- *Corresponding Author:
- S. S. Shidhaye
Bharati Vidyapeeth’s College of Pharmacy, Sector 8, C.B.D., Belapur, Navi Mumbai - 400 614, India
E-mail: supriya.shidhaye@rediffmail.com
| Date of Published | 27 October, 2007 |
| Indian J Pharm Sci, 2007, 69 (5): 729-731 |
Abstract
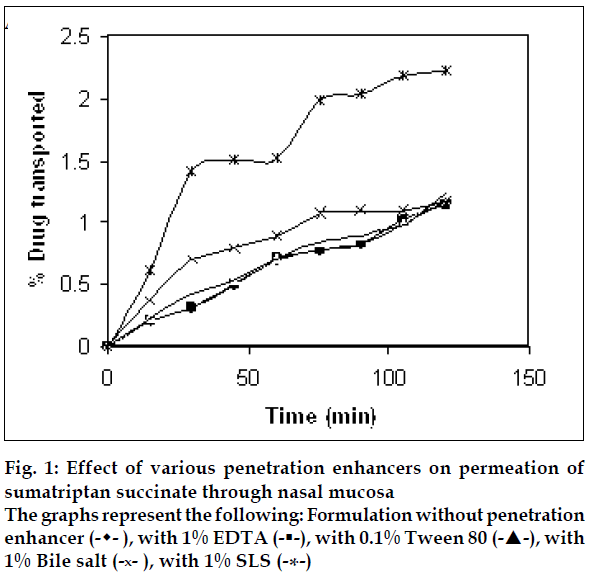
Sumatriptan succinate is a 5-HT1 receptor agonist used in the treatment of migraine. It is administered orally, in doses of 25, 50 or 100 mg as a single dose, nasally in doses of 10 or 20 mg and also subcutaneously, as two 6 mg doses within twenty four hours. Low oral bioavailability (15%) due to high fi rst-pass metabolism [1,2] justifi es a need of nasal drug delivery. To improve the nasal retention time of sumatriptan succinate it has been formulated as in situ mucoadhesive gel [3]. The objective of this work was to improve the nasal bioavailability of sumatriptan succinate by increasing its nasal retention time as well as the nasal permeation. Nasal permeation of sumatriptan succinate was improved by using different penetration enhancers such as SLS, Tween 80, bile salts and EDTA.
| Formulations containing | Permeation coefficient* mg. cm/min | Flux* mg/cm2 min | Enhancement ratio |
|---|---|---|---|
| No penetration enhancer | 0.002652±0.000276 | 0.008593±0.001129 | |
| 1% EDTA | 0.00273±0.000213 | 0.008868±0.000916 | 1.029 |
| 0.1% Tween 80 | 0.002881±0.000129 | 0.009326±0.000628 | 1.055 |
| 1% Bile salts | 0.003114±0.000106 | 0.010078±0.000584 | 1.08 |
| 1% SLS | 0.005348±0.000781 | 0.017303±0.000647 | 2.717 |
| *Each value represents mean ±S.D |
Table 1: Results of Permeation Studies of Sumatriptan Succinate Through Sheep Nasal Mucosa
Materials and Methods
HPLC method development for analysis of sumatriptan succinate for nasal formulation
The evaluation of permeation studies was carried out by HPLC method using phosphate buffer (pH 3): methanol (80:20) as mobile phase using a reverse phase column.
Preparation of mucoadhesive nasal gel of sumatriptan succinate [4] and evaluation
The gel containing drug, Pluronic F127 (18% w/v), 1% propylene glycol and Carbopol 934P (0.3% w/v) was prepared. To this solution, different penetration enhancers (EDTA 1% w/v, Tween 80 0.1% v/v, SLS 1% w/v and bile salt 1% w/v) were added separately to form different formulations. The formulation was studied for the gelation temperature [5] and mucoadhesive strength [6].
In vitro permeation studies
The nasal mucosa was separated from underlying muscular and part of the connective tissue with forceps and scissors. The fresh tissue was kept at 34o in phosphate buffered saline (PBS) pH 6.4 upon removal. Mucosa was used within 45 min of separation. Franz diffusion cell was used for this purpose. Receptor compartment contained 21 ml of pH 6.4 phosphate buffer while donor compartment was filled with 3 ml distilled water. The solution was placed in donor compartment and aliquots were removed at time intervals of 15, 30, 45, 60, 75, 90, 105, 120 min from the receptor compartment while the solution was being stirred continuously using magnetic stirrer, replacing it with fresh medium each time. The experiment was carried out at 34o. The amount of drug permeated was assayed using HPLC method of analysis. The graph of %drug permeated v/s time was plotted and flux, permeability coeffi cient and enhancement ratio was determined.
Results and Discussion
For HPLC analysis, the mixture of phosphate buffer pH 3 and methanol in three different proportions were tried, to resolve the peak of sumatriptan succinate from peaks of protein impurities. The mobile phase containing phosphate buffer pH 3: methanol (80:20) produced satisfactory result with good resolution of drug peak and showed good linearity in the range 5-80 µg/ml (y = 85244x; r2= 0.9986).
Clear transparent mucoadhesive solution was formed with viscosity sufficiently low to administer in the nasal cavity with the help of dropper. The viscosity of the formulation was increased as temperature of the solution increased with gelation at nasal cavity temperature (340). The mucoadhesive strength of the formulation was found to be 10.5 g, which is suffi cient to improve the retention time of solution at nasal mucosa penetrate though the nasal mucosa without permeation enhancer but low permeation was observed due to low Log P value of 0.93. When 1% EDTA and 0.1% Tween 80 was used in formulation these was no significant increase in the permeation of sumatriptan succinate when tested by student’s t-test while formulation containing 1% bile salts showed slight increase in the permeation. The permeation was found to increase two times with 1% SLS with enhancement ratio of 1.72.
It may be concluded that nasal mucosa delivery of sumatriptan succinate could be good alternative when formulated in the in situ mucoadhesive gel. This mucoadhesive gel showed satisfactory mucoadhesive strength and gelation temperature. It was found that use of penetration enhancer was required to achieve permeation through nasal mucosa and SLS was found to be the most effective among various penetration enhancers tried.
References
- Medline Drug Information, U.S. National Library of Medicine andNational Institutes of Health.
- Ryan R, Elkind A, Baker C, Mullican W, DeBussey S, Asgharnejad M. Sumatriptan nasal spray for the acute treatment of migraine: Results oftwo clinical studies. Neurology 1997;49:1225-30.
- Zhou M, Donovan MD. Intranasal mucociliary clearance of putative bioadhesive polymer gels. Int J Pharm 1996;135:115-25.
- Schmolka IR. Artificial skin: Preparation and properties of Pluronic F- 127 gels for the treatment of burns. J Biomed Mater Res 1972;6:571-82.
- Choi H, Oh Y, Kim C. In situ gelling and mucoadhesive liquid suppository containing acetaminophen: enhanced bioavailability. Int J Pharm 1998;165:23-32.
- Noha A, Ismail F. Mucoadhesive drug delivery system, formulation and in vitro evaluation of mucoadhesive tablets containing water soluble drugs. Drug DevInd Pharm 2004;30:995-1004.