoptosis of U87 and U251 cells overexpressing miR- 16-5p was significantly increased, and the difference was statistically significant (p<0.05). At the same time, the results of cell cycle showed that the number of U87 and U251 cells in G1 phase in the miR-16-5p group was significantly higher than that in the control group, while the cells number in S phase was significantly less than that in the control group, indicating that the overexpression of miR-16-5p could inhibit the cell cycle of U87 and U251 (fig. 6).
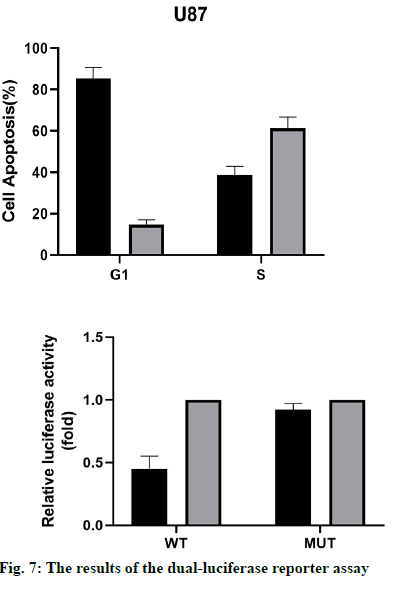
The results of the dual-luciferase reporter assay (fig. 7) showed that the activity of the wild-type p53 luciferase decreased significantly after overexpression of miR-16- 5p, but there was no significant difference in the activity of mutant p53. These results suggest that the miR-16- 5p can only bind to the wild-type p53 and inhibit the luciferase activity, while the mutant p53 gene cannot. Therefore, p53 was the target gene of miR-16-5p.
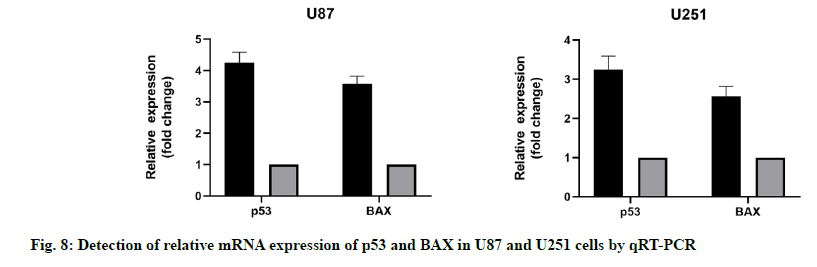
qRT-PCR results showed that the mRNA expression level of p53 and BAX in U87 and U251 cells increased significantly when miR-16-5p was overexpressed. Western blotting also revealed that the miR-16- 5p overexpression could significantly promote the expression of mRNA in U87 and U251 cells, compared with the control group, the difference was statistically significant (p<0.05). These results suggest that the overexpression of miR-16-5p can activate the p53/ BAX signal pathway and inhibit the growth of GM cells (fig. 8).
GM was a malignant tumor in the central nervous system with the highest fatality rate [18]. Although the great progress has been made in the clinical diagnosis and targeted therapy in recent y, the 5 y survival rate of patients was still at a low level. The reason for this outcome was not only that this kind of disease occurs in the human brain, but also related to the high invasive ability of the tumor. Therefore, it is necessary to study the biological characteristics and pathological progression mechanism of the tumor at the molecular level, to provide new ideas and methods for the prevention and clinical diagnosis and treatment.
MicroRNAs was a class of small regulatory RNA, that has been shown to activate or inhibit a variety of carcinogenic activities, such as the proliferation, cell cycle, and apoptosis [19]. Abnormal expression of miRNA has been observed in various tumors, including brain tumors, such as GM and its invasive GM subtypes [20]. Recent studies have shown that the miRNA was involved in the late stage of cancer progression and may be used as an activator or inhibitor of tumorigenesis [21]. MiR-16-5p was a member of the miRNA family and plays different roles in different tumors [22-24]. This shows that the miR-15-5p was specific in the tumor progression. At present, there were few reports on the role of miR-16-5p in GM and its mechanism, and its possible mechanism was not clear. Therefore, in this study, the effect of miR-16-5p on GM and its possible mechanism was studied in vitro cell experiments.
MiR-16-5p has been proved to be a tumor suppressor gene in many tumors. Some studies have found that the miR-16 can target and regulate NF-KB1, MMP-9, and Bcl-2, to inhibit the occurrence and development of GM [25]. Some studies have shown that the miR-16-5p can inhibit the epithelial mesenchymal transition of the transcription factor AP4, thus weakening the invasion and invasion of GM. It was also confirmed that the miR- 16-5p could inhibit the growth of GM. Through the analysis of the pathological tissues and normal tissues in The Cancer Genome Atlas (TGCA) database, it was found that the expression of the miR-16-5p was low in GM, which was verified in the collected pathological tissues and cell lines respectively. In order to explore the mechanism of miR-16-5p inhibiting the growth of GM, the common GM cell lines U87 and U251 were transfected, and the miR-16-5p overexpression group and control group were set up. Then the characteristics of cell function and biological behavior were tested, including cell proliferation, cell invasion, flow cytometry and apoptosis. The results showed that the overexpression of miR-16-5p could inhibit the cell proliferation and invasion significantly, promote apoptosis and delay cell cycle.
Tumor suppressor gene p53 was a key factor in the development of cancer. When DNA damage occurs, p53 [26,27] was induced by different upstream signals and then participates in the regulation of cell cycle arrest, DNA repair, and apoptosis-related pathways. In GM, p53 has also been shown to inhibit the occurrence and progression of tumors [28,29]. p53 was known to activate a variety of effects, including p21 and BAX, and inhibit the cancer cell growth and tumorigenesis [29,30]. Our results showed that the miR-141-3p participates in the tumor inhibition through a variety of mechanisms, including the inhibition of tumor cell growth, promotion of apoptosis, and induction of cell cycle arrest. We have proved that U87 and U251 cells were wild-type p53 cell lines, so the overexpression of miR-16-5p increases the expression of p53 protein and significantly inhibits the growth of U87 and U251 cells. At the same time, the overexpression of mi-16-5p can increase the level of apoptotic protein BAX in U87 and U251 significantly, which can promote the apoptotic process of GM cells, thus blocking the occurrence and development of GM. Therefore, we believe that the miR-16-5p can activate p53/BAX signal pathway to inhibit the growth of GM effectively.
In summary, all the above data show that the miR-16- 5p was a tumor suppressor in the development of GM, and there was a miR-873-5p-p53/BAX regulatory axis in the development of GM, which provides a theoretical basis for the development of alternative strategies for the clinical treatment of GM.
Acknowledgements
None
Conflict of Interests:
The authors declared no conflict of interest.
References
- Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol 2015;17:iv1-62.
- Sakr M, Takino T, Sabit H, Nakada M, Li Z, Sato H. miR-150-5p and miR-133a suppress glioma cell proliferation and migration through targeting membrane-type-1 matrix metalloproteinase. Gene 2016;587:155-62.
- Chen PH, Cheng CH, Shih CM, Ho KH, Lin CW, Lee CC, et al. The Inhibition of microRNA-128 on IGF-1- activating mTOR signaling involves in Temozolomide induced glioma cell apoptotic death. PloS one 2016;11:e0167096.
- Zhou Y, Peng Y, Liu M, Jiang Y. MicroRNA-181b inhibits cellular proliferation and invasion of glioma cells via targeting Sal-like protein 4. Oncol Res 2017;25:947-57.
- Qi Z, Cai S, Cai J, Chen L, Yao Y, Mao Y et al. miR-491 regulates glioma cells proliferation by targeting TRIM28 in vitro. BMC Neurol 2016;16:248.
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215-33.
- Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 2005;65:6029-33.
- Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene 2007;26:2799-803.
- Wang K, Wang X, Zou J, Zhang A, Wan Y, Pu P, et al. miR-92b controls glioma proliferation and invasion through regulating Wnt/beta-catenin signaling via Nemo-like kinase. Neuro Oncol 2013;15:578-88.
- Stegh AH. Targeting the p53 signaling pathway in cancer therapy - the promises, challenges and perils. Expert Opin Ther Targets 2012;16:67-83.
- Baker SJ, Fearon ER, Nigro JM, Preisinger AC, Jessup JM, Ledbetter DH, et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science 1989;244:217-21.
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000;408:307-10.
- Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer 2009;9:749-58.
- Read ML, Seed RI, Modasia B, Kwan PP, Sharma N, Smith VE, et al. The proto-oncogene PBF binds p53 and is associated with prognostic features in colorectal cancer. Mol Carcinog 2016;55:15-26.
- Zhu X, Wang K, Zhang K, Zhang T, Yin Y, Xu F. Ziyuglycoside I inhibits the proliferation of MDA-MB-231 breast carcinoma cells through inducing p53-mediated G2/M cell cycle arrest and intrinsic/extrinsic apoptosis. Int J Mol Sci 2016;17:1903.
- Chen X, Tai L, Gao J, Qian J, Zhang M, Li B, et al. A stapled peptide antagonist of MDM2 carried by polymeric micelles sensitizes glioblastoma to temozolomide treatment through p53 activation. J Control Release 2015;218:29-35.
- Zhang J, Song Y, Zhang C, Zhi X, Fu H, Ma Y, et al. Circulating MiR-16-5p and MiR-19b-3p as two novel potential biomarkers to indicate progression of gastric cancer. Theranostics 2015;5:733-45.
- Rahman M, Lovat F, Romano G, Calore F, Acunzo M, Bell EH, et al. miR-15b/16-2 regulates factors that promote p53 phosphorylation and augments the DNA damage response following radiation in the lung. J Biol Chem 2014;289:26406-16.
- Cai B, Ma M, Chen B, Li Z, Abdalla BA, Nie Q, et al. MiR-16-5p targets SESN1 to regulate the p53 signaling pathway, affecting myoblast proliferation and apoptosis, and is involved in myoblast differentiation. Cell Death Dis 2018;9:1-5.
- Zhan XH, Xu QY, Tian R, Yan H, Zhang M, Wu J, et al. MicroRNA16 regulates glioma cell proliferation, apoptosis and invasion by targeting Wip1-ATM-p53 feedback loop. Oncotarget 2017;8:54788-98.
- Kyritsis AP, Bondy ML, Hess KR, Cunningham JE, Zhu D, Amos CJ, et al. Prognostic significance of p53immunoreactivity in patients with glioma. clinical cancer research 1995;1:1617-22.
- Luan S, Sun L, Huang F. MicroRNA-34a: a novel tumor suppressor in p53-mutant glioma cell line U251. Arch Med Res 2010;41:67-74.
- Sun G, Wang Y, Zhang J, Lin N, You Y. MiR-15b/HOTAIR/p53 form a regulatory loop that affects the growth of glioma cells. J Cell Biochem 2018;119:4540-7.
- Zhou X, Wu W, Zeng A, Nie E, Jin X, Yu T, et al. MicroRNA-141-3p promotes glioma cell growth and temozolomide resistance by directly targeting p53. Oncotarget 2017;8:71080-94.
- Wang Y, Yang J, Zheng H, Tomasek GJ, Zhang P, McKeever PE, et al. Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer cell 2009;15:514-26.
- Liu E, Wu J, Cao W, Zhang J, Liu W, Jiang X, et al. Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J Neurooncol 2007;85:263-70.
- Kyritsis AP, Xu R, Bondy ML, Levin VA, Bruner JM. Correlation of p53 immunoreactivity and sequencing in patients with glioma. Mol Carcinog 1996;15:1-4.
- Wang T, Li X, Sun S L. EX527, a Sirt-1 inhibitor, induces apoptosis in glioma via activating the p53 signaling pathway. Anti Cancer Drugs 2020;31:19-26.
- Zhang Y, Jiang X, Wu Z, Hu D, Jia J, Guo J, et al. Long Noncoding RNA LINC00467 Promotes glioma Progression through Inhibiting p53 Expression via Binding to DNMT1. J Cancer 2020;11:2935-44.
- Liang D, Song Y, Fan G, Ji D, Zhang T, Nie E, et al. Effects of long form of CAPON overexpression on glioma cell proliferation are dependent on AKT/mTOR/P53 signaling. Int J Med Sci 2019;16:614-22.