- *Corresponding Author:
- Y. Qiao
Department of Pulmonary Tuberculosis, Zhe Jiang Jin Hua Guang Fu Tumor Hospital, Jinhua, Zhejiang 321000, China
E-mail: qyl20212310@163.com
| This article was originally published in a special issue, “Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “196-202” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aims to investigate the short- and long-term therapeutic efficacy, as well as the safety profile, of combined treatment with atezolizumab and bevacizumab in patients diagnosed with non-squamous non-small cell lung cancer (NS-NSCLC) characterized by elevated expression of programmed death ligand-1 (PD-L1). Over the period spanning from June 2019 to June 2022, Jinhua Municipal Central Hospitall enrolled 100 patients diagnosed with NS-NSCLC exhibiting PD-L1 overexpression. Among them, 50 individuals were allocated to the observation group and another 50 to the control group as per the treatment strategy they received. The control group was subjected solely to bevacizumab treatment, while the observation group received a combination therapy of atezolizumab along with bevacizumab. Each group underwent therapy administered in cycles spanning 21 days, with a cumulative duration of three cycles. The investigation entailed a juxtaposition of clinical effectiveness, the incidence of unfavorable responses, and one-year survival proportion amid the two cohorts. Moreover, a comparison was conducted concerning alterations in the levels of immunoglobulin G (IgG), immunoglobulin M (IgM), immunoglobulin A (IgA), carcinoembryonic antigen (CEA), vascular endothelial growth factor (VEGF), and carbohydrate antigen 125 (CA125) before and after treatment in both groups. The cumulative response proportion in the observation group (74.00%) surpassed that in the control group (54.00%) with a statistically significant differentiation (P<0.05). Following treatment, IgA, IgG, and IgM levels in the observation group demonstrated conspicuous elevations compared to the pre-treatment levels and the post-treatment levels in the control group, exhibiting statistically significant differences (P<0.05). In addition, the levels of CEA, VEGF, and CA125 in the observation group after treatment showed a substantial decrease compared to their pre-treatment levels and the post-treatment levels in the control group, with disparities holding statistical significance (P<0.05). Nevertheless, despite a slightly higher overall incidence of adverse reactions in the observation group (38.00%) compared to the control group (30.00%), the variance was insignificant (P>0.05). Throughout the course of the one-year follow-up, no instances of follow-up attrition were recorded. The observation group had a mortality rate of 36.00% (18/50) and a one-year survival rate of 64.00% (32/50), whereas the control group exhibited a mortality rate of 40.00% (20/50) and a one-year survival rate of 60.00% (30/50). Kaplan-Meier survival analysis did not reveal any considerable divergence in survival rates between the two groups (Log-Rank=0.018, P=0.893). The combination therapy of atezolizumab and bevacizumab demonstrated favorable outcomes in patients with PD-L1 overexpressing NS-NSCLC, leading to effective enhancement of patient immune function and modulation of CEA, VEGF, and CA125 levels. Furthermore, the treatment exhibited good patient tolerability, thereby warranting attention from clinical practitioners.
Keywords
Non-squamous non-small cell lung cancer, programmed death ligand-1, atezolizumab, bevacizumab, immune function
The occurrence of lung cancer is influenced by various factors, such as smoking, genetics, and atmospheric pollution. In recent years, with the improvement in the quality of life and significant lifestyle changes among the population, the causative factors for lung cancer have increased, leading to a notable rise in the affected population[1,2]. Lung cancer has been reported as the leading type of malignancy in males in terms of both incidence and mortality rates and ranks second among females[3,4]. Typically, in the early stages of the disease, patients may not exhibit noticeable symptoms. However, as the disease progresses, patients may experience a series of symptoms such as cough, chest pain, and chest distress. This often results in the diagnosis of lung cancer occurring in the middle to late stages when the disease has already advanced, causing a missed opportunity for optimal surgical intervention[5,6]. Therefore, actively seeking efficacious treatment methods for lung cancer is of paramount importance.
Currently, the clinical treatment of lung cancer has evolved into a relatively well-established system, including chemotherapy, targeted therapy, and other modalities. Among these, bevacizumab has demonstrated promising efficacy in the treatment of lung cancer patients and has gained recognition from numerous clinicians both domestically and internationally[7-9]. Nevertheless, during the clinical treatment process, it has been observed that some Non-Squamous Non-Small Cell Lung Cancer (NS-NSCLC) patients exhibit high expression of Programmed Death Ligand-1 (PD-L1), defined as PD-L1 profile ≥50 %. Elevated PD-L1 profile can boost tumor invasion and metastasis and may even contribute to immune escape, thereby influencing the clinical therapeutic outcomes of NS-NSCLC to a certain extent. This phenomenon is unfavorable for patient’s prognosis, necessitating the urgent search for alternative effective treatment strategies[10].
PD-L1 is an immune checkpoint protein which bounded to its receptor PD-1, can dampen the immune system’s attack on tumor cells, thus helping tumor cells evade immune responses. High expression of PD-L1 denotes a stronger inhibition of the immune checkpoint by tumor cells, which may lead to a reduced ability of the immune system to resist tumors. For NS-NSCLC patients with PD-L1 overexpression, immune checkpoint inhibitors, like PD-1 inhibitors and PD-L1 inhibitors are commonly utilized in treatment. These drugs can block the binding of PD-L1 to PD-1, restoring the immune system’s ability to recognize and attack tumor cells, leading to enhanced treatment outcomes[11]. Atezolizumab is an immune therapy drug that has been introduced into clinical practice in recent years. It manifests a strong affinity to PD-L1, which hinders the interaction between tumor cell PD-L1 and T cell PD-1, thus impeding immune escape. Based on this, the present study aims to retrospectively analyze the treatment outcomes of atezolizumab combined with bevacizumab for NS-NSCLC patients with PD-L1 overexpression. The study endeavors to furnish a theoretical foundation for the therapeutic management of such individuals.
Materials and Methods
Basic data:
Between June 2019 and June 2022, a cohort of 100 patients diagnosed with NS-NSCLC exhibiting high levels of PD-L1 was meticulously chosen from Jinhua Municipal Central Hospital’s records for this investigation. By utilizing a random number table method, the patients were allocated into two groups, namely the observation group (n=50) and the control group (n=50). The cohort of the observation group comprised 34 male individuals and 16 female individuals, whose ages spanned from 53 y to 64 y, with an average age of (58.19±3.18) y. The Malignant Tumors (TNM) stage includes 14 cases in stage III b and 36 cases in stage IV. The scores on the Karnofsky Performance Scale (KPS) were distributed within the range of 75 to 90, with a mean score of (82.41±5.19). In terms of Body Mass Index (BMI), it spanned from 21 to 25 kg/m2, with an average of (23.16±1.08) kg/ m2.
Meanwhile, the control group comprised 32 males and 18 females, whose ages spanned from 54 y to 62 y, with an average age of (58.27±3.11) y. The TNM stage includes 12 cases in stage III b and 38 cases in stage IV. The KPS scores varied from 76 to 89, and the mean score was calculated as (82.51±5.27). Regarding the BMI, it was distributed in the range of 22 to 25 kg/m2, with an average value of (23.25±1.11) kg/m2. The basic data of both groups were subjected to the χ2 test and t-test (p>0.05). Prior to the initiation of the study, the study protocol had received the imprimatur from Jinhua Municipal Central Hospital’s Medical Ethics Committee following the research discussion.
Inclusion and exclusion criteria:
Inclusion criteria: Patients diagnosed with NSNSCLC based on pathological examination[12]; PD-L1 profile detected by immunohistochemistry (22c3, Dako) showing ≥50 %; age range between 18 and 65 y; TNM staging ranging from stage III b to stage IV and patients or their family members who voluntarily participated in the study after thoroughly understanding the research objectives and methods and provided informed consent.
Exclusion criteria: Predicted survival time less than 3 mo; known allergy to medications used in this research; presence of blood or immune system disorders; existence of blood coagulation disorders, significant organ impairments, or mental illnesses; history of previous targeted drug treatment and presence of severe bleeding tendencies.
Methods:
The control group was treated with bevacizumab (manufactured by Roche Pharmaceuticals, Germany; National Medicine Approved Number: H0192B04; specification: 100 mg/vial) at a dose of 15 mg/kg administered weekly via intravenous infusion. The treatment was administered in cycles of 21 d, with continuous medication for three cycles.
The observation group received atezolizumab (manufactured by Roche Diagnostics GmbH, National Medicine Approved Number: 20200004; specification: 1200 mg/20 ml) in addition to the treatment received by the control group. Atezolizumab was administered at a dose of 1200 mg every 3 w via intravenous injection. The treatment response was evaluated subsequent to three consecutive administrations.
Observation of indicators:
Efficacy assessment: After completion of the treatment, patients were evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST) standards[13], and the responses were categorized as complete remission, partial remission, stable disease, and disease progression. The Overall Response Rate (ORR) was computed employing the following mathematical expression: ORR=(number of complete remission+number of partial remission)/ total number of cases×100 %.
Immunoglobulin (Ig) and tumor marker detection: Upon admission and at the end of the treatment, 5 ml of fasting venous blood was procured from each cohort of patients. Subsequent to centrifugation, IgG, IgM, and IgA were detected using the enzyme kinetic method on the fully automatic biochemical analyzer Erba XL-300 (Germany). The kits were supplied by Pichia Biotechnology (Shenzhen) Co., Ltd. Carbohydrate Antigen 125 (CA125) was detected with the assistance of the fully automated Electrochemiluminescence immunoassay system Roche Cobase601 (Roche, Switzerland) and the corresponding kit. The Enzyme-Linked Immunosorbent Assay (ELISA) measured Carcinoembryonic Antigen (CEA), with the reagent kit supplied by Whenzhou Kemiao Biological Technology Co., Ltd. Vascular Endothelial Growth Factor (VEGF) was examined via ELISA with the use of the Beckman AU5800 fully automated biochemical analyzer, and the reagent kit was supplied by R and D Systems Inc., United States of America (USA).
Drug safety: The incidence of adverse reactions during the treatment duration was contrasted between the two cohorts of patients.
Follow-up: Both groups of patients were followed up for 1 y after completion of the treatment. Followup was conducted through phone calls or outpatient visits, with follow-up visits scheduled every 3 mo. The survival condition of patients during the followup period was documented, and the follow-up was finalized by June 2023.
Statistical analysis:
The data were subjected to analysis using Statistical Package for the Social Sciences (SPSS) 22.0 statistical software. Measurement data were displayed as (x̄ ±s). For comparisons between two groups, the independent-samples t-test was introduced, and the pared-samples t-test was harnessed for within-group comparisons. Enumeration data were represented as (%), and comparisons between groups were conducted using the χ2 test. Survival curves were generated through the Kaplan-Meier method, and the log-rank test was employed. When p<0.05, statistical significance was ascertained.
Results and Discussion
The ORR in the observation group amounted to 74.00 %, considerably exceeding the response rate observed in the control group, which stood at 54.00 %, as indicated by the χ2 test with p<0.05 as shown in Table 1.
| Group | Number of cases | Complete remission | Partial remission | Stable disease | Disease progression | ORR |
|---|---|---|---|---|---|---|
| Observation | 50 | 21 (42.00) | 12 (24.00) | 4 (8.00) | 13 (26.00) | 37 (74.00) |
| Control | 50 | 15 (30.00) | 9 (18.00) | 3 (6.00) | 23 (46.00) | 27 (54.00) |
| χ2 | 4.34 | |||||
| p | 0.037 |
Table 1: Comparison of Clinical Efficacy between the Two Groups [n (%)]
Before treatment, no statistically significant distinctions were observed in the levels of IgA, IgG, and IgM between the two cohorts (p>0.05). Nonetheless, subsequent to treatment, both groups experienced an elevation in IgA, IgG, and IgM levels, with the observation group displaying higher levels than the control group. p<0.05 was confirmed through the t-test as shown in Table 2.
| Group | Number of cases | IgA | IgG | IgM | |||
|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | ||
| Observation | 50 | 2.51±0.55 | 3.91±0.96* | 17.24±4.05 | 25.16±6.45* | 1.66±0.48 | 2.59±0.93* |
| Control g | 50 | 2.49±0.51 | 3.22±0.87* | 17.28±4.10 | 21.60±5.72* | 1.62±0.41 | 2.10±0.85* |
| t | 0.189 | 3.766 | 0.049 | 2.920 | 0.448 | 2.750 | |
| p | 0.851 | 0.000 | 0.961 | 0.004 | 0.655 | 0.007 | |
Note: *p<0.05 compared to pre-treatment levels
Table 2: Comparison of Immunoglobulin Levels before and after Treatment in the Two Groups [g/l, (x̄±s)]
Before commencing treatment, no significant differences were found in the levels of CEA, VEGF, and CA125 between the two groups (p>0.05). However, after the completion of treatment, both groups experienced a decline in CEA, VEGF, and CA125 levels, with the observation group showing lower levels compared to the control group. p<0.05 was ascertained via the t-test as shown in Table 3.
| Group | Number of cases | CEA (μg/l) | VEGF (pg/ml) | CA125 (kU/l) | |||
|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | ||
| Observation | 50 | 118.47±10.57 | 84.16±7.16* | 159.54±12.73 | 119.45±9.12* | 53.48±9.14 | 29.48±6.15* |
| Control | 50 | 118.21±10.43 | 95.12±8.18* | 158.98±12.45 | 132.05±10.26* | 54.02±9.38 | 36.12±7.05* |
| t | 0.124 | 7.129 | 0.222 | 6.49 | 0.292 | 5.019 | |
| p | 0.902 | 0.000 | 0.825 | 0.000 | 0.771 | 0.000 | |
Note: *p<0.05 compared to pre-treatment levels
Table 3: Comparison of Tumor Marker Levels before and after Treatment in the Two Groups (x̄±s)
The overall occurrence of adverse reactions in the observation group was 38.00 %, showing a marginal increase compared to the occurrence of 30.00 % in the control group. Nevertheless, the χ2 test demonstrated that p>0.05 as shown in Table 4.
| Group | Number of cases | Loss of appetite | Diarrhea | Rash | Fever | Fatigue | Joint pain | Urinary tract infection | Overall incidence |
|---|---|---|---|---|---|---|---|---|---|
| Observation | 50 | 5 (10.00) | 4 (8.00) | 3 (6.00) | 3 (6.00) | 1 (2.00) | 2 (4.00) | 1 (2.00) | 19 (38.00) |
| Control | 50 | 4 (8.00) | 3 (6.00) | 3 (6.00) | 2 (4.00) | 1 (2.00) | 1 (2.00) | 1 (2.00) | 15 (30.00) |
| χ2 | 0.713 | ||||||||
| p | 0.398 |
Table 4: Comparison of Adverse Reaction Incidence between the Two Groups [n (%)]
Following the conclusion of treatment, a 1 y followup was conducted for both cohorts of patients, and no instances of follow-up loss were encountered. Throughout the observation group’s follow-up duration, it was observed that 36.00 % (18/50) of the patients had passed away, resulting in a 1 y survival rate of 64.00 % (32/50). Within the control group, during the follow-up period, the mortality rate was recorded as 40.00 % (20/50), with a corresponding 1 y survival rate of 60.00 % (30/50). Kaplan-Meier survival analysis suggested no distinct differences in survival rates between the two groups (Log- Rank=0.018, p=0.893). The survival curves for both groups are presented in fig. 1.
NSCLC represents a predominant histological type among lung cancer cases, encompassing a considerable fraction of diagnosed lung cancer patients. Individuals afflicted with this cancer type are frequently detected at an advanced stage, rendering radical surgical intervention unattainable. Targeted therapy is a novel treatment approach that has been discovered and applied in clinical practice in recent years. It can specifically target and kill certain cancer cells without causing damage to other healthy tissues[14,15]. Bevacizumab is one of the targeted drugs clinically applied, which exerts anti-tumor effects by repressing VEGF generation. Clinical studies have unraveled the occurrence of PD-L1 overexpression in NS-NSCLC patients. In such cases, conventional anti-tumor therapies often fail to achieve satisfactory clinical outcomes. Therefore, it is essential to actively explore alternative effective treatment strategies for this patient group. In this retrospective research, we delved into the use of atezolizumab in combination with bevacizumab for the treatment of NS-NSCLC patients with PD-L1 overexpression.
The findings of this investigation substantiated that the clinical effectiveness in the observation group surpassed that in the control group, implying that the co-administration of atezolizumab and bevacizumab in NS-NSCLC patients with PD-L1 overexpression yielded more favorable therapeutic results. Bevacizumab, as a VEGF monoclonal antibody, efficaciously suppresses VEGF activity, attenuating neovascularization and tumor blood supply, thereby dampening tumor growth and exerting therapeutic effects[16,17]. Previous studies by Shi et al.[18], Yu et al.[19], and others have substantiated the therapeutic effects of bevacizumab in NS-NSCLC patients. Atezolizumab, on the other hand, belongs to PD-L1 inhibitors and is an immune therapy drug recently applied in clinical practice. Unlike conventional PD-1/PD-L1, atezolizumab not only blocks the binding of PD-1 and PD-L1 but also impedes the binding of PD-L1 and B7-1 (CD80), thereby strengthening the immune response[20,21]. It also activates the body’s immune system, enabling the immune system to attack tumor cells, leading to tumor cell-killing effects[22]. Studies by Finn et al.[23], Lee et al.[24], and others have also indicated that the combination of atezolizumab and bevacizumab is effective for patients with inoperable malignant tumors, and international scholars such as Seto et al.[25] and Provencio et al.[26] have confirmed the efficacy of this combination therapy for NS-NSCLC patients with PD-L1 overexpression. They believe that this treatment approach is a potential option for treating such patients, further corroborating the outcomes of this study. Additionally, this study observed the tumor markers CEA, VEGF, and CA125 commonly used in clinical practice and discovered that their levels in the observation group after treatment were remarkably lower than those before treatment and those in the control group subsequent to treatment. This denotes that the joint application of atezolizumab and bevacizumab can better suppress tumor growth and exert tumor cell-killing effects in NS-NSCLC patients. This may be due to that the two drugs exert their respective therapeutic effect through different pathways, supporting the aforementioned research findings.
The immune function plays a crucial part in the formation and development of malignant tumors. After the onset of the disease, tumor cells can exert an impact on the body’s normal immune function, leading to a weakening of the patient’s immune function. IgA, IgG, and IgM are essential indicators for evaluating immune function[27-29]. Therefore, in this research, the alterations in IgA, IgG, and IgM levels before and after treatment in both groups were observed. The results unveiled that the improvement in IgA, IgG, and IgM levels subsequent to treatment was more pronounced in the observation group compared to the control group, reflecting that the combination of atezolizumab and bevacizumab in treating NS-NSCLC with overexpressed PDL1 can enhance the immune function of patients. Regarding the more significant improvement in immune function observed in the observation group, several factors could account for this. Atezolizumab, as one of the immune therapy drugs, can ameliorate or even reverse the impairment and exhaustion of T cells. It exerts an activating effect on T cells, thereby eliminating the inhibitory impact of malignant tumors on immune function and overcoming the immune escape triggered by high PD-L1 expression, contributing to the eradication of tumor cells[30].
Furthermore, this study also conducted a statistical analysis of the adverse reactions and 1 y survival rates during the medication period in both groups. The outcomes confirmed that the safety and 1 y survival rates were comparable between the two groups, indicating that the combination of atezolizumab and bevacizumab in the treatment of NS-NSCLC with PD-L1 overexpression is well-tolerated and has a high level of safety. Rittmeyer et al.[31] also pointed out that atezolizumab treatment in lung cancer patients not only enhances their prognosis and survival but also displays good drug safety. Nonetheless, in this research, there was no considerable variance in 1 y survival rates between the two groups, which may be attributed to the relatively short follow-up period in this study or differences in the pathological types of lung cancer patients included in the research.
To summarize, the treatment with the combination of atezolizumab and bevacizumab for NS-NSCLC patients with high PD-L1 expression can improve the expression levels of CEA, VEGF, CA125, IgA, IgG, and IgM, bringing about favorable clinical treatment outcomes and a good safety profile. This innovative therapeutic modality has the potential to emerge as a novel alternative for managing patients afflicted with this specific medical condition.
Conflict of interests:
The authors declared no conflict of interests.
References
- Cui L, Qiu YN, Xu Y, Teng YO, Yu P, Liu Z. Effect and mechanism of aspirin combined with vinorelbine on non-small cell lung cancer. Zhongguo Zhong yao za zhi 2020;45(24):6012-9.
[Crossref] [Google Scholar] [PubMed]
- Chen J, Liu X, Zhang J, Huang Z, Zeng W, Hu J, et al. Frontline anti-PD-1/PD-L1 vs. bevacizumab in advanced non-small-cell lung cancer: A network meta-analysis. Future Oncol 2022;18(13):1651-64.
[Crossref] [Google Scholar] [PubMed]
- Bade BC, Cruz CS. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin Chest Med 2020;41(1):1-24.
[Crossref] [Google Scholar] [PubMed]
- Wu F, Wang L, Zhou C. Lung cancer in China: Current and prospect. Curr Opin Oncol 2021;33(1):40-6.
[Crossref] [Google Scholar] [PubMed]
- Herbst RS, Majem M, Barlesi F, Carcereny E, Chu Q, Monnet I, et al. COAST: An open-label, phase II, multidrug platform study of durvalumab alone or in combination with oleclumab or monalizumab in patients with unresectable, stage III non–small-cell lung cancer. J Clin Oncol 2022;40(29):3383-93.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Zeng L, Li Y, Xu Q, Yang H, Lizaso A, et al. Anlotinib combined with PD-1 blockade for the treatment of lung cancer: A real-world retrospective study in China. Cancer Immunol Immunother 2021;70(9):2517-28.
[Crossref] [Google Scholar] [PubMed]
- Akamatsu H, Toi Y, Hayashi H, Fujimoto D, Tachihara M, Furuya N, et al. Efficacy of osimertinib plus bevacizumab vs osimertinib in patients with EGFR T790M–mutated non–small cell lung cancer previously treated with epidermal growth factor receptor–tyrosine kinase inhibitor: West Japan oncology group 8715L phase 2 randomized clinical trial. JAMA Oncol 2021;7(3):386-94.
[Crossref] [Google Scholar] [PubMed]
- Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer 2023;22(1):1-37.
[Crossref] [Google Scholar] [PubMed]
- Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol 2021;32(9):1137-47.
[Crossref] [Google Scholar] [PubMed]
- Perol M, Felip E, Dafni U, Polito L, Pal N, Tsourti Z, et al. Effectiveness of PD-(L) 1 inhibitors alone or in combination with platinum-doublet chemotherapy in first-line (1L) non-squamous non-small-cell lung cancer (Nsq-NSCLC) with PD-L1-high expression using real-world data. Ann Oncol 2022;33(5):511-21.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Li C, Seery S, Yu J, Meng X. Identifying optimal first-line interventions for advanced non-small cell lung carcinoma according to PD-L1 expression: A systematic review and network meta-analysis. Oncoimmunology 2020;9(1):1746112.
[Crossref] [Google Scholar] [PubMed]
- Latimer KM, Mott TF. Lung cancer: Diagnosis, treatment principles, and screening. Am Family Phys 2015;91(4):250-6.
[Google Scholar] [PubMed]
- Therese P. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst 2000;92(3):205-16.
[Crossref] [Google Scholar] [PubMed]
- Han P, Zhou J, Xiang J, Liu Q, Sun K. Research progress on the therapeutic effect and mechanism of metformin for lung cancer. Oncol Rep 2023;49(1):1-21.
[Crossref] [Google Scholar] [PubMed]
- Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol 2021;16(11):1909-24.
[Crossref] [Google Scholar] [PubMed]
- Zhou Q, Xu CR, Cheng Y, Liu YP, Chen GY, Cui JW, et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study. Cancer Cell 2021;39(9):1279-91.
[Crossref] [Google Scholar] [PubMed]
- Andrini E, Lamberti G, Mazzoni F, Riccardi F, Bonetti A, Follador A, et al. A phase II, open-label, single-arm trial of carboplatin plus etoposide with bevacizumab and atezolizumab in patients with extended-stage small-cell lung cancer (CeLEBrATE study): Background, design and rationale. Future Oncol 2022;18(7):771-9.
[Crossref] [Google Scholar] [PubMed]
- Shi Y, Lei K, Jia Y, Ni B, He Z, Bi M, et al. Bevacizumab biosimilar LY01008 compared with bevacizumab (Avastin) as first‐line treatment for Chinese patients with unresectable, metastatic, or recurrent non‐squamous non–small‐cell lung cancer: A multicenter, randomized, double‐blinded, phase III trial. Cancer Commun 2021;41(9):889-903.
[Crossref] [Google Scholar] [PubMed]
- Yu H, Chen P, Xia L, Fu S, Chen C, Zhang X, et al. PD-1/PD-L1 inhibitor plus chemotherapy vs. bevacizumab plus chemotherapy in first-line treatment for non-squamous non-small-cell lung cancer. J Immunother Cancer 2021;9(11):e003431.
[Crossref] [Google Scholar] [PubMed]
- Tugnait M, Gupta N, Hanley MJ, Venkatakrishnan K, Sonnichsen D, Kerstein D, et al. The effect of a high‐fat meal on the pharmacokinetics of brigatinib, an oral anaplastic lymphoma kinase inhibitor, in healthy volunteers. Clin Pharmacol Drug Dev 2019;8(6):734-41.
[Crossref] [Google Scholar] [PubMed]
- Ahn HK, Sim SH, Suh KJ, Kim MH, Jeong JH, Kim JY, et al. Response rate and safety of a neoadjuvant pertuzumab, atezolizumab, docetaxel, and trastuzumab regimen for patients with ERBB2-positive stage II/III breast cancer: The neo-PATH phase 2 nonrandomized clinical trial. JAMA Oncol 2022;8(9):1271-7.
[Crossref] [Google Scholar] [PubMed]
- Negrao MV, Papadimitrakopoulou VA, Price AC, Tam AL, Furqan M, Laroia ST, et al. Vidutolimod in combination with atezolizumab with and without radiation therapy in patients with programmed cell death protein 1 or programmed death-ligand 1 blockade–resistant advanced NSCLC. JTO Clin Res Rep 2023;4(3):100423.
[Crossref] [Google Scholar] [PubMed]
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382(20):1894-905.
[Crossref] [Google Scholar] [PubMed]
- Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol 2020;21(6):808-20.
- Seto T, Nosaki K, Shimokawa M, Toyozawa R, Sugawara S, Hayashi H, et al. Phase II study of atezolizumab with bevacizumab for non-squamous non-small cell lung cancer with high PD-L1 expression (@ Be Study). J Immunother Cancer 2022;10(2).
[Crossref] [Google Scholar] [PubMed]
- Provencio M, Ortega AL, Coves-Sarto J, Calvo V, Marsé-Fabregat R, Domine M, et al. Atezolizumab plus bevacizumab as first-line treatment for patients with metastatic nonsquamous non–small cell lung cancer with high tumor mutation burden: A nonrandomized controlled trial. JAMA Oncol 2023;9(3):344-53.
[Crossref] [Google Scholar] [PubMed]
- Cheng R, Li Y, Su L, Wang L, Cao Y. Clinical effect of cold and heat ablation on patients with advanced lung cancer and its influence on immune function. Am J Transl Res 2023;15(4):2939.
[Google Scholar] [PubMed]
- Pan J, Yu L, Wu Q, Lin X, Liu S, Hu S, et al. Integration of IgA and IgG autoantigens improves performance of biomarker panels for early diagnosis of lung cancer. Mol Cell Proteomics 2020;19(3):490-500.
[Crossref] [Google Scholar] [PubMed]
- Yu L, Lin X, Zhang L, Wu Q, Zhang S, Chen D, et al. The combination of IgA and IgG autoantibodies against transcriptional intermediary factor-1γ contributes to the early diagnosis of lung cancer. Int J Med Sci 2020;17(11):1561.
[Crossref] [Google Scholar] [PubMed]
- Hecht JR, Raman SS, Chan A, Kalinsky K, Baurain JF, Jimenez MM, et al. Phase Ib study of talimogene laherparepvec in combination with atezolizumab in patients with triple negative breast cancer and colorectal cancer with liver metastases. ESMO Open 2023;8(2):100884.
[Crossref] [Google Scholar] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, Von Pawel J, et al. Atezolizumab vs. docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389(10066):255-65.
[Crossref] [Google Scholar] [PubMed]
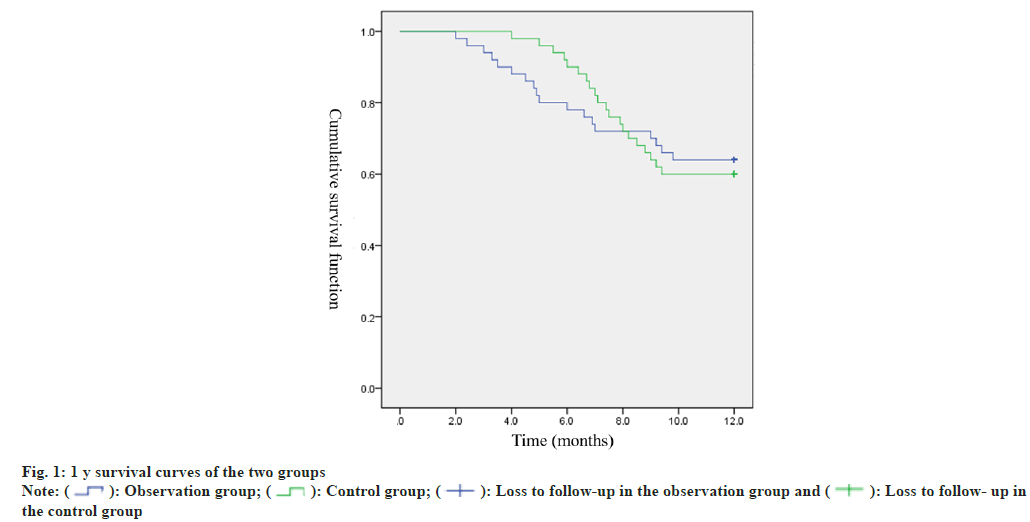
 : Observation group;
: Observation group;  Loss to follow-up in the observation group and
Loss to follow-up in the observation group and  : Loss to follow- up in
the control group
: Loss to follow- up in
the control group



