- *Corresponding Author:
- R. M. Nejem
Department of Analytical Chemistry, Alaqsa University, P.O.Box 4051, Gaza-76888, Palestine
E-mail: rafatnejem@alaqsa.edu.ps
| Date of Submission | 25 June 2007 |
| Date of Revision | 05 March 2008 |
| Date of Acceptance | 08 June 2008 |
| Indian J Pharm Sci 2008, 70 (3): 344-350 |
Abstract
A novel atomic absorption spectrometric method and two highly sensitive spectrophotometric methods were developed for the determination of paracetamol. These techniques based on the oxidation of paracetamol by iron (III) (method I); oxidation of p-aminophenol after the hydrolysis of paracetamol (method II). Iron (II) then reacts with potassium ferricyanide to form Prussian blue color with a maximum absorbance at 700 nm. The atomic absorption method was accomplished by extracting the excess iron (III) in method II and aspirates the aqueous layer into air-acetylene flame to measure the absorbance of iron (II) at 302.1 nm. The reactions have been spectrometrically evaluated to attain optimum experimental conditions. Linear responses were exhibited over the ranges 1.0-10, 0.2-2.0 and 0.1-1.0 µg/ml for method I, method II and atomic absorption spectrometric method, respectively. A high sensitivity is recorded for the proposed methods I and II and atomic absorption spectrometric method value indicate: 0.05, 0.022 and 0.012 µg/ml, respectively. The limit of quantitation of paracetamol by method II and atomic absorption spectrometric method were 0.20 and 0.10 µg/ml. Method II and the atomic absorption spectrometric method were applied to demonstrate a pharmacokinetic study by means of salivary samples in normal volunteers who received 1.0 g paracetamol. Intra and inter-day precision did not exceed 6.9%.
Keywords
Spectrophotometry, atomic absorption spectrometry, paracetamol, pharmacokinetic, within-day variability, between-day variability
Paracetamol (N-acetyl-p-aminophenol) has been in use as analgesic and antipyretic drug for over 50 years. It has been accepted as a very effective medication for the relief of pain and fever in adults and children [1]. A review of broad scope analytical interest such as chemical, physical and biopharmaceutical properties on paracetamol has been published [2,3]. Several spectrophotometric methods have been reported for the determination of paracetamol based on nitration [4,5], oxidation6-8 and hydrolysis to p-aminophenol followed by diazotization and phenolic coupling [9-13].
The reduction process of iron (III) by diclofenac sodium [14], salbutamol sulfate [15], captopril [16], amoxycillin [17] and ciprofloxacin [17] to iron (II) were used to quantify these drugs colorimetrically. The reversible redox system iron (III)/iron (II) was used as indicating aspect for the indirect flow-injection biamperometric determination of paracetamol18 and in simultaneous spectrophotometric determination of paracetamol by a differential kinetic method [19].
Liu and Oka described a spectrophotometric technique for the detection of paracetamol in serum and plasma based on the reduction of ferric 2,4,6-tris(2-pyridyl)- 5-triazine, to ferrous 2,4,6-tris(2-pyridyl)-5-triazine complex [20]. The method was specific to compounds that contain a phenolic hydroxyl group located in either Meta or para position on the benzene ring over a linear range from 25 to 400 mg/l [20]. The sensitivity of their method was not adequate to perform a pharmacokinetic study. Also some other drugs which have no phenolic hydroxyl group can reduce iron (III), like diclofenac [14], captopril [16] and ciprofloxacin [17]. Diclofenac and captopril are reducing agents due to the presence of aromatic amine (-NH) and thiol (-SH) groups in their structure.
In the present study, three methods were proposed for the determination of paracetamol based on spectrophotometric and atomic absorption spectrometry (AAS) techniques. The spectrophotometric methods (I and II) were based on the direct reduction of iron (III) with paracetamol and the hydrolysis of paracetamol to p-aminophenol, respectively. A Prussian blue color was formed due to the reaction of iron (II) with potassium ferricyanide, whose intensity is proportional to the concentration of paracetamol. P-aminophenol is a reducing agent due to the presence of phenolic hydroxyl and aromatic amine groups. The present study also aimed to develop a novel AAS method for the quantitation paracetamol in salivary samples where the literature reveals no researches cover the determination of paracetamol based on AAS. The AAS method based on the extraction of iron (III) from 6.0 M hydrochloride acid with diethyl ether (probably as the solvated complex [H3O(R2O)2+,FeCl4-] [21] and the aqueous layer was used for atomic absorption measurement of iron (II).
The spectrophotometric method II and AAS techniques were applied for the determination and assessment of the bioavailability of paracetamol using saliva samples. The pharmacokinetic study of paracetamol by means of saliva was considered feasible, since the ease of samples collection and analysis besides the good correlation between saliva and plasma levels of paracetamol [22].
Materials and Methods
Paracetamol was supplied by the Middle East pharmaceuticals and Cosmetics Laboratories, Palestine (Kempex BV Holland). Paracetamol tablets, 500 mg, were purchased from the pharmacy, Decamol (Megapharm) and Dexamol (Dexxon). Standard solutions of paracetamol (10 µg/ml) were prepared in deionized water and diluted as required. Potassium ferricyanide solution (1.0 mM) and ferric sulfate solution (1.0 mM) was also prepared in deionized water. All chemicals and reagents used were of analytical grade.
A spectro 20 D plus, Labomed Inc. USA spectrophotometer with 1.0 cm rectangular glass cell was used for all spectrophotometric measurements. Atomic absorption measurements were performed using Perkin-Elmer AAS, model A Analyst 100 spectrophotometer equipped with an iron hollow cathode lamp, under the following conditions: wave length, 302.1 nm, slit-width 0.2 nm, lamp current 30.0 mA, air flow rate and acetylene flow rate are adjusted at the standard conditions.
Spectrophotometric method I
Six different portion of paracetamol standard concentrations (0, 1.0, 2.0, 4.0, 6.0, 8.0 and 10.0 µg/ml), 2.5 ml each, were pipetted into a series of 5 ml measuring flasks. To each flask, 1 ml of ferric sulfate solution (1.0 mM) and 1.0 ml of potassium ferricyanide solution (1.0 mM) were added and completed with deionized water. Absorbances were recorded at 700 nm after 24 min [16].
Spectrophotometric method II
Six different portion of paracetamol standard concentrations (0, 0.2, 0.4, 0.8, 1.2, 1.6 and 2.0 µg/ ml), 2.5 ml each portion, were pipetted into a series of 5.0 ml measuring flasks. To each flask, 0.5 ml of 1.0 M HCl solution was added, followed by 1.0 ml of ferric sulfate. The mixtures were heated using a boiling water bath at 100° for 10 min. After cooling, 1.0 ml of potassium ferricyanide was added and diluted with water. Absorbances were recorded at 700 nm after 24 min [16].
AAS method
Six different portion of paracetamol standard concentrations (0, 0.1, 0.2, 0.4, 0.6, 0.8, and 1.0 µg/ ml), 2.5 ml each, were pipetted into a series of 10 ml measuring flasks. To each flask, 0.5 ml of 1.0 M HCl solution was added, followed by 1.0 ml of ferric sulfate. The mixtures were heated using a boiling water bath at 100° for 10 min and after cooling 4.0 ml of 12.0 M HCl solution was added. Iron (III) was extracted with three 25.0 ml portions of diethyl ether. The aqueous layer was aspirated into the air-acetylene flame, and the absorbance of iron (II) was measured at 302.1 nm.
Saliva standard solutions for calibration curve were prepared by adding (0, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.6 and 2.0 ml) of 2.5 µg/ml of paracetamol to 0.25 ml drug free saliva using micropipette and complete the volume to 2.5 ml. The final concentrations of paracetamol each saliva sample were 0, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.6 and 2.0 µg/ml.
Extraction of saliva samples
The extraction is performed based on the procedure described by Dordoni et al. [23] and Spooner et al. [24]. An amount of 1.0 g Na2SO4 and 10 ml of diethyl ether were added to 0.25 ml saliva aliquot or standard and mixed thoroughly. The diethyl ether layer was separated and evaporated. The residue was dissolved in 2.5 ml water and determined by spectrophotometric method II or AAS method.
Analysis of paracetamol from tablet dosage form in pharmaceutical preparations
Twenty tablets of paracetamol (500 mg paracetamol active ingredient) were accurately weighed and powdered, Decamol (Megapharm, Palestine) and Dexamol (Dexxon, Israel). A portion equivalent to 50 mg was dissolved in distilled water and analyzed by the recommended procedures.
Pharmacokinetic application
The pharmacokinetic study has been approved by the Ministry of Health, PNA. Five healthy female volunteers, with ages ranging from 20-24 years and weights 55-75 kg, were enrolled in the pharmacokinetic study. In a randomized two-way crossover design, each subject received 1.0 g dose of paracetamol (2 tablets) on an empty stomach and overnight fast. Tablets were administered with 200 ml of water. No food was allowed for 4 h after which a light standard lunch was served. The volunteers were instructed to drink water regularly during the study to keep saliva flow. Three ml saliva samples were collected before drug administration as Blank and at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 4, 6 and 8 h, a wash period of 7 days separated each two consecutive phases of the crossover. Saliva samples were centrifuged for 10 minutes and 0.25 ml of the supernatant was taken and analyzed using spectrophotometric method II or AAS method.
Results and Discussion
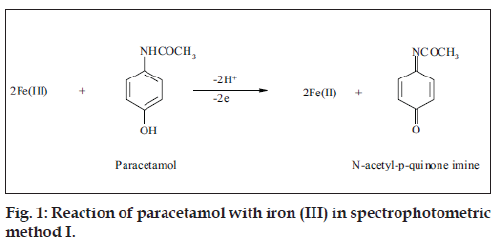
Oxidation of paracetamol with iron (III) followed by potassium ferricyanide to form a Prussian blue color whose intensity is proportion to the concentration of paracetamol was performed. Paracetamol was quantitatively oxidized at room temperature (25°) without any significant effect of pH. It was found that keeping paracetamol concentration constant and altering the concentration of iron (III), caused an increase in the absorbance up to mole ratio of 2:1 iron (III) and paracetamol, respectively. The probable reaction as shown in fig. 1, N-acetyl-p-quinone imine was only produced by the oxidation of paracetamol, 2,2-dihydroxy-5,5’-diacetylaminobiphenyl is formed only in alkaline medium [25,26].
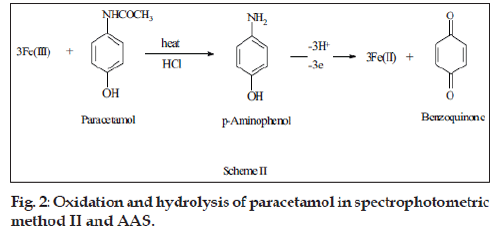
Paracetamol was hydrolyzed in acidic medium using boiling water bath to give p-aminophenol which reduced iron (III). Heating intervals and pH were optimized to ensure complete hydrolysis and oxidation. Different heating intervals were investigated where 10 min were found to be enough for the complete reaction. It was also observed that the absorbance remained constant in the pH range 1.0-3.0. Therefore, all the studies were performed at pH 1.0. The stoichiometry of the reaction was established as in method I and the iron (III) to paracetamol mole ratio was found 3:1 (fig. 2).
Different aliquots of the standard iron (III) were dissolved in 6.0 M HCl solutions and each sample was extracted with three 25 ml portions of diethyl ether. Iron (III) in the aqueous layer was determined colorimetrically using KSCN at 480 nm or by AAS method at 302.1 nm. The overall extraction efficiency was approximately 100% and 99.99% using colorimetric and AAS method, respectively.
Detection limit (LOD) of the proposed methods was determined by analysis of the peak baseline noise in five blank samples, which was considered as three times the variation in measured response. The LOD were 0.12, 0.05 and 0.026 µg/ml for spectrophotometric method I and II and AAS method, respectively. The estimated limit of quantification (LOQ) was calculated as ten times the variation in measured response. The LOQ were 0.40, 0.20 and 0.10 µg/ml for spectrophotometric method I and II and AAS method, respectively. The LOQ were confirmed for saliva using calibrators with nominal concentration of 0.20 and 0.10 µg/ml. The sensitivity was determined as the concentrations that gave an absorbance reading of 0.0044. The sensitivity of the proposed methods were 0.05, 0.022 and 0.012 µg/ml methods I, and II and AAS, respectively.
Typical calibration curves were constructed for the three proposed methods based on linear regression analysis of absorbance versus concentration. The slope, intercept, sensitivity and correlation coefficient were summarized (Table 1). Linear responses were displayed in the range 1.0-10, 0.2-2.0 and 0.1-1.0 µg/ ml for paracetamol using spectrophotometric method I, and II and AAS method, respectively. The molar absorptivities were 1.35×104 and 2.72×104 l/mol.cm for paracetamol using spectrophotometric method I and II, respectively.
| Parameters | Spectrophotometric method | AAS | |
|---|---|---|---|
| I | II | ||
| λ (nm) | 700 | 700 | 302.1 |
| pH | - | 1 | 6 |
| Molar absorptivity (L/mol.cm) | 1.35×104 | 2.72×104 | - |
| LOD (µg/ml) | 0.12 | 0.05 | 0.026 |
| LOQ (µg/ml) | 0.40 | 0.20 | 0.10 |
| Sensitivity (µg/mL) | 0.05 | 0.022 | 0.012 |
| Linear response (µg/ml) | 1-10 | 0.2-2 | 0.1-1 |
| RSD (n=5) | 3.86 | 5.0 | 2.55 |
| Regression equation | |||
| Slope | 0.089 ± 0.007 | 0.155 ± 0.012 | 0.40 ± 0.033 |
| Intercept | 0.011 ± 0.001 | 0.049 ± 0.003 | 0.025 ± 0.002 |
| Correlation coefÞcient (R) | 1 | 0.9920 | 0.9982 |
Table 1: Response Characteristics Of The Proposed Methods
Five replicate measurements were performed using the three proposed methods. The relative standard deviations (RSD) were 3.86, 5.0 and 2.55 for spectrophotometric method I, and II and AAS method, respectively. The proposed methods were applied for the recovery of paracetamol in its dosage forms. The obtained results using the proposed methods are in good concordance with the standard method of British pharmacopoeia [27]. The comparison through using t- and f- statistical tests confirm the high accuracy and precision of the proposed methods (Table 2).
| Sample | Recovery % ± SD based on different methods a | ||||
|---|---|---|---|---|---|
| Spectrophotometric method | AAS | Standard [27] method | |||
| I | II | ||||
| Paracetamol bulk | 100.2 ± 0.31 | 98.5 ± 0.22 | 99.6 ± 0.44 | 99.7 ± 0.47 | |
| Decamol (500 mg/tablet) | 98.8 ± 0.52 | 100.8 ± 0.71 | 98.9 ± 0.51 | 99.9 ± 0.74 | |
| Dexamol (500 mg/tablet) | 99.2 ± 0.47 | 99.6 ± 0.18 | 99.4 ± 0.69 | 100.1 ± 0.55 | |
| t-test / f-test | |||||
| Paracetamol bulk | 0.29 / 2.3 | 1.53 / 1.7 | 1.12 / 2.6 | ||
| Decamol (500 mg/tablet) | 1.33 / 2.9 | 1.24 / 2.8 | 1.34 / 3.8 | ||
| Dexamol (500 mg/tablet) | 1.85 / 1.3 | 1.87 / 3.2 | 1.67 / 1.6 | ||
Table 2: Assay of paracetamol in bulk and dosage forms by the proposed methods and official Method [27]
Within-day variability of the proposed methods for the analysis of paracetamol were determined by repeating the analysis of three quality control samples at low, medium and high concentrations on the same day. The results indicate that these methods are reproducible within the same day (Table 3). Between-day variability of the proposed methods were determined by repeated analysis of three quality control samples at low, medium and high concentration on three different days. The results are shown in Table 3. This data proves the reproducibility of the proposed methods within different days.
| Paracetamol (µg/ml) | Within-day variability ( ± SDa) | RSD | SAEb | CLc | ||
|---|---|---|---|---|---|---|
| Spectrophotometric | ||||||
| I | II | AAS | ||||
| 1 | 1.08 ± 0.088 | - | - | 8.15 | 0.039 | 1.08 ± 0.109 |
| 6 | 5.9 ± 0.324 | - | - | 5.49 | 0.145 | 5.9 ± 0.402 |
| 10 | 10.18 ± 0.50 | - | - | 4.91 | 0.224 | 10.18 ± 0.621 |
| 0.2 | - | 0.2 ± 0.012 | - | 6.00 | 0.005 | 0.2 ± 0.015 |
| 0.8 | - | 0.84 ± 0.062 | - | 7.38 | 0.028 | 0.84 ± 0.077 |
| 2.0 | - | 1.95 ± 0.056 | - | 2.86 | 0.250 | 1.95 ± 0.700 |
| 0.1 | - | - | 0.1 ± 0.007 | 7.00 | 0.003 | 0.1 ± 0.009 |
| 0.6 | - | - | 0.59 ± 0.026 | 4.40 | 0.120 | 0.59 ± 0.032 |
| 1.0 | - | - | 1.01 ± 0.020 | 1.98 | 0.009 | 1.01 ± 0.025 |
| Between-day variability ( ± SDa) | ||||||
| 1 | 1.1 ± 0.130 | - | - | 11.82 | 0.058 | 1.1 ± 0.161 |
| 6 | 6.2 ± 0.267 | - | - | 4.30 | 0.119 | 6.2 ± 0.330 |
| 10 | 10.6 ± 0.496 | - | - | 4.68 | 0.222 | 10.6 ± 0.616 |
| 0.2 | - | 0.22 ± 0.012 | - | 5.45 | 0.005 | 0.22 ± 0.139 |
| 0.8 | - | 0.85 ± 0.052 | - | 6.12 | 0.023 | 0.85 ± 0.640 |
| 2.0 | - | 1.96 ± 0.053 | - | 2.70 | 0.023 | 1.96 ± 0.640 |
| 0.1 | - | - | 0.11 ± 0.007 | 6.38 | 0.003 | 0.11 ± 0.008 |
| 0.6 | - | - | 0.61 ± 0.020 | 3.30 | 0.009 | 0.61 ± 0.025 |
| 1.0 | - | - | 1.01 ± 0.026 | 2.57 | 0.012 | 1.01 ± 0.033 |
Table 3: Within- and between-day variability of proposed methods for paracetamol Determination
The effect of the presence of common excipients such as dextrose, glucose, saccharine sodium, starch, talc and magnesium stearate were investigated. There were insignificant interferences from these excipients. The most of drugs (ascorbic acid, caffeine, salbutamol, diclofenac sodium, amoxycillin and ciprofloxacin) and metabolites were reduced by iron (III) and paracetamol should therefore be extracted and separated before applying the methods.
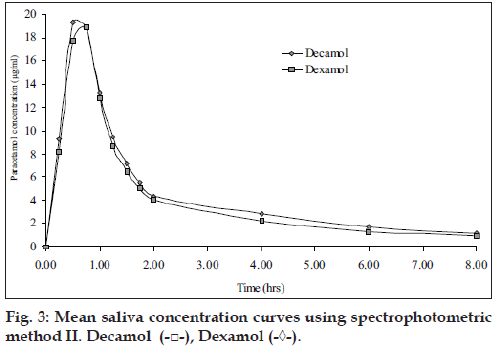
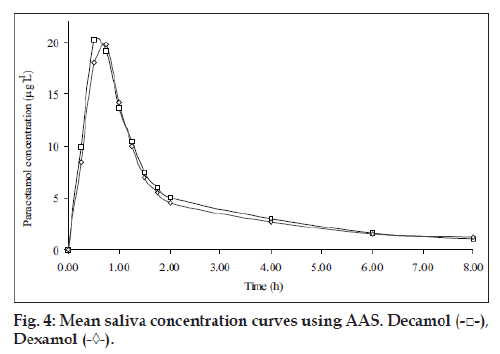
Five healthy female volunteers were administrated 1 g of Dexamol and Decamol separately. The recovery investigations of standard saliva samples spiked with paracetamol are shown in Table 4. The recovered concentrations of paracetamol using the three proposed methods are highly correlated to the spiked amounts of paracetamol. The mean saliva concentrationtime curves based on spectrophotometric method II and AAS are shown in figs. 3 and 4, respectively.
| Saliva (ml) | Paracetamol Spiked (µg/ml) | Recovery based on spectrophotometric Method IIa | Recovery based on | ||
|---|---|---|---|---|---|
| AAS Methoda | |||||
| (µg/ml) | % | (µg/ml) | % | ||
| 0.25 | 0.1 | - | - | 0.105 ± 0.002 | 105 |
| 0.25 | 0.2 | 0.21 ± 0.003 | 105 | 0.19 ± 0.005 | 95 |
| 0.25 | 0.4 | 0.39 ± 0.007 | 97.5 | 0.38 ± 0.008 | 95 |
| 0.25 | 0.6 | - | - | 0.59 ± 0.012 | 98 |
| 0.25 | 0.8 | 0.82 ± 0.015 | 102 | 0.820 ± 0.013 | 103 |
| 0.25 | 1.0 | - | - | 1.01 ± 0.033 | 101 |
| 0.25 | 1.2 | 1.18 ± 0.041 | 98 | - | - |
| 0.25 | 1.6 | 1.56 ± 0.053 | 97.5 | - | - |
| 0.25 | 2.0 | 1.88 ± 0.067 | 94 | - | - |
Table 4: Recovery Of Spiked Paracetamol In The Control Saliva Samples
Paracetamol saliva concentration did not show any significant difference between the two brands at each sampling.
The pharmacokinetic parameters for the two brands, maximum salivary concentration (Cmax, µg/ml), time to maximum saliva concentration (Tmax, h) and area under the saliva concentration-time curve (AUC0-8 µg/ml h) are summarized (Table 5). The mean values of AUC0-8 oral bioavailability of Decamol tablet was 103% relative to Dexamol tablet (innovator brand) in each described assay. Thus, the bioavailability of the two brands was not significantly different; therefore the two products are considered bioequivalent.
| Parameters | Spectrophotometric method II | AAS method | ||
|---|---|---|---|---|
| Dexamol tablet | Decamol tablet | Dexamol tablet | Decamol tablet | |
| Cmax (µg/ml) | 18.9 | 19.3 | 19.1 | 20.2 |
| Tmax (h) | 0.6 | 0.5 | 0.6 | 0.5 |
| T½ (h) | 2.76 | 2.55 | 2.82 | 2.69 |
| AUC0-8 (µg/ml.h) | 34.7 | 35.9 | 35.2 | 36.3 |
| % AUC | 100 | 103 | 100 | 103 |
Table 5: Pharmacokinetic Parameters In Healthy Female Volunteers
All the proposed methods are simple, rapid, accurate, and exhibit higher sensitivity compared to Zarei et al and Liu and Oka methods [19,20] and the AAS is a novel method. The extraction of paracetamol from saliva into diethyl ether improves the specificity of the assay. The statistical parameters and the recovery study data clearly indicate the reproducibility and accuracy of the methods. The proposed methods are suitable for the analysis of paracetamol in its pharmaceuticals formulations without any interference from the exipients normally found in commercial preparations. Spectrophotometric method II and AAS method are highly sensitive for the application in the pharmacokinetic studies.
Acknowledgements
Authors are thankful to the Middle East pharmaceuticals and cosmetics laboratories, Palestine and Alaqsa University. As well, we thank Prof. Abd Elrahem Ashor and the participant staff for their assistance.
References
- Blacow NW, editor. Martindale: The Extra Pharmacopoeia, 26th ed. London: The Pharmaceutical Press; 1972. p. 245.
- Fairbrother JE. Analytical profiles of drug substances, Vol. 3, Orlando, FL: Academic Press; 1972. p. 2.
- El-Obeid A, Al-Badr A. Analytical profiles of Drug substances, Vol. 14, Orlando, FL: Academic Press; 1985. p. 551.
- Glynn JP, Kendal SE. Paracetamol measurement. Lancet 1975;305: 1147-8.
- Belal SF, El Sayed MA, Elwalily HA, Abdine H. Spectrophotometric Determination of Acetaminophen and Salicylamide through Nitrosation and Subsequent Chelation. Analyst 1979;104:919-27.
- Sultan SM. Spectrophotometric determination of paracetamol in drug formulations by oxidation with potassium dichromate. Talanta 1987;34:605-8.
- Calatayud JM, Pascal MC, Sagrado SV. Determination of paracetamol by a ßow injection-spectrophotometric method. Anal Lett 1986;19:2023-8.
- Verma KK, Gulati AK, Poland S, Tyagi P. Spectrophotometric determination of paracetamol in drug formulations with 2-Iodylbenzoate. Analyst 1984;109:735-7.
- Bhowal SK, Das TK. Estimation of some analgesic and antipyretic drugs alone and in drug formulations of spectrophotometrically using uranyl acetate. Indian Drugs 1990;27:475-80.
- Nagaraja P, Murthy KCS, Rangappa KS. Spectrophotometric method for the determination of paracetamol and phenacetin. J Pharm Biomed Anal 1998;17:501-6.
- Bouhsain Z, Garrigues S, Rubio AM, Guardia MD. Flow injection spectrophotometric determination of paracetamol in pharmaceuticals by means of on-line microwave-assisted hydrolysis and reaction with 8-hydroxyquinoline (8-quinolinol). Ana Chim Acta 1996;330:59-69.
- Lavorante AF, Pires CK, Reis BF. Multicommuted flow system employing pinch solenoid valves and micro-pumps: Spectrophotometric determination of paracetamol in pharmaceutical formulations. J Pharm Biomed Anal 2006;42:423-9.
- Afshari JT, Liu TZ. Rapid Spectrophotometric Method for the Quantitation of Acetaminophen in Serum. Anal ChimActa 2001; 443: 165-69.
- El- Sousi KS, Ahmed MA, Issa MM. Bioavailability of oral paracetamol formulations. Bull Fac Pharm (Cairo Univ Press) 2002;40:195-201.
- Kanakapura B, Chandrashekar PH. Spectrophotometric determination of salbutamol sulfate and acyclovir using iron (III) and ferricynide. Science Asia 2003;29:141-6.
- Rahman N, Anwar N, Kashif M, Hoda N. A Sensitive kinetic spectrophotometric method for the determination of captopril in bulk and dosage forms. Acta Pharm 2006;56:347-57.
- Nagaralli BS, Seetharamappa J, Melwanki MB. Sensitive spectrophotometric methods for the determination of amoxycillin, ciproßoxacin and piroxicam in pure and pharmaceutical formulations. J Pharm Biomed Anal 2002;29:859-64.
- Galves AM, Mateo JVG, Calatayud JM. Study of various indicating redox systems on the indirect flow-injection biamperometric determination of pharmaceuticals. Anal Chim Acta 1999;396:161-70.
- Zerai AR, Afkhami A, Sarlak AN. Simultaneous spectrophotometric determination of paracetamol and salicylamide in human serum and pharmaceutical formulations by a differential kinetic method. J AOAC Int 2005;88:1695-701.
- Liu TZ, Oka KH. Spectrophotometric screening method for aacetaminophen in serum and plasma. Clin Chem 1980;26:69-71.
- Vogel AI. In: Bassett J. editor. Vogel’s Text Book of Quantitative Inorganic Analysis, 4th ed. New York: John Wiley & Sons; 1978. p. 157.
- Cardot JM, Aiache JM, Renoux R, Kantelip JR. Correlation between saliva and plasma concentrations of paracetamol relevance for bioavailability studies. STP Pharma 1985;1:114-20.
- Dordoni B, Willson RA, Thompson RP, Williams R. Reduction of absorption of paracetamol by activated charcoal and cholestyramine: A possible therapeutic measure. Br Med J 1973;3:86-7.
- Spooner RJ, Reavey PC, McIntosh L. Rapid estimation of paracetamol in plasma. J Clin Pathol 1976;29:663.
- Murillo JA, Garcia LF. Application of first derivative fluorescence spectrometry to the simultaneous determination of paracetamol and salicylamide in pharmaceuticals. Anal Lett 1996;29:423-38.
- Vilchez JL, Balnc R, Avidad R, Navalon A. Spectrofluorimetric determination of paracetamol in pharmaceuticals and biological ßuids. J Pharm Biomed Anal 1995;13:1119-25.
- British Pharmacopoeia, British Pharmacopoeia Commission, London: HMSO; 1993. p. 483.