- *Corresponding Author:
- Li Yan
Department of Orthopedics,
Wuhan Fourth Hospital,
Puai Hospital,
Tongji Medical College,
Huazhong University of Science and Technology,
Hanzheng,
Qiaokou,
Wuhan 430033,
China
E-mail: swyanli666@hotmail.com
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3) Spl Issue “144-151” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Neochamaejasmin A is an individual compound isolated from Stellera chamaejasme and exerts antitumor effects on several types of human cancer cells. However, the effect of neochamaejasmin A on human osteosarcoma cells has not yet been investigated. The main aim was to detect the in vitro antitumor effect of neochamaejasmin A on human osteosarcoma MG-63 cells. MG-63 cells were treated with different concentrations of neochamaejasmin A (20, 40, 80 μg/ml). Cell viability was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Cell apoptosis was detected through cell morphology observation and Annexin V/propidium iodide staining. Mitochondrial membrane potential was measured by rhodamine 123 staining. The expressions of apoptosis-related proteins and phosphatidylinositol 3 kinase/protein kinase B signaling proteins were analyzed by western blotting. The results showed that neochamaejasmin A significantly inhibited proliferation and induced apoptosis of MG- 63 cells. Neochamaejasmin A triggered the disorder of mitochondrial membrane potential, increased the expression of Bcl-2-associated X protein and cleaved-caspase 3, decreased expression of B-cell lymphoma 2, and induced apoptosis through the inactivation of phosphatidylinositol 3 kinase/protein kinase B/glycogen synthase kinase-3-beta signaling pathway. These new findings may indicate that neochamaejasmin A has potential to be chosen as a candidate for osteosarcoma chemotherapy.
Keywords
Neochamaejasmin A, osteosarcoma, apoptosis, mitochondria, phosphatidylinositol 3 kinase/protein kinase B pathway
Osteosarcoma is the primary malignant bone tumor, which mainly originates from the long bone of children and adolescents. The rapid growth, easy relapse and metastasis are the main characters of osteosarcoma. Although the treatment of osteosarcoma has been prominently improved with the development of surgical resection, adjunctive chemotherapy and radiotherapy, the mortality rate of patients with metastatic disease or relapse is still high[1,2]. Thus, it is a hotspot to seek novel therapeutic methods or new drugs that may be applied in the treatment of osteosarcoma.
Stellera chamaejasme Linn., known as Rui Xiang Lang Du in China, has been used as a traditional Chinese herb medicine for many years. This herb has been found to exhibit therapeutic effects in the treatment of skin ulcers, scabies, tinea and tuberculosis[3,4]. Moreover, the extractive compounds of Stellera chamaejasme have also been shown to possess antitumor activities in several types of human cancer cell lines, including human lung cancer cell lines (NCI-H157), epithelial, human breast cancer cell line (MDA-MB-231), osteosarcoma cell line (MG-63) and human immortalized myelogenous leukemia cell line (K562) cells[5-9]. Recent studies also reported that chamaejasmenin B and neochamaejasmin C (two active constituents of Stellera chamaejasme) could inhibit the in vitro proliferation of various types of human cancer cells, such as human liver cancer cell line (HepG2), human hepatocarcinoma cell line (SMMC-7721), human lung adenocarcinoma cell line (A549), human bone osteosarcoma epithelial cell line (U2OS), human bone osteosarcoma cell line (KHOS), human colorectal carcinoma cell line (HCT-116) and immortal cell line (HeLa) cells[10].
Neochamaejasmin A (NCA) is another new individual compound isolated from the dried root of Stellera chamaejasme. In one previous study, it was reported to exert antitumor effects on human prostate cancer, Lymph Node Carcinoma of the Prostate (LNCaP) cells. Exposure with NCA could block cell cycle progression at the Growth 1 (G1) phase by activating cyclindependent kinase inhibitor p21 and induce apoptosis through Fas Cell Surface Death Receptor (FAS)/ caspase dependent pathway[11]. Recently, Ding et al. reported that NCA could inhibit the proliferation of human hepatoma HepG2 cells and induce mitochondrialmediated apoptosis via Extracellular Signal-Regulated Kinase 1/2 (ERK1/2)/c-Jun N-terminal Kinase (JNK) signaling pathway[12]. However, the antitumor effect of NCA on human osteosarcoma cells has not yet been investigated.
In this study, we for the first time reported the antitumor activity of NCA against human osteosarcoma MG- 63 cells. The results indicated that NCA significantly inhibited proliferation and induced apoptosis of MG- 63 cells. NCA triggered the disorder of mitochondrial membrane potential, increased the expression of Bcl- 2-associated X protein (Bax) and cleaved-caspase 3, decreased expression of B-cell lymphoma 2 (Bcl-2), and induced apoptosis through the inactivation of Phosphatidylinositol 3 Kinase (PI3K)/Protein Kinase B (AKT)/Glycogen Synthase Kinase-3-beta (GSK-3β) signaling pathway.
Materials and Methods
Materials:
NCA with 98 % purity was purchased from Wuhan ChemFaces Biochemical Co., Ltd. (Wuhan, China) and dissolved in 0.05 % Dimethylsulfoxide (DMSO). Roswell Park Memorial Institute (RPMI) 1640 medium was obtained from HyClone. Fetal Bovine Serum (FBS) was obtained from Gibco. 3-(4,5-Dimethylthiazolyl-2)-2,5-Diphenyltetrazolium Bromide (MTT) and rhodamine 123 staining solution were from Sigma-Aldrich. Bicinchoninic Acid (BCA) protein assay kit and Radioimmunoprecipitation Assay (RIPA) lysis buffer were purchased from Beyotime Institute of Biotechnology (Suzhou, China). Annexin V/ Propidium Iodide (PI) apoptosis kit was from KeyGen Biotech Co., Ltd. (Nanjing, China). Anti-PI3K (# 4255, 1:1000), anti-AKT (# 9272, 1:1000), anti-phospho-AKT (# 4060, 1:500) and anti-phospho-GSK-3β (# 5558, 1:500) were obtained from cell signaling technology, Inc. (Danvers, Massachusetts (MA), United States of America (USA)). Anti-Bax (# BM3964, 1:1000), anti-Bcl-2 (# BM0200, 1:1000), anti-pro-caspase-3 (# BM4340, 1:500), anti-cleaved caspase-3 (# BA3592, 1:1000), beta (β)-action (# BM0627, 1:1000) and goat anti-rabbit/mouse secondary antibodies (# BA1056, 1:1000) were all purchased from Boster Biotechnology Co. Ltd. (Wuhan, China).
Cell culture:
Human osteosarcoma MG-63 cell line was provided by the cell bank of the Chinese academy of science (Shanghai, China). The cells were negative for bacteria, yeast, fungi and mycoplasma. MG-63 cells were cultured as our previous report[13]. Briefly, cells were incubated in complete medium containing RPMI 1640 medium supplemented with 10 % FBS, 100 units/ml penicillin and 100 μg/ml streptomycin. The environment of incubator was maintained at 37° with 5 % Carbon dioxide (CO2) and 95 % humidified air. All cell cultures were carried out by one single person strictly adhere to good and sterile practices.
Cell viability analysis:
Cell viability was detected by a MTT assay. MG- 63 cells were seeded on a 96-well plate at a density of 1×104 cells per well and incubated with complete medium for 24 h. Then the cells were treated with different concentrations of NCA (20, 40, 80 μg/ml) for 24, 48 and 72 h. The control group contained 0 μg/ml NCA where complete medium containing with 0.05 % DMSO. After that, the medium of every well was changed with 40 μl MTT solution (5 mg/ml). The plates were incubated for 4 h at 37° with 5 % CO2. The MTT solution was removed and 100 μl DMSO was added per well to dissolve the formazan product. The absorbance was read at 570 nm by a microplate reader (Bio-Rad 680, Hercules, California (CA), USA).
Cell morphology observation:
MG-63 cells were seeded on a 24-well plate at a density of 2×104 cells per well and incubated with complete medium for 24 h. Then the cells were treated with 0, 20, 40, 80 μg/ml NCA for 48 h. The cell morphology was observed using an inverted phase contrast microscope (Olympus CX23, Japan; magnification, 200×). The images were captured by a digital camera (Canon 600D, Japan).
Cell apoptosis assay:
The apoptosis ratio of MG-63 cells was detected by Annexin V/PI staining followed with a flow cytometry. MG-63 cells were treated with 0, 20, 40, 80 μg/ml NCA for 24 h. Then the cells were digested and resuspended in 1× binding buffer at a concentration of 1×106 cells/ ml. After that, the cells in 500 μl suspension were incubated with 5 μl Annexin V and 5 μl PI for 15 min. The apoptosis ratio was analyzed by a flow cytometry (BD FACScan, San Diego, CA, USA).
Mitochondrial membrane potential analysis:
The mitochondrial membrane potential was measured by rhodamine 123 staining. MG-63 cells were seeded on a 6-well plate at a density of 2×105 cells per well and incubated with complete medium for 24 h. Then the cells were treated with 0, 20, 40, 80 μg/ml NCA for 48 h. The cells were harvested and stained with rhodamine 123 solution (1 μg/ml) in dark at 37° for 20 min. After that, the cells were washed with Phosphate Buffered Saline (PBS) twice and centrifuged at 500 g for 10 min. The absorbance was read at 505 nm (excitation wavelength) and 534 nm (emission wavelength) respectively, using a spectrofluorometer (F-2500, Hitachi, Ltd., Japan).
Western blot analysis:
MG-63 cells were treated with 0, 20, 40, 80 μg/ml NCA for 24 h. Then, the cells were harvested and lysed using a RIPA lysis buffer on ice for 30 min. The supernatants were collected and the concentrations of extracted protein were measured by a BCA protein assay kit. Western blot assay was performed as our previous report[13]. The protein bands were examined using an electrochemiluminescence imaging system (ChemiScope Mini 3400, CLINX, Shanghai, China) and further quantified by Image J 1.46r software.
Statistical analysis:
All experimental data were expressed as mean±standard deviation. All detections were performed for three times. The data were analyzed by one-way Analysis of Variance (ANOVA) followed with Tukey’s post hoc test using GraphPad Prism 5 software, p<0.05 was considered to be statistically significant and different.
Results and Discussion
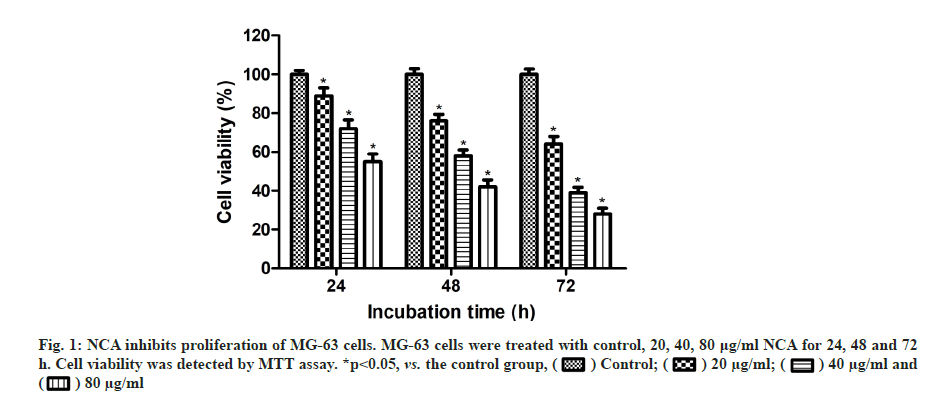
MTT assay was carried out to examine the antitumor effect of NCA on MG-63 cells. As shown in fig. 1, NCA could significantly decrease the viability of MG- 63 cells in a time and concentration-dependent manner (p<0.05). When the concentration of NCA reached 80 μg/ml, the viability was reduced to 55.4 %, 42.7 % and 28.8 % after treatment for 24, 48 and 72 h respectively, compared with the control group.
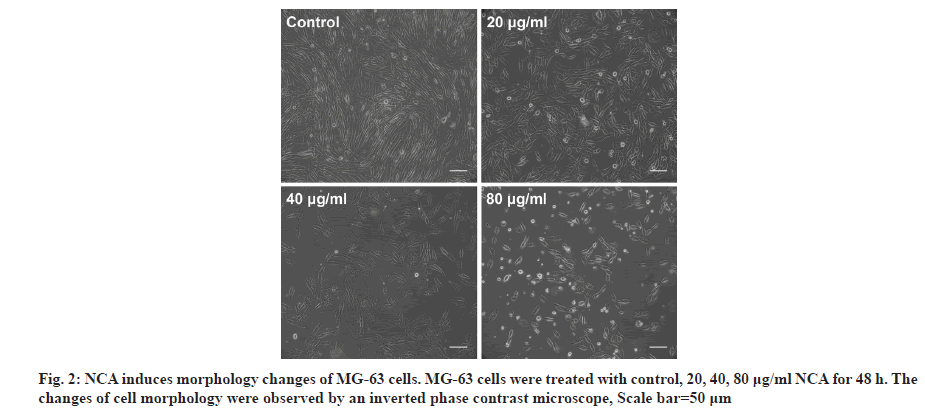
MG-63 cells were treated with different concentrations of NCA (0, 20, 40, 80 μg/ml) for 48 h and cell morphology observation was performed to detect whether the inhibited proliferation was due to apoptosis. As shown in fig. 2, treatment with NCA could decrease the total cell number and increase the floating cells. Moreover, cell morphology in NCAtreated group showed remarkable changes with cell shrinkage, chromatin condensation and apoptotic bodies formation. The changes were more obvious with increasing concentration of NCA. These results indicated that NCA might induce apoptotic cell death in MG-63 cells.
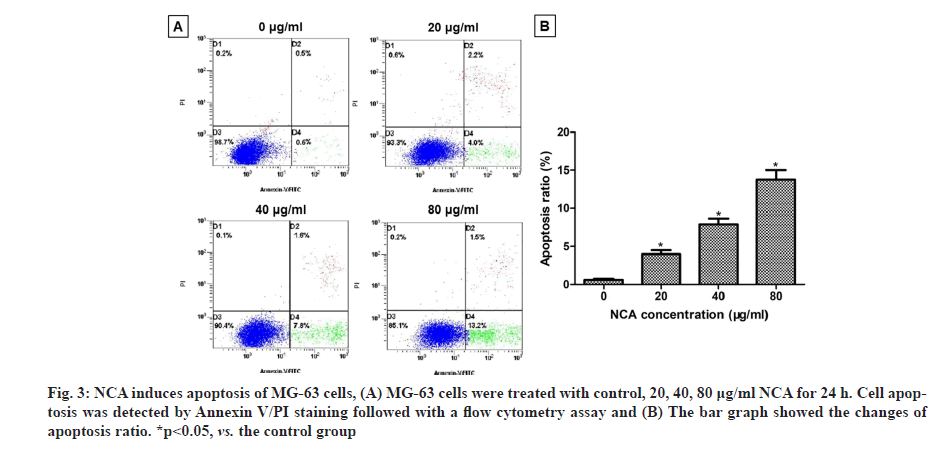
The apoptosis rates of MG-63 cells were detected by Annexin V/PI staining followed with a flow cytometry assay. In our results, the apoptosis ratio in the control group was 0.5 %, whereas, after treatment with NCA for 24 h, the apoptosis ratio increased from 4 % to 13.2 % with increasing drug concentration (fig. 3A). The apoptosis ratios of MG-63 cells were significantly increased in NCA group compared with the control group (p<0.05) (fig. 3B). These results showed that NCA could induce apoptosis occurred in MG-63 cells.
Fig. 3: NCA induces apoptosis of MG-63 cells, (A) MG-63 cells were treated with control, 20, 40, 80 μg/ml NCA for 24 h. Cell apoptosis was detected by Annexin V/PI staining followed with a flow cytometry assay and (B) The bar graph showed the changes of apoptosis ratio. *p<0.05, vs. the control group
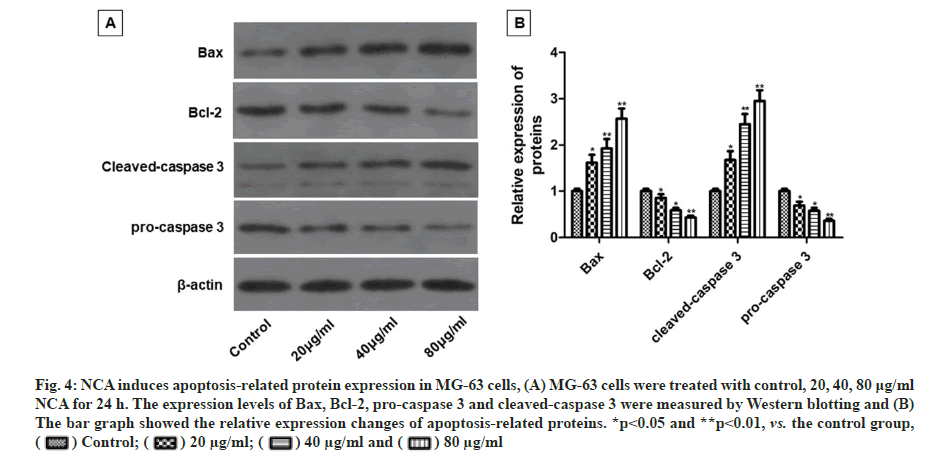
The impacts of NCA on apoptosis-related protein expression in MG-63 cells were investigated by Western blotting. Compared with the control group, NCA treatment dose-dependently increased the expression of Bax and cleaved-caspase 3, but decreased the expression of Bcl-2 and pro-caspase 3 (fig. 4A and fig. 4B).
Fig. 4: NCA induces apoptosis-related protein expression in MG-63 cells, (A) MG-63 cells were treated with control, 20, 40, 80 μg/ml NCA for 24 h. The expression levels of Bax, Bcl-2, pro-caspase 3 and cleaved-caspase 3 were measured by Western blotting and (B) The bar graph showed the relative expression changes of apoptosis-related proteins. *p<0.05 and **p<0.01, vs. the control group, 
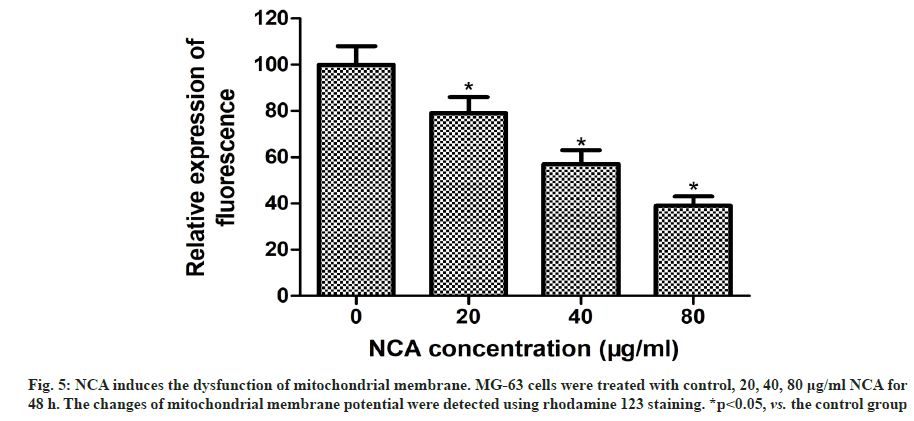
Previous studies have demonstrated that the damage of mitochondrial membrane is a vital stage during apoptosis. The loss of mitochondrial membrane potential is associated with mitochondrial dysfunction[14-16]. To explore the role and mechanism of NCA induced apoptosis in MG-63 cells, we detected the changes of mitochondrial membrane potential using rhodamine 123 staining. Compared with the control group, the fluorescence intensity in NCA-treated cells was significantly decreased (fig. 5). This indicated that NCA triggered the disorder of mitochondrial membrane potential and induced apoptosis through mitochondrial dependent pathway.
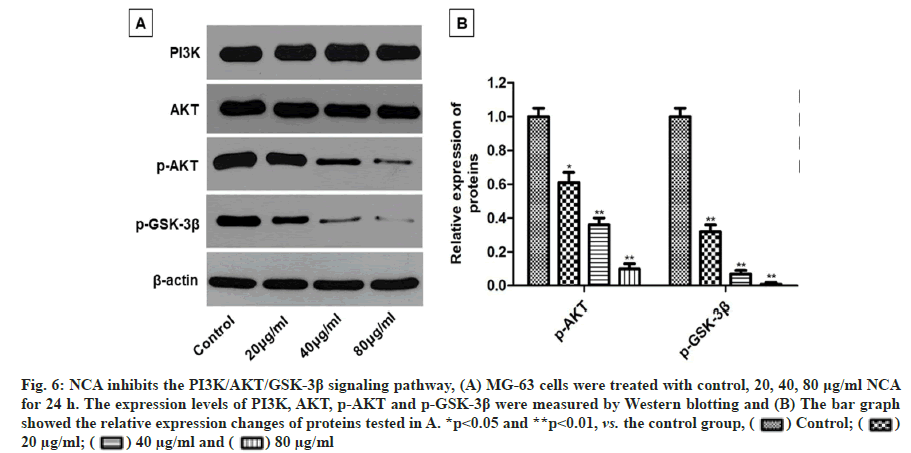
Some studies have reported that the progression of many human malignancies is linked with the aberrant activation of PI3K/AKT signaling pathway. Inhibition of PI3K/AKT pathway may serve as an effective approach for the treatment of some malignancies[17]. To further explore the molecular mechanism of NCA induced apoptosis in MG-63 cells, we investigated the effects of NCA on PI3K/AKT signaling pathway. Western blotting analysis showed that the expression levels of phosphorylated AKT (p-AKT) and phosphorylated GSK-3β (p-GSK-3β) were decreased in MG-63 cells after treatment with NCA for 24 h. Meanwhile, the expression levels of total PI3K and AKT were not changed (fig. 6A and fig. 6B).
Fig. 6: NCA inhibits the PI3K/AKT/GSK-3β signaling pathway, (A) MG-63 cells were treated with control, 20, 40, 80 μg/ml NCA for 24 h. The expression levels of PI3K, AKT, p-AKT and p-GSK-3β were measured by Western blotting and (B) The bar graph showed the relative expression changes of proteins tested in A. *p<0.05 and **p<0.01, vs. the control group, 
Osteosarcoma is the most common bone tumor with high-grade nausea, which often occurs in children and adolescents. During past decades, the progression of osteosarcoma has been significantly delayed due to the development of surgical resection and adjuvant chemotherapy. However, the mortality rates of patients remain high[1]. Chemotherapy is one important approach in the treatment of osteosarcoma. There are some bottlenecks in the application of traditional chemotherapy, such high toxicity, minimal effect and drug resistance[2]. Therefore, it is urgent to discover effective and safe agents to improve the therapeutic outcome of osteosarcoma.
In recent years, numerous studies have reported the antitumor effects of compounds extracted from natural products[18,19]. NCA is a new biologically active compound isolated from Stellera chamaejasme. NCA has been reported to possess antitumor effects on several types of human cancers[11,12]. However, few studies focused on its effect on osteosarcoma. Our study aimed to investigate the effect of NCA on human osteosarcoma MG-63 cells and to speculate whether it could be applied as a potential therapeutic agent for osteosarcoma. The effect of NCA on the proliferation of MG-63 cells was detected by MTT assay. Our results showed that NCA could significantly inhibit the proliferation of MG-63 cells in a time and concentration-dependent manner. In order to explore whether the inhibitory proliferation was due to apoptosis, we observed morphology changes in MG- 63 cells after treatment with NCA. We found that NCA-treated cells showed remarkable changes with cell shrinkage, chromatin condensation and apoptotic bodies formation and these changes were more obvious with increasing concentration of NCA. To measure the apoptotic ratio, a flow cytometry was performed. The apoptosis rate of MG-63 cells significantly increased after incubation with NCA. These findings indicated that NCA inhibited the proliferation of MG-63 cells, possibly via inducing apoptosis.
Bcl-2 family proteins play an important role in the regulation of apoptosis, which comprise pro-apoptotic factors, such as Bax and Bcl2 associated agonist of cell death (Bad) and anti-apoptotic factors, such as Bcl-2 and B-cell lymphoma-extra-large (Bcl-xL). Apoptosis is regulated depending on the ratio between pro-apoptotic factors and anti-apoptotic factors[20]. It was proven that upregulation of Bax and downregulation of Bcl-2 could significantly promote cellular apoptosis[21]. In the present study, we demonstrated that NCA significantly increased the expression of Bax and decreased the expression of Bcl-2, and therefore promoted apoptosis of MG-63 cells. Western blotting assay also showed the effect of NCA on caspase 3. Cleaved-caspase 3 is the active form of caspase 3 and the main cleavage enzyme to promote apoptosis[22]. The expression level of cleaved-caspase 3 was significantly upregulated after NCA treatment. The detection of apoptosis-related protein suggested that NCA could induce apoptosis in MG-63 cells through regulating these apoptosis-related factors.
Mitochondria can promote the metabolism of carbohydrates and provide energy for cell growth. The damage of mitochondrial function may mediate intrinsic pathway and lead to apoptosis of cells. The disruption of mitochondrial outer membrane is associated with the loss of mitochondrial membrane potential, which is a vital stage during mitochondria-mediated intrinsic apoptotic pathway[14]. In the present study, we detected the changes of mitochondrial membrane potential in MG-63 cells using rhodamine 123 staining. The results revealed that NCA could trigger the dysfunction of mitochondrial membrane and might induce apoptosis of MG-63 cells through mitochondrial dependent pathway. To further explore the molecular mechanism of NCA induced apoptosis of MG-63 cells, we investigated the effects of NCA on PI3K/AKT signaling pathway. PI3K/ AKT pathway is widely involved in the occurrence, development and treatment of tumors. GSK-3β is a crucial downstream regulator in PI3K/AKT pathway, which plays an important role in protein synthesis and apoptosis. The activation of PI3K/AKT pathway is finished through the cascaded phosphorylation of PI3K and AKT, and further inhibits the apoptosis of tumor cells[23,24]. Numerous studies have demonstrated the important role of PI3K/AKT pathway in the proliferation, migration, invasion and apoptosis of osteosarcoma cells. Inhibiting the phosphorylation of AKT can decrease the expression of downstream signaling factors, such as GSK-3β and caspase 3, thus induces cell apoptosis[25-28]. In the present study, we found that the expression levels of p-AKT and p-GSK- 3β were significantly decreased in MG-63 cells after treatment with NCA for 24 h. Therefore, NCA could inactivate the PI3K/AKT signal and promote apoptosis of MG-63 cells through the PI3K/AKT/GSK-3β signaling pathway.
Nevertheless, some limitations still exist in this study. Firstly, human osteosarcoma includes several types of cell lines, thus the effects of NCA on other osteosarcoma cell lines (e.g., U2OS, human osteosarcoma cell lines (HOS)) should be studied. Second, only in vitro experiments were carried out, the antitumor effects of NCA should be further verified using an osteosarcoma xenografted in vivo model. Thirdly, the inhibitory effects of NCA were investigated in MG-63 cells, however the cytotoxicity of NCA on normal cells was not examined. In addition, other signaling pathway should also be investigated to systematically screen the molecular mechanism of NCA induced cell death.
In conclusion, the results of this study revealed that NCA significantly inhibited proliferation and induced apoptosis of MG-63 osteosarcoma cells. Apoptosis might be induced by the upregulation of Bax and cleaved-caspase 3 and downregulation of Bcl-2 in mitochondrial dependent pathway. The antitumor effect of NCA on osteosarcoma might be mediated through the inhibitory effect of NCA on the PI3K/AKT/GSK- 3β signaling pathway. These new findings may indicate that NCA has potential to be chosen as a candidate for osteosarcoma chemotherapy.
Acknowledgements
This work was supported by Youth Foundation of Wuhan Municipal Health Commission (Grant no.WX20Q15).
Conflict of Interests
The authors declared no conflict of interest.
References
- Kumar R, Kumar M, Malhotra K, Patel S. Primary osteosarcoma in the elderly revisited: Current concepts in diagnosis and treatment. Curr Oncol Rep 2018;20(2):1-6.
[Crossref] [Google Scholar] [PubMed]
- Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma-connecting aetiology, biology and therapy. Nat Rev Endocrinol 2017;13(8):480-91.
[Crossref] [Google Scholar] [PubMed]
- Xu ZH, Qin GW, Li XY, Xu RS. New biflavanones and bioactive compounds from Stellera chamaejasme L. Yao Xue Xue Bao 2001;36(9):669-71.
[Google Scholar] [PubMed]
- Wang L, Duan H, Wang Y, Liu K, Jiang P, Qu Z, et al. Inhibitory effects of Lang-du extract on the in vitro and in vivo growth of melanoma cells and its molecular mechanisms of action. Cytotechnology 2010;62(4):357-66.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Li Y, Yang Q, Chen Y, Weng X, Wang Y, et al. In vitro inhibitory and pro-apoptotic effect of Stellera chamaejasme L. extract on human lung cancer cell line NCI-H157. J Tradit Chin Med 2012;32(3):404-10.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Zhao Y, Liu Y, Tian L, Jin D. Chamaejasmine inactivates Akt to trigger apoptosis in human HEp-2 larynx carcinoma cells. Molecules 2011;16(10):8152-64.
[Crossref] [Google Scholar] [PubMed]
- Yang D, Wang P, Ren X. Apoptosis induced by chamaejasminein human osteosarcoma cells through p53 pathway. Tumor Biol 2015;36(7):5433-9.
[Crossref] [Google Scholar] [PubMed]
- Zhang SD, Lei SH, Wei LI, Hong-Lin LI, Zhang WD. Isochamaejasmin induces apoptosis in leukemia cells through inhibiting Bcl-2 family proteins. Chin J Nat Med 2015;13(9):660-6.
[Crossref] [Google Scholar] [PubMed]
- Zhang T, Yu H, Dong G, Cai L, Bai Y. Chamaejasmine arrests cell cycle, induces apoptosis and inhibits nuclear NF-κB translocation in the human breast cancer cell line MDA-MB-231. Molecules 2013;18(1):845-58.
[Crossref] [Google Scholar] [PubMed]
- Zhang C, Zhou SS, Feng LY, Zhang DY, Lin NM, Zhang LH, et al. In vitro anti-cancer activity of chamaejasmenin B and neochamaejasmin C isolated from the root of Stellerachamaejasme L. Acta Pharmacol Sin 2013;34(2):262-70.
[Crossref] [Google Scholar] [PubMed]
- Liu WK, Cheung FW, Liu BP, Li C, Ye W, Che CT. Involvement of p21 and FasL in induction of cell cycle arrest and apoptosis by neochamaejasmin A in human prostate LNCaP cancer cells. J Nat Prod 2008;71(5):842-6.
[Crossref] [Google Scholar] [PubMed]
- Ding Y, Xie Q, Liu W, Pan Z, Fan X, Chen X, et al. Neochamaejasmin a induces mitochondrial-mediated apoptosis in human hepatoma cells via ROS-dependent activation of the ERK1/2/JNK Signaling Pathway. Oxid Med Cell Longev 2020;2020:1-12.
[Crossref] [Google Scholar] [PubMed]
- Jin Z, Aixi Y, Baiwen Q, Zonghuan L, Xiang H. Inhibition of hypoxia-inducible factor-1 alpha radiosensitized MG-63 human osteosarcoma cells in vitro. Tumori 2015;101(5):578-84.
[Crossref] [Google Scholar] [PubMed]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 2004;305(5684):626-9.
[Crossref] [Google Scholar] [PubMed]
- Singh PK, Roukounakis A, Frank DO, Kirschnek S, Das KK, Neumann S, et al. Dynein light chain 1 induces assembly of large Bim complexes on mitochondria that stabilize Mcl-1 and regulate apoptosis. Genes Dev 2017;31(17):1754-69.
[Crossref] [Google Scholar] [PubMed]
- Márquez-Jurado S, Díaz-Colunga J, das Neves RP, Martinez-Lorente A, Almazán F, Guantes R, et al. Mitochondrial levels determine variability in cell death by modulating apoptotic gene expression. Nat Commun 2018;9(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Park JH, Pyun WY, Park HW. Cancer metabolism: Phenotype, signaling and therapeutic targets. Cells 2020;9(10):2308.
[Crossref] [Google Scholar] [PubMed]
- Sharifi-Rad J, Ozleyen A, Boyunegmez Tumer T, Oluwaseun Adetunji C, El Omari N, Balahbib A, et al. Natural products and synthetic analogs as a source of antitumor drugs. Biomolecules 2019;9(11):679.
[Crossref] [Google Scholar] [PubMed]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 2020;83(3):770-803.
[Crossref] [Google Scholar] [PubMed]
- Delbridge AR, Grabow S, Strasser A, Vaux DL. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat Rev Cancer 2016;16(2):99-109.
[Crossref] [Google Scholar] [PubMed]
- Warren CF, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis 2019;10(3):1-2.
[Crossref] [Google Scholar] [PubMed]
- Wang W, Zhu M, Xu Z, Li W, Dong X, Chen Y, et al. Ropivacaine promotes apoptosis of hepatocellular carcinoma cells through damaging mitochondria and activating caspase-3 activity. Biol Res 2019;52(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Yang Q, Jiang W, Hou P. Emerging role of PI3K/AKT in tumor-related epigenetic regulation. Semin Cancer Biol 2019; 59:112-24.
[Crossref] [Google Scholar] [PubMed]
- Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B. The relation between PI3K/AKT signalling pathway and cancer. Gene 2019;698:120-8.
[Crossref] [Google Scholar] [PubMed]
- Wang B, Li J. Piceatannol suppresses the proliferation and induced apoptosis of osteosarcoma cells through PI3K/AKT/mTOR pathway. Cancer Manag Res 2020;12:2631-40.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Yu XH, Yan YG, Wang C, Wang WJ. PI3K/Akt signaling in osteosarcoma. Clin Chim Acta 2015;444:182-92.
[Crossref] [Google Scholar] [PubMed]
- Li M, Hong L. Pseudolaric acid B exerts antitumor activity via suppression of the Akt signaling pathway in HeLa cervical cancer cells. Mol Med Rep 2015;12(2):2021-6.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Weng Q, Han J, Chen J. Alantolactone suppresses human osteosarcoma through the PI3K/AKT signaling pathway. Mol Med Rep 2020;21(2):675-84.
[Crossref] [Google Scholar] [PubMed]