- *Corresponding Author:
- K. S. Santh
Department of Zoology, Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore, Tamil Nadu 641043, India
E-mail: dr.santhyanandan@gmail.com
| Date of Received | 28 November 2020 |
| Date of Revision | 12 January 2023 |
| Date of Acceptance | 04 April 2023 |
| Indian J Pharm Sci 2023;85(2):501-510 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Hygrophila auriculata is a thorny sub-shrub of the family Acanthaceae. It is a potent medicinal plant in the Indian systems of medicine with various therapeutic uses, from simple management of infections to treatment of diseases. The present study was conducted to investigate the phytochemical and pharmacological properties of the leaves of Hygrophila auriculata. Various phytochemicals were screened using a standard procedure, and the characterization was analyzed with spectral studies. The pharmacological investigation includes antioxidant and antimicrobial activities of the extract. Antioxidant activity was carried out using 2, 2-diphenylpicrylhydrazyl free radical scavenging assay and reducing power assay. Antimicrobial activity was done by the agar well diffusion method. The phytochemical screening detected the presence of alkaloids, flavonoids, terpenoids, tannins, carbohydrates and saponins. The 2,2-diphenylpicrylhydrazyl antioxidant assay reveals that the extract had a considerable scavenging capacity. It was found that the reducing power of different concentrations of the leaf extract was remarkable. Antimicrobial tests showed that the leaf extract was active against selected Gram-positive bacteria (Bacillus subtilis, Staphylococcus aureus) and few fungal species (Aspergillus sp.). These findings disclosed that the Hygrophila auriculata leaves have various phytotherapeutic activities and the compounds identified using the gas chromatography were responsible for the above actions. Thus, it would be helpful to isolate the compounds responsible for the bioactivities in the future for the treatment of various ailments.
Keywords
Hygrophila auriculata, phytochemicals, antioxidant, antimicrobial
Millennia-old Traditional Medicines (TMs) offer empirical practices, including medication with natural compounds and focusing on overall wellness. The acceptance of TM by the scientific community is limited by the lack of ground-breaking scientific evidence of its benefits and efficiency, coupled with the ignorance of its inherent medical basis. Nearly 80 % of the world’s population relies on TMs for primary health care, most involving plant extracts. This has resulted in using many medicinal plants with curative properties to treat various diseases[1].
Indian medicinal plants have contributed much to academic curiosity, as apparent from the number of publications. Yet, the main reason for the scientific abandonment is unclear evidence for their mechanism of action. But at times, it has been recommended that drug discovery should not permanently be restricted to a single molecule and the existing statement ‘one disease-one drug’ method might be indefensible. The rationally synthesized polyherbal formulations could also be evaluated as an alternative in multitarget therapeutics and prevention[2].
The plants prevent the consequences of the oxidative stress-derived compounds with the assistance of essential substances known as antioxidants. The role of medicinal plants in disease prevention has been attributed to the antioxidant properties of their constituents. Therefore, the isolation of natural antioxidants mainly of plant origin has increased immensely during the last decade. The modes of action for curing diseases vary from plant to plant. The mixture form may exhibit enhanced activity than individual plants, known as synergistic action[3].
The number of multi-drug resistant microbial strains and the appearance of strains with reduced susceptibility to antibiotics is continuously increasing[4]. Antimicrobial compounds from potential plants should be explored to alleviate this problem. The side effects of these drugs are scanty, less toxic and cost-effective. They effectively treat infectious diseases while mitigating many side effects of synthetic antimicrobials[5]. Hence, in the present study, the same efforts are continued in searching for novel therapeutics.
Hygrophila auriculata (H. auriculata) (Buch. -Ham) is a thorny sub-shrub of the family Acanthaceae that grows widely throughout India, Sri Lanka, Myanmar, Indo-China, Tropical Africa and Malaya. It grows naturally in moist places on the banks of ditches and paddy fields. In Ayurvedic literature, it was described as Ikshura, Ikshugandha, Kokilaksha, or Indian Cuckoo, and in the Ayurvedic system of medicine as Seethaveryam and Mathuravipaka. Leaves of this shrub are whorled, linear-lanceolate, with undulating margins. Traditionally, the leaves were used as/in diuretic, jaundice, antibacterial, dropsy, rheumatism, diseases of the urinogenital tract, aphrodisiac, hypnotic, diarrhea, dysentery, urinary calculi, urinary discharge, anti-inflammatory, joint pain, eye diseases, anemia, anuria, cough, stomachic, lumbago, arthritis, gastric disorder and leucorrhea[6].
With the above context, the present investigation aimed to evaluate H. auriculata for its potential use as a source of phytochemicals, antioxidant compounds and other biological agents, including those which may show antimicrobial activities.
Materials and Methods
Plant collection and preparation of extract:
The fresh plant of H. auriculata was collected from Kuttanad wetlands, Alappuzha (Dist.), Kerala. The plant material was identified, and its authenticity was confirmed at the herbarium of Botanical Survey of India, Southern Regional Centre, Coimbatore, Tamil Nadu (BSI/SRC/5/23/2015/Tech-1422).
The fresh leaves were cleaned and dried for 5-7 d in the shade at room temperature. The dried samples were then powdered using an electric grinder and stored in screw cap bottles until further analysis. Ten grams of powder from the dried sample of H. auriculata leaves were taken, to which 100 ml of different solvents (chloroform, ethyl acetate, methanol, and water) were added, mixed and kept for 4 d. They were intermittently shaken with an electrical shaker and further concentrated by evaporation.
Qualitative phytochemical analysis:
As the extractive index and the intensity of phytochemicals were shown to be high in the methanol extract of H. auriculata, the methanol extract was subjected to various spectral analyses to identify the chemical nature of the active components present in the plant.
Chemical characterization:
As the extractive index and the intensity of phytochemicals were shown high in methanol extract of H. auriculata, methanol extract was subjected to various spectral analyses to identify the chemical nature of the active components present in the plant.
Ultra Violet (UV)-visible Spectral analysis:
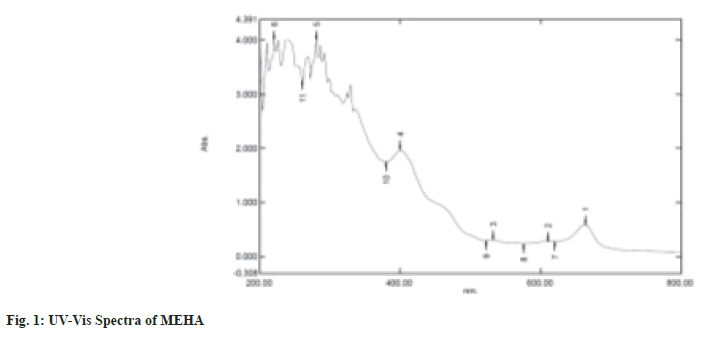
UV-Visible spectrum profile of the sample was detected with a Shimadzu UV-1700 spectrophotometer at a wavelength ranging from 200-800 nm (fig. 1).
Fourier Transform Infrared (FT-IR) spectral analysis:
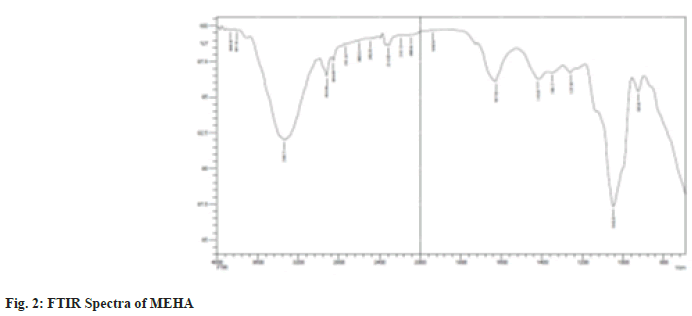
The powdered sample was loaded in an FTIR spectroscope (Shimadzu, IR AFFINITY-1, Japan), with a scan range from 800-4000 cm-1. The peaks obtained were plotted as percentage transmittance on the X-axis and wavenumber (cm-1) in the Y-axis (fig. 2).
X-Ray diffraction analysis:
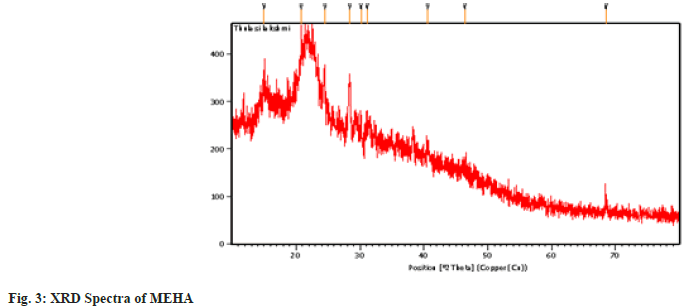
The X-ray diffraction pattern of the powder samples was recorded on a PANalytical X’PERT PRO X-ray diffractometer using Cu K-alpha radiation (λ=1.54060 Å). The particle characterization was done by measuring the crystallite size of the sample from the line broadening analyses using the Debey Schherer formula after accounting for instrumental broadening (fig. 3).
Gas Chromatography–Mass Spectrometry (GCMS) analysis:
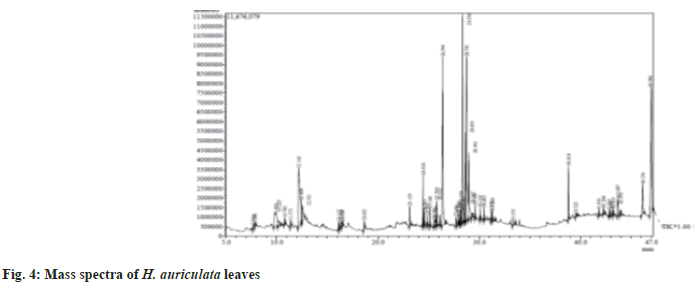
Chromatographic analysis was conducted using thermo GC-Trace Ultra Ver: 5.0 GC-MS (Model Thermo MS DSQ II gas chromatograph). The GC temperature program was as follows; the initial temperature was 75°, held for 2 min, increased to 150° at a rate of 2°/min, then to 220° at a rate of 3°/ min, and finally to 260° at a rate of 6°/min and held for 10 min. The split ratio was 1:12, the injection temperature was 250°, the transfer line temperature was 270° and the mass spectrometer was operated at 70 eV in a run time of 29 min. The individual components were identified by computerized matching of their mass spectra of peaks with those gathered in the NIST 08 and WILEY 8-Mass Spectral library of the GC-MS data software system (fig. 4).
Antioxidant studies:
2,2-Diphenyl-1-Picryl-Hydrazyl-Hydrate (DPPH) assay[8]: The diluted working solutions of the test extracts were prepared in methanol. About 3 ml of graded concentration (10-50 μg/ml) of extracts were taken in different test tubes. 1 ml of 0.3 mM DPPH methanol solution was added to these test tubes and shaken vigorously. Methanol served as the blank, and DPPH in methanol, without the leaf extracts, was the negative control, whereas ascorbic acid was a positive control. After 30 min incubation of samples at 25° in the dark, the absorption was measured at 517 nm. The change in absorbance of each sample was determined and the following equation calculated the scavenging activity.
Scavenging activity (%)=(Abscontrol–Abssample)/ (Abscontrol)×100
Where Abscontrol is the absorbance of DPPH radical with methanol and Abssample is the absorbance of DPPH radical with sample extract.
Reducing power assay[9]: Reaction mixtures were prepared by adding 2.5 ml of phosphate buffer (0.2 M, pH 6.6), 2.5 ml potassium ferricyanide (1 %), and varying concentrations of extracts (10-50 μg/ml). After the reaction, mixtures were incubated at 50° in the water bath for 30 min, cooled at room temperature (28°), and 2.5 ml of 10 % Trichloroacetic Acid (TCA) were added to each reaction mixture and then centrifuged at 2000 rpm for 10 min. The supernatant (2.5 ml) was separated in the test tube, and 2.5 ml of distilled water was added. This was further added with 0.5 ml Ferric chloride (FeCl3) (1.0 %) and allowed to react for 10 min at room temperature. Ascorbic acid served as the control. The absorbance was measured at 700 nm.
Antimicrobial activity: The antimicrobial sensitivity assay was carried out against six bacterial strains (Bacillus subtilis (B. subtilis), Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), Klebsiella sp., Salmonella typhi (S. typhi) and Proteus sp.) and four fungal strains Aspergillus fumigatus (A. fumigatus), Aspergillus niger (A. niger), Aspergillus flavus (A. flavus) and Trichoderma sp. by the agar well diffusion method by Barry et al.[10]. Petri plates containing 20 ml Muller-Hinton medium were swabbed with the 24 h bacterial culture and similarly, the fungal cultures to be tested were evenly spread over Rose Bengal chloramphenicol agar plates. The wells were bored into the plate. Each well was filled with different doses of methanolic extract of H. auriculata (10, 15, 20, 25 and 30 μg/ml) and the last well with standard antibiotics (Chloramphenicol for bacteria and nystatin for fungi). The bacterial plates were subjected to incubation at 37° for 24 to 48 h and the fungal plates were kept at room temperature for 3-5 d to assess the fungal activity. After incubation, the results were observed and the zones of inhibition thus developed were measured with the scale to the nearest in mm. The experiment was done in triplicates and the mean values were presented.
Results and Discussion
Therapeutic properties of the herbs are closely related to their chemical components, which are classified into some major groups like alkaloids, acids, essential oils, steroids, saponins and tannins, where the effectiveness of those as herbal remedies depends upon their solubility in various solvents[11]. The present study was designed to document the pharmacological properties of H. auriculata in association with its phytocompounds. In the present investigation, among the four extracts analyzed, the methanolic extract showed the maximum concentration of phytochemicals such as alkaloids, flavonoids, terpenoids, phenols, tannins, carbohydrates, cardiac glycosides and saponins as represented in Table 1. These attributes in utilizing the Methanol Extract of H. auriculata (MEHA) for further analysis. Alkaloids were absent in the aqueous extract but present in all the other three extracts. The absence of alkaloids in the aqueous extract might be due to the relative insolubility of alkaloids in water compared to organic solvents[12]. These results corroborate with Rastogi et al.[13] who reported high intensity of phytochemicals in the MEHA. Further, many previous reports reveal the presence of specific isolated compounds such as flavonoids, alkaloids, triterpenes, sterols, and other compounds that belong to minerals, aliphatic esters, amino acids, fatty acids, and essential oils[14]. Also, the results reveal the occurrence of cardiac glycosides which might prevent the pump of Na+/K+, consequently raising the availability of calcium ions for the heart muscles contraction, reducing congestive heart failure, cardiac arrhythmia and cardiac arrhythmia and distension of the hear[15].
| S. no. | Constituents | Solvents | ||||
|---|---|---|---|---|---|---|
| Chloroform | Ethyl acetate | Methanol | Aqueous | |||
| 1 | Alkaloids | + | + | + | - | |
| 2 | Flavonoids | + | + | + | + | |
| 3 | Sterols | - | - | - | + | |
| 4 | Phenols | - | - | + | - | |
| 5 | Saponins | - | - | + | - | |
| 6 | Tannins | + | + | + | - | |
| 7 | Quinones | - | - | - | - | |
| 8 | Proteins | + | + | - | + | |
| 9 | Carbohydrates | - | - | + | - | |
| 10 | Cardiac glycosides | - | + | + | + | |
| 11 | Terpenoids | - | - | + | - | |
Table 1: Qualitative phytochemical Analysis of the Extracts of H. auriculata
Researchers are bound to discover the curative properties of medicinal plants and authenticate them to be nontoxic using analytical tools and experiments. This is an essential quality criterion for reporting efficacy and safety. This type of authentication focuses on identifying and recording the profiles of the secondary metabolic fingerprints by chromatography and spectroscopy which provides valuable information about the qualitative and quantitative pattern of the composition of plant compounds[16]. In the present study, the UV-Visible spectrum profile of MEHA was chosen at a 200-800 nm wavelength. The profile showed the peaks at 664, 610, 532, 400, 280 and 220 nm with absorption of 0.585, 0.280, 0.302, 1.967 and 4.00 respectively. MEHA showed significant and minor peaks indicating the presence of wide range of phytocomponents. UV-Vis spectra had absorption utmost in the region of 200-400 nm. Generally, the flavonoid spectra comprise two absorption bands maxima in 230-285 nm and 300-350 nm[17]. Hence the band occurring at 280 nm typically represents flavonoid groups. The FTIR spectra produce the sample profile, a distinctive molecular fingerprint that can be used to screen and scan samples for many different components. FTIR is an effective analytical instrument for detecting functional groups and characterizing covalent bonding information. The FT-IR spectrum revealed mainly hydroxyl groups, implying Alcohols, Alkanes, Amines, and Carboxylic acid compounds in MEHA (Table 2). It is discovered through the literature survey that the FTIR spectra for the MEHA have not been reported previously. The bands identified in this research are similar to those found by Mohammed et al.[18], who studied the Methanolic Extract of Selected Medicinal Plant of Anethum graveolens and Plantago major. Additionally, the results of the present study corroborate with the findings of Bharathi et al.[19], who reported the presence of functional groups such as amines and Carboxylic acid through FTIR characterization of aqueous extract of H. auriculata. X-ray powder diffraction (XRD) technique utilizes the X-ray scattering phenomenon to determine the nature of the materials as crystalline or amorphous. Powder diffraction data of the present study showed nine prominent diffraction peaks at 20 positions of 14.9, 20.8, 24.51, 28.38, 30.16, 31.12, 40.60, 46.42 and 68.48.
| Peak value | Functional group |
|---|---|
| 3340.71 | Alcohols |
| 2924.09 | Alkane |
| 2854.65 | Alkane |
| 1627.92 | Amine |
| 1049.28 | Amine |
| 925.83 | Carboxylic acid |
Table 2: Peak Values and Functional Groups Present in MEHA
The Braggs reflections indicated the presence of sets of lattice planes and further. The presence of broad peaks in the spectrum of extract suggests the predominance of amorphous nature. The broadening and noise of the observed peak were probably indicating the presence of macromolecules present in the plant powder. This information can be used to establish parameters for developing of Phytotherapeutics products, helping to ensure their quality, safety and efficacy.
Mass spectrometric examination plays a vital role in the phytochemical analysis and chemotaxonomic investigation of medicinal plants containing biologically active components. Many researchers have applied GC-MS analysis to identify the possible bioactive components present in the plant extracts and herbal preparations, which might be helpful for the identification of lead compounds for the development of new pharmaceutical drugs. In the present study the results of the GC-MS analysis exhibited the presence of twenty pharmacologically important compounds from MEHA with nine major peaks (Table 3). Among all, hexadecanoic acid, octadecanoic acid and phytol were previously reported to have antimicrobial activities in the plant Carduus pycnocephalus L.[20]. Also, these compounds have been shown to possess many aspects of ideal chemo preventive agents, such as availability, low cost, oral bioavailability, and low toxicity, making it feasible to consider them for chemoprevention testing. Ravisankar et al.[21] reported compounds such as 2-Furancarboxaldehyde, 2,6,10-Trimethyl,14-Ethylene-, Tetradecanoic acid in H. auriculata, which was similar to the present study. The compound phytol was found to have antimicrobial, antioxidative and antidiabetic properties[22]. Also, n-Hexadecanoic acid possesses antioxidant, antimicrobial and anti-inflammatory properties[23]. Likewise, stigma sterol has some reports regarding its anticancer efficacies[24].
| S. no | RT | Compound name | Molecular formula | Area % |
|---|---|---|---|---|
| 1 | 28.74 | 9,12,15-Octodecatrienoic acid | C18H30O2 | 14.27 |
| 2 | 46.98 | Stigma sterol | C29H48O | 12.92 |
| 3 | 26.39 | n-Hexadecanoic acid | C16H32O2 | 11.48 |
| 4 | 12.16 | 2-Furancarboxaldehyde | C6H6O3 | 9.03 |
| 5 | 28.35 | Phytol | C20H40O | 8.60 |
| 6 | 28.65 | 9,12-Octadecadienoic acid | C18H32O2 | 4.99 |
| 7 | 28.96 | Octadecanoic acid | C18H26O2 | 4.43 |
| 8 | 9.92 | Cyclopentanone | C7H14N2 | 3.47 |
| 9 | 48.13 | Ergost-5-En-3-01, (3. Beta.,24R) | C28H48O | 2.91 |
| 10 | 38.81 | 2,6,10,14,18,22-Tetracosahexaene | C30H5O | 2.34 |
| 11 | 12.53 | Nonionic acid | C9H18O2 | 1.70 |
| 12 | 24.45 | 2,6,10-Trimethyl,14-Ethylene- | C20H38 | 1.67 |
| 13 | 43.68 | Cholesterol | C27H46O | 1.40 |
| 14 | 10.22 | 1,2,6-Trimethyl-hexane | C9H20O3 | 1.05 |
| 15 | 28.48 | Octadecanoic acid, Methyl ester | C19H38O2 | 1.03 |
| 16 | 23.13 | Tetradecanoic acid | C14H28O2 | 1.00 |
| 17 | 25.79 | Hexadecanoic acid, methyl ester | C17H34O2 | 0.96 |
| 18 | 28.19 | 9,12,15-Octadecatrienoic acid | C19H32O2 | 0.80 |
| 19 | 25.14 | 3,7,11,15-Tetramethyl-2-hexadecen | C20H40O | 0.61 |
| 20 | 25.55 | n-Hexadecanoic acid | C16H32O2 | 0.60 |
Table 3: Phytocomponents Identified in the Leaves of H. auriculata by GC-MS
The major groups of the phytochemicals obtained from the plant showed antioxidant activities and were known to prevent degenerative diseases. There is a growing interest in natural antioxidants that might attenuate oxidative stress and paves the way for safe therapeutics[25]. Medicinal plants are believed to be a potential source of reactive oxygen species scavenger molecules[26]. Antioxidants might work either alone or in association with each other against different types of free radicals.
DPPH is a stable free radical, which has been widely used in phytomedicine to assess the scavenging activities of bioactive compounds. The DPPH scavenging activity was shown in the Table 4. MEHA showed a significant free radical scavenging capacity. The inhibition percentage of DPPH radical increases with an increase in concentration. The antioxidant capacity ranged from 43 % to 70 %, which was relatable to the standard. The antioxidant effect is proportional to the disappearance of the purple color of DPPH in test samples. Antioxidant molecules present in the extracts can quench DPPH free radicals by providing hydrogen atoms or electrons and then forming a colorless stable molecule[27]. Similar significant results were reported by Anusha et al.[28] and Raaman et al.[29] for various concentrations using the MEHA. The reducing power activity of the MEHA of different concentrations was remarkable. As the concentration gradually increased, the absorbance of each concentration was found to rise. Maximum absorbance in 50 μg/ml, shown in Table 5. In the reducing power assay, the yellow color of the test solution changed to multiple shades of green and blue depending on the reducing power of MEHA and showed excellent reducing power ability. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity. The reducing ability is generally associated with reductions, which break the free radical chain by donating a hydrogen atom[30]. These effects of the extract could be attributed to the presence of bioactive compounds in MEHA. Several synthetic antibiotics and drugs are employed to treat microbial infections and infectious diseases, but microbial pathogens develop resistance to synthetic antibiotics. The increasing incidence of antibiotic resistance and its side effects on organ systems functioning necessitate finding substitutes for antibiotics[31]. In the present study, the same efforts are continued in searching for novel therapeutics against antibiotic activity. The methanol leaf extract of H. auriculata had promising activity against selected Gram-positive bacteria such as B. subtilis, S. aureus, E. coli, Klebsiella sp., S. typhi and Proteus sp. (Table 6). The zone of inhibition increased with increased concentration of the extracts in the well. This showed the concentrationdependent activity. The highest concentration showed maximum inhibition activity against species such as B. subtilis (18.0±1.0), S. aureus (17.66±1.52), moderate activity against E. coli (17.3±0.57), Klebsiella (17.0±1.52), but mild activity against S. typhi. (16.6±0.57) and Proteus sp. (15.8±1.0) when compared with the standard antibiotic. The results reveal variability in the inhibitory nature of the extract against specific bacteria. The inhibition of bacterial growth was dose-dependent since the inhibitory action of the extract was found to increase with an increase in concentration against all bacterial strains, as evidenced by the higher zone of inhibitions at higher concentrations of MEHA.
| Drug | Concentration (μg/ml) | Inhibition (%) |
|---|---|---|
| Sample | 10 | 43±2.22 |
| 20 | 56±1.43 | |
| 30 | 60±1.22 | |
| 40 | 65±2.34 | |
| 50 | 70±1.77 | |
| Ascorbic acid | 10 | 48±1.21 |
| 20 | 53±1.86 | |
| 30 | 60±1.43 | |
| 40 | 64±1.25 | |
| 50 | 73±2.72 |
Note: Values are mean±Standard Deviation (SD) of four replicates
Table 4: DPPH Radical Scavenging Activity of H. auriculata
| Concentration (μg/ml) | Absorbance at 517 nm | |
|---|---|---|
| Standard | Sample | |
| 10 | 0.32±0.01 | 0.29±0.005 |
| 20 | 0.38±0.04 | 0.31±0.15 |
| 30 | 0.41±0.02 | 0.32±0.017 |
| 40 | 0.46±0.01 | 0.38±0.005 |
| 50 | 0.48±0.03 | 0.39±0.01 |
Note: Values are mean±SD of four replicates
Table 5: Reducing Power Activity of H. auriculata
| Test organisms | Zone of inhibition in mm | |||||
|---|---|---|---|---|---|---|
| 10 (µl) | 15 (µl) | 20 (µl) | 25 (µl) | 30 (µl) | Control* | |
| B. subtilis | 13.0±0.57 | 14.3±1.15 | 15.3±0.57 | 16.3±1.0 | 18.0±1.0 | 21.6±1.15 |
| S. aureus | 12 ±1.0 | 13.6±0.57 | 14.6±1.52 | 15.0±1.73 | 17.66±1.52 | 20.0±1.0 |
| E. coli | 13.3±1.52 | 14.0±1.0 | 15.7±1.0 | 16.5±1.73 | 17.3±0.57 | 20.3±1.52 |
| Klebsiella sp. | 13.0±2 | 14.0±1.0 | 15.0±1.0 | 16.3±1.0 | 17.0±1.52 | 21.0±1.73 |
| S. typhi | 11.0±1.0 | 12.33±0.57 | 13.3±1.52 | 14.0±5 | 16.6±0.57 | 21.3±2.30 |
| Proteus sp. | 11.30±1.52 | 12.0±1.0 | 13.3±1.15 | 14.6±0.57 | 15.8±1.0 | 20.6±2.08 |
Table 6: Diameter of Zones of Inhibition (MM) Of MEHA against Microorganisms
In the antifungal activity, the MEHA was more active against Aspergillus species than Trichoderma. The MEHA significantly inhibited the growth of all the Aspergillus species (A. fumigatus, A. niger and A. flavus) and Trichoderma species in a concentrationdependent manner (Table 7). The maximum inhibition was exhibited against the A. fumigatus (23.8±2.3) followed by A. niger (22.2±2.3) and minimum inhibition was found against A. flavus (21±1.0) followed by Trichoderma sp. (18.8±2.2). The bioactive compounds present in H. auriculata showed the highest promising activity profile against B. subtilis, S. aureus, E. coli, and Aspergillus sp. MEHA has established a moderate inhibition zone over standard antibiotics, chloramphenicol, and nystatin.
| Test organisms | Zone of inhibition in diameter (mm) | Control (Nystatin) | ||||
|---|---|---|---|---|---|---|
| 10 (µg) | 15 (µg) | 20 (µg) | 25 (µg) | 30 (µg) | ||
| A. fumigatus | 17±1.0 | 18.5±1.0 | 19±1.0 | 21±1.0 | 23.8±2.5 | 27.6±3.2 |
| A. niger | 16.6±1.5 | 18±1.0 | 19±3.3 | 21.3±1.2 | 22.2±2.3 | 29.3±1.5 |
| A. flavus | 16±1.0 | 17.5±1.5 | 19.6±1.5 | 20.5±1.0 | 21±1.0 | 26.3±1.5 |
| Trichoderma sp. | 14±1.0 | 15±1.0 | 16±1.0 | 17±2.5 | 18.8±2.2 | 24.3±2.4 |
Note: Values are mean±SD of triplicates
Table 7: Antifungal Activity of H. auriculata
Previous reports on the antibacterial activities of H. auriculata showed selective inhibition against Gram-positive bacteria. Usually, Gram-positive bacterial strains are more susceptible to plant extracts than Gram-negative bacteria. This is mainly attributed to the difference in cell wall composition and thickness. The peptidoglycan layer in Gram-positive bacteria makes them more sensitive to plant extracts, which are permeable to the extracts[32]. The presence of phytochemicals such as flavonoids, alkaloids, tannins and phenols might have interrupted the bacterial cell wall and complexed with the extracellular proteins, thus inhibiting growth. Tannins precipitate the microbial proteins that lack healthy proteins for microbial growth[33]. Likewise, fungus development might have been suppressed due to phenols in the extract. The phenols swell the hyphae, causing plasma seeping and leakage, distortion, abnormal branching or fusion, and wrinkling[34]. The present work reveals various phytochemical constituents in the plant extract that contribute to the antioxidant and antimicrobial properties, which provides scientific validation for its use in folk medicine. Other progressive research activities on the separation of the compounds from the plant extract can be explored.
To summarize, the methanol leaf extract of H. auriculata exhibited high antioxidant activities and selective inhibition against Gram-positive bacteria and few fungal strains. These findings suggest the leaf extract as a potential source for the isolating bioactive compounds for future therapeutics. Also, it is worthwhile to test the extract using human cell lines.
Acknowledgements:
This work was supported by Department of Zoology, Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore.
Conflict of interests:
The authors declared no conflict of interests.
References
- Lemonnier N, Zhou GB, Prasher B, Mukerji M, Chen Z, Brahmachari SK, et al. Traditional knowledge-based medicine: A review of history, principles, and relevance in the present context of P4 systems medicine. Prog Prev Med 2017;2(7):e0011.
- Bhutani KK, Gohil VM. Natural products drug discovery research in India: Status and appraisal. Ind J Exp Biol 2010;48:199-207.
[Google Scholar] [PubMed]
- Valsala P, Deepika E, Santhy KS. Enzymatic and non-enzymatic antioxidant activities of methanol extract of Mentha piperital. J Adv Sci Res 2021;12(1):140-4.
- Sharma A, Patel VK, Chaturvedi AN. Vibriocidal activity of certain medicinal plants used in Indian folklore medicine by tribals of Mahakoshal region of central India. Indian J Pharmacol 2009;41(3):129-33.
[Crossref] [Google Scholar] [PubMed]
- Harishchandra MR, Rajan PR, Singh Satyendrakumar RP. Study of krimighna effect of nimba (Azadiracta indica A. Juss.) Patra as rakshoghna dhoopan by culture and sensitivity method WSR to pyogenic bacteria. Int Res J Pharm 2012;3(6):142-6.
- Dhanalakshmi S, Harikrishnan N, Srinivasan N, Pandian P, Tanisha BA, Kumar MT, et al. A perspective overview on Hygrophila auriculata. Pharmacogn J 2020;12(6s).
- Raman N. Phytochemical Technique. New Indian Publishing Agencies. New Delhi; 2006. p. 19.
- Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TC, Coube CS, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 2001;15(2):127-30.
[Crossref] [Google Scholar] [PubMed]
- Oyaizu M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Japan J Nutr Diet 1986;44(6):307-15.
- Barry AL. Principle and practice of Microbiology. Lea and Fabager; Philadelphia. 1976;3:21-25.
- Al-Daihan S, Al-Faham M, Al-shawi N, Almayman R, Brnawi A, shafi Bhat R. Antibacterial activity and phytochemical screening of some medicinal plants commonly used in Saudi Arabia against selected pathogenic microorganisms. J King Saud Univ Sci 2013;25(2):115-20.
- Ding K, Liu L, Cheng X, Wang C, Wang Z. Investigation on representation methods of dissolubility property of total alkaloid extract from Peganum harmala. Zhongguo Zhong Yao Za Zhi 2010;35(17):2250-3.
[Google Scholar] [PubMed]
- Rastogi A, Shankar SR, Mahalingam GA. Phytochemical screening, antioxidant activity and in vitro anti-diabetic activity of aqueous, methanolic, ethanolic and chloroformic extracts of Hygrophila auriculata. Int J Pharm Pharm Sci 2014;6(5):557-60.
- Sethiya NK, Ahmed NM, Shekh RM, Kumar V, Singh PK, Kumar V. Ethnomedicinal, phytochemical and pharmacological updates on Hygrophila auriculata (Schum.) Hiene: An overview. J Integ Med 2018;16(5):299-311.
[Crossref] [Google Scholar] [PubMed]
- Kren V, Martínková L. Glycosides in medicine: The role of glycosidic residue in biological activity. Curr Med Chem 2001;8(11):1303-28.
[Crossref] [Google Scholar] [PubMed]
- Geethu MG, Suchithra PS, Kavitha CH, Aswathy JM, Dinesh B, Murugan K. Fourier-transform infrared spectroscopy analysis of different solvent extracts of water hyacinth (Eichhornia crassipes Mart Solms.) an allelopathic approach. World J Pharm Pharm Sci 2014;3(6):1256-66.
- Dhivya SM, Kalaichelvi K. Uv-Vis spectroscopic and FTIR analysis of Sarcostemma brevistigma, wight. and Arn. Int J Herbal Med 2017;9(3):46-9.
- Mohammed NK. Phytochemical screening by FTIR spectroscopic analysis and anti-bacterial activity of methanolic extract of selected medicinal plant of Anethum graveolens and Plantago major. Annl Roman Soc Cell Biol 2021:3110-22.
- Bharathi S, Kumaran S, Suresh G, Ramesh B, Sundari MN. Phytosynthesis of silver nanoparticles using Hygrophila auriculata leaf extract and assessment of their antibacterial and antioxidant properties. Int J Appl Pharm 2018:112-8.
- Al-Shammari LA, Hassan WH, Al-Youssef HM. Chemical composition and antimicrobial activity of the essential oil and lipid content of Carduus pycnocephalus L. growing in Saudi Arabia. J Chem Pharm Res 2012;4(2):1281-7.
- Ravisankar M, Ester VC. Determination of bioactive compounds in Hydrophila auriculata leaf extracts using GC MS. Int J Chem Stud 2017;5(3):729-33.
- Kumar PP, Kumaravel S, Lalitha C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr J Biochem Res 2010;4(7):191-5.
- Nathiya S, ShaishtaJabeen N, Jagapriya L, Senthilkumar B, Devi K. Estimation of bioactive compound of Catharanthus roseus Leaf extract by phytochemical screening and GC-MS analysis. Intern J Trend Scie Res Dev 2017; 2(1):417.
- Saha S, Paul S. Potential of Hygrophila auriculata (Schumach.) Heine as a source of future anti-cancer drugs: A comprehensive review. J Pharmacogn Phytochem 2017;6(4):1725-40.
- Bhatt ID, Rawat S, Rawal RS. Antioxidants in medicinal plants. In Biotechnology for Medicinal Plants: Micro propagation and Improvement; Berlin, Heidelberg: Springer Berlin Heidelberg; 2012. p. 295-326.
- Anandjiwala S, Bagul MS, Parabia M, Rajani M. Evaluation of free radical scavenging activity of an ayurvedic formulation, Panchvalkala. Indian J Pharm Sci 2008;70(1):31.
[Crossref] [Google Scholar] [PubMed]
- Seal T. Antioxidant activity of some wild edible plants of Meghalaya state of India: A comparison using two solvent extraction systems. Int J Nutr Metab 2012;4(3):51-6.
- Anusha P, Immanuel SR. Antioxidant and antibacterial activities of leaves extract of Hygrophila auriculata (Schumach.) Heine. J Pharmacogn Phytochem 2019;8(2):1784-9.
- Raaman N. Antioxidant activites and phytochemical analysis of methanol extract of leaves of Hygrophila auriculata (Schumach) heine. Int J Curr Pharm Res 2015;7(4):100-5.
- Subhashini N, Thangathirupathi A, Lavanya N. Antioxidant activity of Trigonella foenum-graecum using various in vitro and ex vivo models. Int J pharm pharm Sci 2011;3(2):96-102.
- Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: Antioxidants or signalling molecules? Free Radical Biol Med 2004;36(7):838-49.
[Crossref] [Google Scholar] [PubMed]
- Lekha GS, Deepika E, Swetha S, Kanagarajan A, Gayathridevi V, Santhy KS. In vivo evaluation of antimicrobial, antipyretic, analgesic, and anti?inflammatory activities of Nilavembu kudineer capsule in comparison with Siddha classical Nilavembu kudineer. Pharmacogn Res 2020;12(4):387-93.
- Thenmozhi DC, Ramalakshmi V. Preliminary phytochemical screening and antibacterial activity of Pergularia daemia. Int J Pharm Bio Sci 2011;2:162-6.
- Huang J, Chung W. Management of vegetable crop diseases with plant extracts. Adv Plant Dis Manag 2003:153-63.