- *Corresponding Author:
- Qun ChenDepartment of Pharmacology, Ningbo College of Health Sciences, No. 51, Xuefu Road, Ningbo, Zhejiang 315100, ChinaE-mail: sunnyxq@zju.edu.cn
| This article was originally published in a special issue, “Therapeutic Perspectives in Biomedical Research and Pharmaceutical Sciences and their Nursing Methods” |
| Indian J Pharm Sci 2021:83(4)Spl issue “127-132” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Depression is a common mental disorder affecting approximately 17 % of the world population. Current therapeutic options are suboptimal due to a lower efficacy and high rates of side effects. Traditional Chinese medicine such as Radix Polygalae has been applied to depressed patients with encouraging outcomes. The main ingredient extracted from Radix Polygalae is onjisaponin B. The aim of this study was to investigate the therapeutic effects of onjisaponin B in experimental depression in mice. 30 male C57/Bl6 mice aged between 6 and 8 w old (25-30 g upon arrival) were purchased from Animal Centre of Shanghai Branch, Chinese Academy of Sciences (Shanghai, China) in this study and inducted to depression mice model. Tail suspension test, forced swimming test, measurement of monoamines and monoamine oxidase methods were used to detect the function of onjisaponin B in depression mice model. Our data showed that in depression mice model, onjisaponin B attenuates the severity of experimental depression. Onjisaponin B inhibits activity of monoamine oxidase A and increases the abundance of 5-hydroxytryptamine and noradrenaline in the brain. These preclinical results indicated the application of onjisaponin B in depressed mice and provided a new potential treatment strategy for patients with clinical depression.
Keywords
Depression, mice, major depressive disorder, onjisaponin B, monoamine oxidase
Depression is devastating mental disorder that affects approximately 17 % of the population worldwide[1,2], which is a heavy burden on those affected and the society as a whole. Depression is usually associated with sleep disorders, eating disorders, anxiety and other disorders[3]. Major depressive disorder (MDD) is characterized as the persistent depressed mood for longer than 2 w or the loss of pleasure or interest in usually enjoyable activities[4], which has a negative influence on the society. Currently, administration of antidepressants, such as serotonin selective reuptake inhibitors (SSRIs), tricyclic antidepressant (TCAs), noradrenaline reuptake inhibitors (NRIs), serotonin and noradrenaline reuptake inhibitors (SNRIs) and monoamine oxidase inhibitors (MAOIs), has been widely applied to depressed patients[5]. However, SSRIs often take weeks even months to perform therapeutic roles with unwanted side effects[6]. Therefore, it is important to develop novel therapeutic options for patients with depression.
The precise pathogenesis of depression has yet to be fully understood, which may include genetic and environmental factors[7]. In addition, monoamine neuromodulators play an important role in the process, as depression caused by monoamine depletion was observed in hypertension patients treated with reserpine in the 1960s[8]. Recently, monoamine neuromodulators have been shown to have three core effects in emotion regulation: Dopamine-reward, Norepinephrine-stress and serotonin-punishment[9,10]. Thus, modulation of monoamines is a major therapeutic means and target. Monoamine oxidase (MAO) is a major enzyme that degrades monoamine transmitters, including 5-hydroxytryptamine (5-HT), noradrenaline (NA, also known as norepinephrine) and dopamine (DA)[10]. MAO is located at the mitochondrial outer membrane, which has two isotypes: MAO-A and MAO-B[11]. MAO-A oxidizes serotonin or 5-HT as well as NA, whereas MAO-B degrades β-phenylethylamine[12].
Traditional Chinese medicinal herbs, including Radix Polygalae, have been applied for emotion disorders and neurological diseases for years. Onjisaponin B is the major active ingredient of Radix Polygalae. It has been shown that onjisaponin B is protective against a mouse model of Parkinson’s disease[13]. However, whether onjisaponin B is the effective ingredient that attenuates depressive symptoms in patients remains unknown. In this study, we tested our hypothesis that onjisaponin B could attenuate preclinical depression in mice by modulating the concentrations of monoamines. Here we report that onjisaponin B is protective against experimental depression by increasing the abundance of 5-HT and NA, but not DA, in the brain of mice.
Materials and Methods
Animals:
The ethics committee of Ningbo College of Health and Science, Ningbo, Zhejiang Province, China approved and supervised this animal protocol in line with the National Institutes of Health (NIH) guide for the care and use of laboratory animals. Male C57/Bl6 mice, aged between 6 and 8 w old (25-30 g upon arrival) were purchased from controlled specific pathogen-free environment, with fixed temperature (23°), humidity (55 %) and Animal Centre of Shanghai Branch, Chinese Academy of Sciences (Shanghai, China). Mice were kept in a 12 h cycles of light/dark. Mice were allowed to have water and standard lab chow ad libitum.
Induction of experimental depression:
Reserpine (purchased from Sigma-Aldrich, St Louis, MO, USA) was dissolved in the vehicle (phosphatebuffered saline (PBS) containing 0.1 % dimethyl sulfoxide (DMSO) and 0.3 % Tween-80) to a final concentration of 1 mg/kg. Reserpine was administered to mice intraperitoneally with a dose of 1 mg/kg/d for 3 consecutive days, according to a published paper[14].
Administration of Onjisaponin B:
Onjisaponin B (fig. 1) was dissolved in vehicle (50 % polyethylene glycol-40 (PEG40) in autoclaved doubledistilled water (ddH2O))[15]. Onjisaponin B solution was administered to mice with different dosages via oral gavage once a 14 d for consecutive days. At the end of treatment with onjisaponin B, mice were assessed for the severity of experimental depression.
Tail suspension test (TST):
TST is commonly used to measure a medication’s effect in mouse models of depression[2,16]. All tests were performed in an isolated room without any acoustical or visual interference. Mice were suspended 50 cm above the floor with a piece of tape about 1 cm long from the tail tip. The total time of immobility in the last 4 min of the 6 min test period was recorded and analyzed by using software Any-maze (Stoeling Co. Ltd., IL, USA).
Forced swimming test (FST):
FST is also frequently used in assessment of preclinical depression in mice[2,16]. A clear glass jar with a dimension of 20×20×40 cm was used for FST. Water in the jar was set at 30 cm deep with temperature of 22°. A mouse was removed from the cage and place alone in the jar for swimming for 6 min. In the end, the animal was removed from water, dried with paper towels and put back to the cage. Total immobility time was measured during the last 4 min of 6 min swimming time by using software Any-maze.
Measurement of monoamines:
Mice were sacrificed by cervical dislocation. The brain was rapidly removed and immediately put into liquid nitrogen. The frontal cortex and hippocampus were dissected on an ice-cold petri dish. Tissues were weighted and stored at -80° until assessments.
We tested the amount of 5-HT, NA and DA in the above mentioned brain tissues using high-performance liquid chromatography (HPLC)[17]. Briefly, frozen tissues were homogenized in 200 μl of 0.4 M solution A, (perchloric acid). The sample was placed on ice for 1 h and then centrifuged at 2400 g at 4° for 20 min. The supernatant was collected, 160 μl of which was added to 80 μl solution B, 0.2 M potassium citrate, 0.3 M dipotassium hydrogen phosphate and 0.2 M ethylenediaminetetraacetic acid (EDTA)). A total of 20 μl of solution was injected into an HPLC system (ESA CoulArray, MA, USA). The flow rate was set to 1.0 ml/ min. Levels of monoamine were normalized as ng/g tissue.
Monoamine oxidase (MAO) assay:
MAO activity in frozen whole brain tissue was measured according to previous publications[18,19]. Briefly, frozen brain tissues were suspended in ten volumes of cold sodium phosphate buffer (200 mM, pH=7.4) containing 320 mM sucrose and homogenized. Nucleus and cell debris were removed by centrifugation at 600 g for 10 min at 4°. The fraction containing mitochondria was achieved by centrifugation at 15 000 g for 30 min at 4°. Protein concentration was measured by the Lowry method. The protein concentration of the mitochondrial fraction was diluted to 1 mg/ml. Activity of MAO-A or MAO-B was measured as the ability to catalyze 5-HT or β-phenylethylamine, respectively.
The organic phase of mixtures was collected and the absorbance at 280 nm for MAO-A and 242 nm for MAO-B was recorded. Activity of MAO was normalized as nmol/mg protein/h.
Statistical analysis:
Data were presented as mean±SEM (standard error of means). Comparisons among more than two groups were performed using one-way Analysis of variance (ANOVA) tests with Bonferroni post-hoc tests. Statistical analysis was conducted using GraphPad Prism 5.0 (GraphPad Software Inc., CA, USA), p value of less than 0.05 was considered as statistical significance.
Results and Discussion
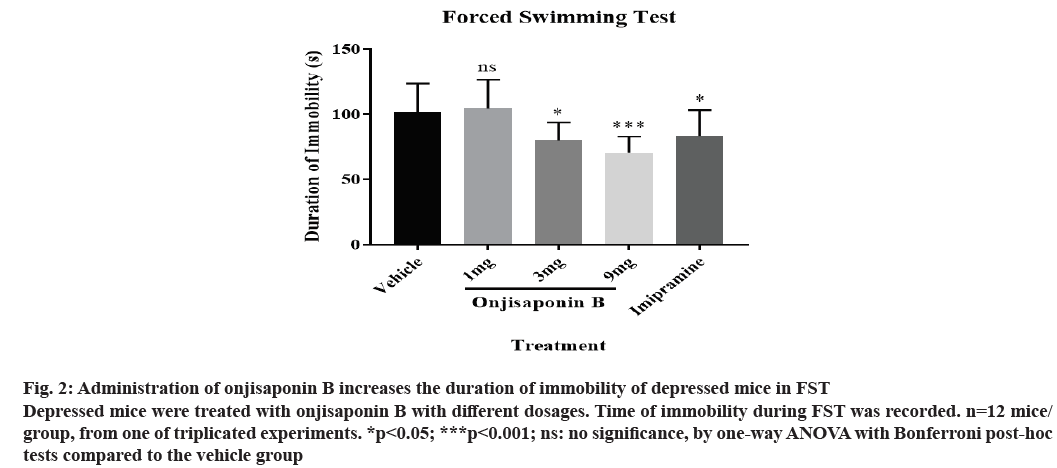
Administration of onjisaponin B shortens the immobility time in FST. We induced the preclinical depression model in mice by injecting reserpine. Depressed mice were treated with various dosages of onjisaponin B. The severity of depression was measured by FST. As shown in fig. 2, depressed mice treated with onjisaponin B showed reduced time of immobility with a dosedependent effect. The most significant effect was seen in those treated with 9 mg onjisaponin B.
Figure 2: Administration of onjisaponin B increases the duration of immobility of depressed mice in FST Depressed mice were treated with onjisaponin B with different dosages. Time of immobility during FST was recorded. n=12 mice/ group, from one of triplicated experiments. *p<0.05; ***p<0.001; ns: no significance, by one-way ANOVA with Bonferroni post-hoc tests compared to the vehicle group
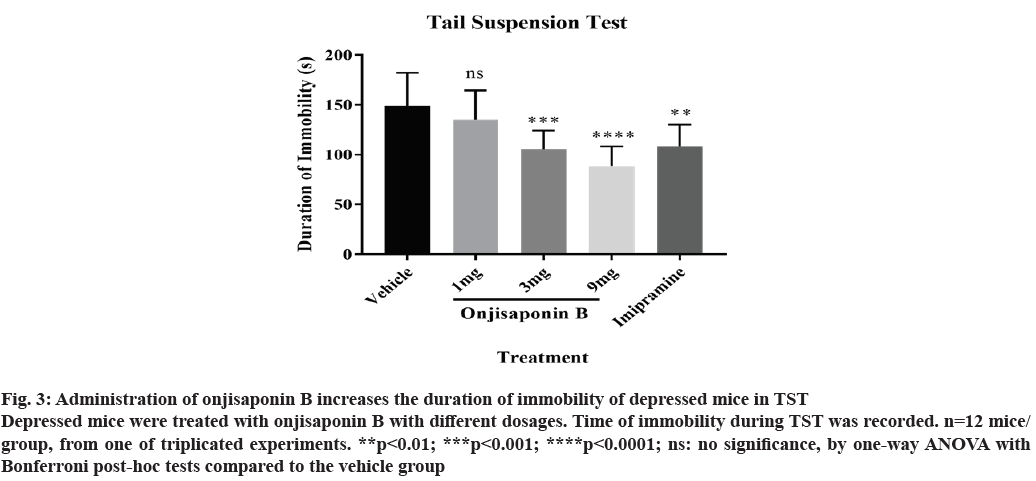
TST was also utilized to measure the effects of onjisaponin B in depressed mice. As demonstrated in fig. 3, treatment with onjisaponin B reduces the time of immobility in TST, indicating an attenuated severity of depression. It is worth noting that effects of onjisaponin B in the immobility time during TST are also dosagedependent, where the most significant effect is seen in those treated with 9 mg onjisaponin B.
Figure 3: Administration of onjisaponin B increases the duration of immobility of depressed mice in TST Depressed mice were treated with onjisaponin B with different dosages. Time of immobility during TST was recorded. n=12 mice/ group, from one of triplicated experiments. **p<0.01; ***p<0.001; ****p<0.0001; ns: no significance, by one-way ANOVA with Bonferroni post-hoc tests compared to the vehicle group
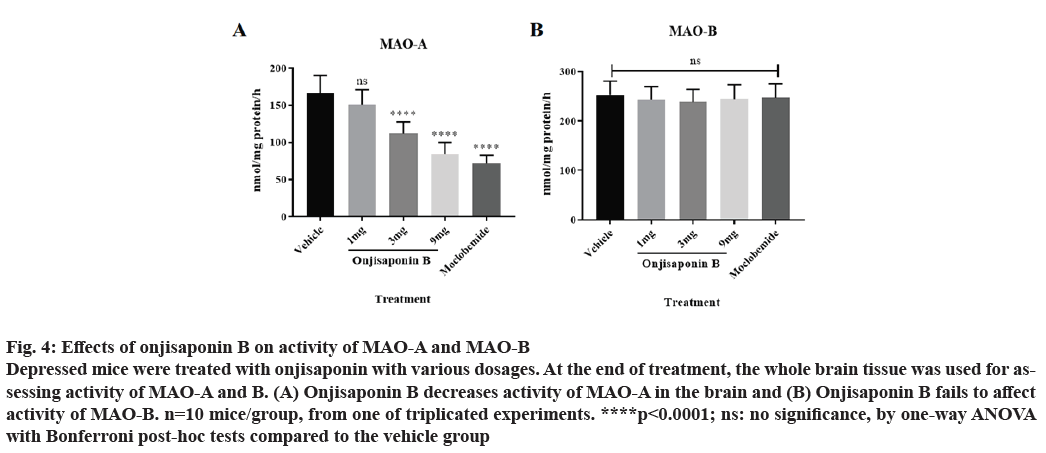
Depressed mice were treated with different dosages of onjisaponin B. By the end of treatment the whole brain tissue was collected for assessing activity of MAO-A and MAO-B. As shown in fig. 4A, treatment with onjisaponin B reduces activity of MAO-A. However, onjisaponin B has no effect on activity of MAO-B (fig. 4B).
Figure 4: Effects of onjisaponin B on activity of MAO-A and MAO-B Depressed mice were treated with onjisaponin with various dosages. At the end of treatment, the whole brain tissue was used for assessing activity of MAO-A and B. (A) Onjisaponin B decreases activity of MAO-A in the brain and (B) Onjisaponin B fails to affect activity of MAO-B. n=10 mice/group, from one of triplicated experiments. ****p<0.0001; ns: no significance, by one-way ANOVA with Bonferroni post-hoc tests compared to the vehicle group
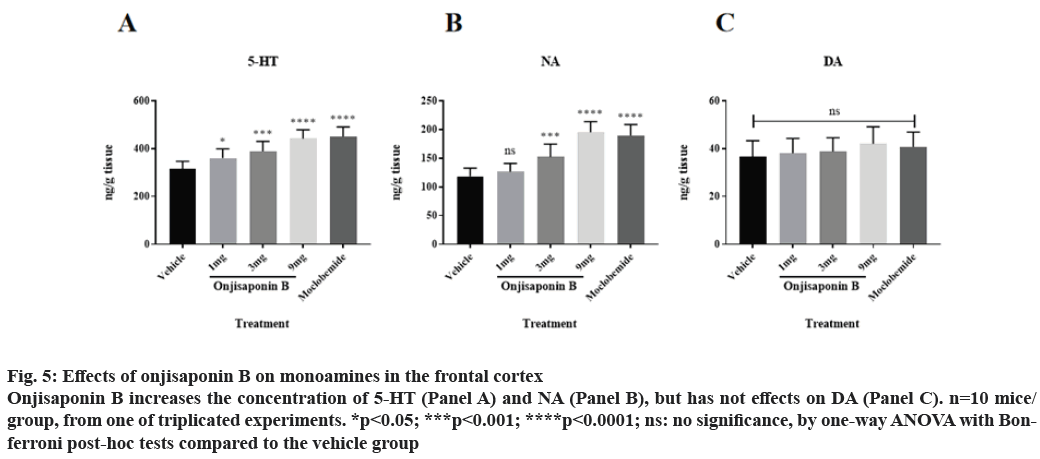
Onjisaponin B increases the abundance of 5-HT and NA, but not DA, in the frontal cortex. Subsequently, we checked the concentrations of the substrates of MAO in the frontal cortex of depressed mice treated with onjisaponin B, as onjisaponin B decreases activity of MAO-A. As shown in fig. 5A and fig. 5B, administration of onjisaponin B increases the concentrations of 5-HT and NA in the frontal cortex with a dose-dependent manner. It is worth noting that onjisaponin B does not alter the concentration of DA (fig. 5C).
Figure 5: Effects of onjisaponin B on monoamines in the frontal cortex Onjisaponin B increases the concentration of 5-HT (Panel A) and NA (Panel B), but has not effects on DA (Panel C). n=10 mice/ group, from one of triplicated experiments. *p<0.05; ***p<0.001; ****p<0.0001; ns: no significance, by one-way ANOVA with Bonferroni post-hoc tests compared to the vehicle group
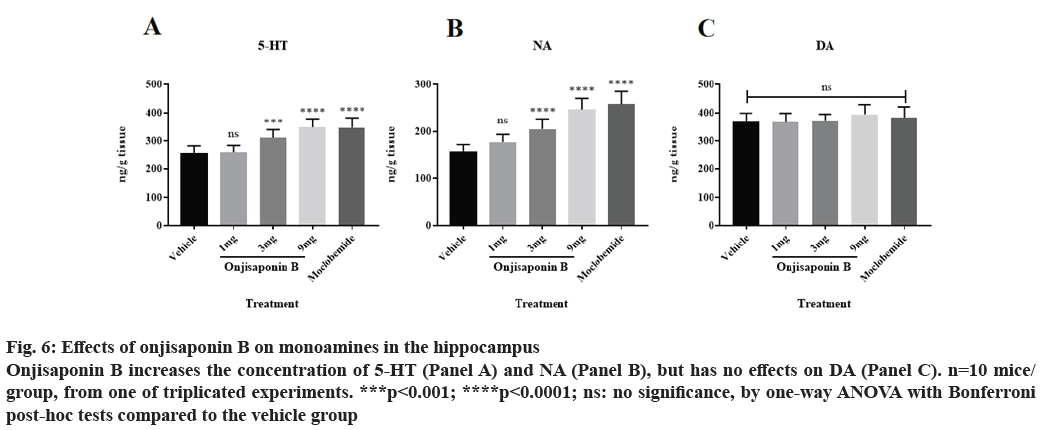
Onjisaponin B increases the abundance of 5-HT and NA, but not DA, in the hippocampus. Finally, we assessed the concentrations of 5-HT, NA and DA in the hippocampus using the same methods of fig. 5. As demonstrated in fig. 6A and fig. 6B, application of onjisaponin B increases the concentrations of 5-HT and NA in the hippocampus of mice with preclinical depression with a dose-dependent manner. However, onjisaponin does not increase the concentration of DA (fig. 6C).
Figure 6: Effects of onjisaponin B on monoamines in the hippocampus Onjisaponin B increases the concentration of 5-HT (Panel A) and NA (Panel B), but has no effects on DA (Panel C). n=10 mice/ group, from one of triplicated experiments. ***p<0.001; ****p<0.0001; ns: no significance, by one-way ANOVA with Bonferroni post-hoc tests compared to the vehicle group
In this study, we demonstrate that: administration of onjisaponin B attenuates experimental depression in mice measured by immobility time during FST and TST; treatment with onjisaponin B decreases activity of MAO-A, but not MAO-B and supplement of onjisaponin B increases the abundance of 5-HT and NA in the brain tissue.
Traditional Chinese medicinal herbs have been widely used to treat emotion disorders as well as neurological diseases. For a long time, however, the exact ingredient that mediates pharmacological effects in herbs and the precise mechanisms are poorly documented. Radix Polygalae has been used in the formulas that treat depression, although clinical evidence is still warranted for the efficacy and safety (reviewed by Zhao et al.[20]). Onjisaponin B is the major active ingredient extracted from Radix Polygalae. It has given the encouraging results from clinical observations in traditional Chinese medicine, it is worth exploring the effects of onjisaponin B in preclinical models of depression in order to document its mechanisms for the future applications.
Onjisaponin B has been shown to be effective in protection against Parkinson’s disease and other cognitive impairment in rodents[13,15,21], suggesting its effects occur in the central nervous system. For the best of our knowledge, the current paper is the first to show the protective effects of onjisaponin B in mice with depression. These data encourage the further application of onjisaponin B in clinical applications in patients with depression, with support of investigations in mechanisms in the near future.
In this study, we demonstrate that application of onjisaponin B decreases activity of MAO-A in the brain tissue to increase the abundance of substrates of MAO-A including 5-HT and NA, which play an essential role in alleviating depression. However, how exactly onjisaponin B acts on MAO-A remains unclear. It is possible that onjisaponin B works as a MAO-A inhibitor to decrease the enzyme’s activity. It is also possible that onjisaponin B has a direct effect on production of MAO-A in mitochondria. Future investigations regarding these areas are required before early-stage clinical trials.
Onjisaponin B attenuates experimental depression in mice. The protection of onjisaponin B might be mediated by reducing activity of MAO-A to increase levels of 5-HT and NA in the brain tissue. Applications of onjisaponin B in depression patients are expected in the near future.
Acknowledgements:
This work was supported by the Ningbo College of Health & Science. Gao Wen Li and Ling Zhang are contributed equally to this work, Dong Ying Tao and Qun Chen are considered as co-corresponding authors.
Conflicts of interest:
The authors report no conflicts of interest.
References
- Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 2018;554:317-22.
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010;329:959-64.
- Kok RM, Reynolds CF. Management of depression in older adults: a review. JAMA 2017;317(20):2114-22.
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289(23):3095-105.
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function Nat Rev Neurosci 2003;4(1):13-25.
- Joshi A. Selective serotonin re-uptake inhibitors: an overview. Psychiatr Danub 2018;30(7):605-9.
- Hidaka BH. Depression as a disease of modernity: explanations for increasing prevalence. J Affect Disord 2012;140(3):205-14.
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry 1965;122(5):509-22.
- Gu S, Wang F, Cao C, Wu E, Tang YY, Huang JH. An integrative way for studying neural basis of basic emotions with fMRI. Front Neurosci 2019;13:628.
- Wang F, Yang J, Pan F, Bourgeois JA, Huang JH. Early life stress and depression. Front Psychiatry 2020;10:964.
- Xu Q, Jiang M, Gu S, Wang F, Yuan B. Early Life Stress Induced DNA Methylation of Monoamine Oxidases Leads to Depressive-Like Behavior. Front Cell Dev Biol 2020;8:920.
- Grimsby J, Toth M, Chen K, Kumazawa T, Klaidman L, Adams JD, et al. Increased stress response and β–phenylethylamine in MAOB–deficient mice. Nat Genet 1997;17(2):206-10.
- Peng F, Lu L, Wei F, Wu D, Wang K, Tang J. The onjisaponin B metabolite tenuifolin ameliorates dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. Neuroreport. 2020;31(6):456-65.
- Park BK, Kim NS, Kim YR, Yang C, Jung IC, Jang IS, et al. Antidepressant and Anti-Neuroinflammatory Effects of Bangpungtongsung-San. Front Pharmacol 2020;11:958.
- Li X, Cui J, Yu Y, Li W, Hou Y, Wang X, et al. Traditional Chinese nootropic medicine radix polygalae and its active constituent onjisaponin B reduce β-amyloid production and improve cognitive impairments. PLoS One 2016;11(3):e0151147.
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011;475(7354):91-5.
- Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, et al. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav 2005;82(1):200-6.
- Min CH, You-Ping LU, Yan-Lin ZO, Ling-Hu LA, Dao-Feng CH. Heteroclitins RS: new dibenzocylooctadiene lignans from Kadsura heteroclita. Chin J Nat Med 2014;12(9):689-92.
- Yu ZF, Kong LD, Chen Y. Antidepressant activity of aqueous extracts of Curcuma longa in mice. J Ethnopharmacol 2002;83(1-2):161-5.
- Zhao X, Cui Y, Wu P, Zhao P, Zhou Q, Zhang Z, et al. Polygalae Radix: A review of its traditional uses, phytochemistry, pharmacology, toxicology, and pharmacokinetics. Fitoterapia 2020:104759.
- Li X, Sun Y, Wei Y, Zhou L, Liu L, Yin P, et al. Onjisaponin B (OB) is neuroprotective during cognitive loss through immune-mediated and SIRT1 pathways. Curr Neurovasc Res 2018;15(2):94-102.