- Corresponding Author:
- C. Liu
The National Laboratory of Pharmacodynamics and Pharmacokinetics, Tianjin Institute of Pharmaceutical Research, Tianjin 300193
E-mail: liuchangxiao@163.com
| Date of Submission | 8 February 2010 |
| Date of Revision | 11 May 2011 |
| Date of Acceptance | 15 May 2011 |
| Indian J Pharm Sci, 2011, 73 (3): 276-281 |
Abstract
In order to increase the dissolution rate and bioavailability, solid dispersions of evodiamine in PVP K30 with different enriched samples of evodiamine to PVP K30 ratios were prepared by solvent method. Our studies showed that the dissolution rate of evodiamine was significantly higher in the solid dispersion system in comparison with that in enriched samples of evodiamine or physical mixtures. The increase of the dissolution rate was evidently related to the ratio of evodiamine to PVP K30. The solid dispersion system (enriched samples of evodiamine/PVP K30= 1/6, w/w) gave the highest dissolution rate: about 27.7-fold higher than that of enriched samples of evodiamine in hard capsules. Powder X-ray diffraction studies showed that enriched samples of evodiamine presented a total chemical stability after its preparation as solid dispersions. In vivo administration studies indicated that solid dispersions of evodiamine in hard capsules had a higher Cmax and a shorter Tmax than those of physical mixture in hard capsules, and the differences of Cmax and Tmax between them were significant. These results suggest that solid dispersions of evodiamine in hard capsules has a notably faster and greater absorption rate than enriched samples of evodiamine in physical mixture hard capsule and corresponds with the in vitro dissolution.
Keywords
Dissolution rate, evodiamine, pharmacokinetics, solid dispersions, X-ray powder diffraction
Evodiamine (EV; C19H17N3O; MW 303.36), an indolequinoline alkaloid, is the major component in the fruit of Evodia rutaecarpa, which has been widely used for a long time in various kinds of herbal medicines to treat abdominalgia, hernia and menorrhalgia [1]. It has shown various biological effects, such as testosterone secretion [2], catecholamine secretion [3], antinociceptive [4], antiinflammatory [5], antiobesity [6], vasodilatory [7], thermoregulatory [8] and uterotonic effects [9]. In particular, it has drawn many researchers’ attention in recent years to the anticancer action of evodiamine. In 2001, Ogasawara et al. [10] examined the effects of 75 kinds of natural compounds on in vitro migration and proliferation of colon 26-L5 cells, demonstrating distinct inhibitory activities of EV on tumor cells. Further studies demonstrated that evodiamine had anti-tumor potential by inhibiting proliferation, inducing apoptosis and reducing invasion and metastasis of a wide variety of tumor cells, including breast cancer cells [11], prostate cancer cells [12-14], leukemia T-lymphocyte cells [15,16], melanoma cells [17], cervical cancer cells [18], colon cancer cells [19] and lung cancer cells [20]. More importantly, EV not only sensitizes chemo resistant breast cancer cells to adriamycin, but also shows little toxicity to normal human peripheral blood cells [11].
However, EV has poor water solubility. The oral bioavailability of EV is estimated to be about 0.1% in the conscious rat system, and EV levels in feces are much higher than those in plasma. The data also indicates that a large amount of evodiamine is unabsorbed in the gastrointestinal tract [21]. Currently, EV as a new anticancer drug candidate is undergoing the pre-clinical stage of the research and development process. As poor water solubility and low bioavailability of EV are key problems to solve in order to educe an anticancer effect better in vivo, it is necessary to increase the drug solubility in the gastrointestinal tract, thus increasing the oral absorption of poorly water-soluble drug [22]. The solid dispersion (SD) technique, which has been widely used to improve the dissolution rate, solubility and oral absorption of poorly water-soluble drugs, is a method that can achieve a super saturation of drugs and improve their bioavailability [23-28].
In order to solve the aforementioned problems, EV solid dispersions were prepared by solvent method and shown to improve the solubility in vitro and the bioavailability in vivo in this study.
Materials and Methods
PVP K30 was purchased from Tianjin Tiantai Fine Chemicla Co., Ltd. (Tianjin, China). EV was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Evodiae rutaecarpa were purchased from a pharmaceutical company in Hebei, China. HPLC-grade methanol was obtained from the Tianjin Concord Technology Co., Ltd. Deionized water (Milli-Q water system, Millipore Bedford, MA, USA) was used in the preparation of the samples and buffer solution. The other materials were of analytical reagent grade.
Extraction and purification of EV from Evodia rutaecarpa (Juss). Benth
The extraction conditions of Evodia rutaecarpa (Juss). Benth was added at 8 times the amount of 70% ethanol, with circumfluence distilling 3 times and 2 h each time. The extraction solution was filtrated and dried under reduced pressure. Then those extracts were added to 24 times the amount of water of pH 3 in the water precipitation process. These sediments were put in the aluminum oxide column. The chromatography conditions were as follows: loading amounts were 0.4 g/ml, eluant were acetoacetate/dichlormethane mixed solution at ratio of 70:30, loading volume were 5 bed volume (BV) and eluant flow rate were 2 BV/h. Finally, enriched samples of EV (ESEV) were acquired, with a content of evodiamine of 11.5 percent.
Preparation of physical mixtures and solid dispersions
Solid dispersions of EV (SDEV) were prepared with ESEV:PVP K30 in 1:2, 1:4, 1:6, 1:8, and 1:10 weight ratios by solvent method. For example, 2 g of ESEV and 4 g of PVP K30 were accurately weighed and dissolved in 200 ml of alcohol. Then, the solvent was evaporated at 60° and dried under vacuum in the lyophilizer. After being dried, the sample was pulverized, sieved and the fractions ≤187.5 μm were selected. Physical mixtures were prepared by grinding ESEV and PVP K30 in a mortar (the weight ratios of ESEV to PVP K30 was 1:2, 1:4, 1:6, 1:8, and 1:10). The particle size fractions (≤187.5 μm) of physical mixtures were collected for further investigation.
Preparation of enteric capsules
For dissolution and animal experiments, SDEV hard capsules (SDEV-HC), ESEV in physical mixtures hardcapsules (PMEV-HC) and ESEV hardcapsules (EV-HC) were prepared by filling their powder into the hard capsules, respectively. Each hard capsule contained 6.25 mg of EV.
Dissolution studies
Dissolution studies were carried out according to the Chinese Pharmacopoeia 2005 apparatus No. 2 (oar method) with a RCZ-5A dissolution apparatus (Tianjin, China) equipped with eight dissolution beakers. The solubility of evodiamine in pH 6.8 phosphate buffer is 3.8 μg/ml at 37±0.5° according to the equilibrium method. Nine hundred milliliters of pH 6.8 phosphate buffer was used as dissolution medium. One SDEV-HC was used to investigate the dissolution profiles under sink conditions. The dissolution tests were carried out at 37±0.5° at a rotation speed of 100 rpm. Samples of 5 ml were withdrawn at predetermined times and the amount taken was immediately replaced with the same amount of fresh dissolution medium maintained at the same temperature. The samples were filtrated through a 0.45 μm membrane filter (Millipore, cellulose acetate) and the concentration of drug was determined by a HPLC-MS/MS. PMEV-HC and ESEV-HC was studied with the same method, respectively.
X-ray powder diffraction
Powder X-ray diffraction (PXRD) was performed using a Rigaku D/max 2500v/pc X-ray diffract meter equipped with a high-frequency 18 kW X-ray generator (Rigaku Corp., Japan). Data was processed using DMSNT software (version 1.37, Scintag Inc.). The X-ray source was a copper filament X-ray tube operated at 40 kV and 200 mA. The alignment of the goniometry was checked daily using a corundum standard. Samples were continuously spun and scanned at a rate of 1° 2θ/min over a range of 3-50 degrees.
In vivo administration studies
Eight beagle dogs were divided into two groups: the experimental group (EG) and the reference group (RG). After an overnight fast for 12 h, each beagle dog in the EG swallowed eight SDEV-HC (ESEV/ PVP K30=1/6, w/w), while each beagle dog in the RG swallowed eight PMEV-HC (ESEV /PVP K30=1/6, w/w), with 150 ml water on an empty stomach. Food and drink were not allowed during the following 4-h period of test after administration of the drug. The cross-over test was performed 1 week later after the first administration.
For pharmacokinetic analysis, 3 ml venous blood samples were collected in heparin tubes at the following time: 0.5 h before administration, and l0, 30, 60, 90, 120, 180, 240, 360, 480, 720 and 1440 min after administration. The blood samples were centrifuged at 2500 rpm for 10 min at room temperature to obtain plasma. Plasma from each sample was transferred to a 2 ml tube, immediately frozen with 1 h (−20º), and maintained at this temperature until analyzed.
HPLC-MS/MS analysis
HPLC-MS/MS analyses were carried out using a Finnigan HPLC instrument (Finnigan, San Jose, CA) consisting of a Surveyor autosampler (Thermo Finnigan, San Jose, USA) and a TSQ Quantum Discovery MaxTM triple-quadruple mass spectrometer (Thermo Finnigan, San Jose, USA). Xcalibur software (version1.4, Thermo Finnigan, San Jose, USA) was used to control the instruments, and for data acquisition and processing. The HPLC operation conditions: mobile phase: methanol to 0.02 mol/l ammonium acetate (including 1 percent formic acid) =80:20 (v/v); flow rate: 0.4 ml/min; chromatographic column: Symmetry C18 (5 μm, 4.6×100 mm, Serial No. 186002616); injection volume 10 μl. Finnigan TSQ Quantum Discovery MAX (Thermo Finnigan, San Jose, USA) was operated with an electrospray ionization (ESI) source under the following conditions: positive mode; ion spray voltage: 4000 V; capillary temperature: 280º; sheath gas pressure: 40 psi; auxiliary gas pressure: 10 psi. Quantization was achieved using selective reaction monitoring (SRM) based on m/z=304.1→160.8 for Ev with the collision energy of 20 V.
Two hundred microliters of plasma were placed in a glass tube and 100 μl of methanol containing glibenclamide (500 ng/ml) were added as internal standard, 3000 μl of EtoAc were added, mixed and centrifuged (2000 rpm) for 10 min. The clear supernatant liquid (2.4 ml) was transferred to a new glass tube and evaporated till dry under nitrogen flow at 40º. The residue was dissolved in the mobile phase (100 μl) and was determined by HPLC-MS/MS. Blank plasma and quality control samples were assayed as per the above method for the authentic samples. All determinations were performed in triplicate. The concentration of evodiamine and glibenclamide in the plasma was calculated using regression equation.
Pharmacokinetic analysis
Pharmacokinetic analyses were performed using valuable data to estimate pharmacokinetic parameters for single dosing. The AUC(0–24 h) and T1/2 of the two pharmaceutical preparations were calculated using a two-compartmental approach with 3P87 software. The Cmax and Tmax were obtained directly from the actual observed data. T1/2 was calculated from the terminal straight portion of 9 data points. AUC was calculated by the trapezoidal method.
Statistical analysis
Differences in the pharmacokinetic parameters between the two groups were compared by analysis of variance. A P-value of less than 0.05 was considered statistically significant.
Results and Discussion
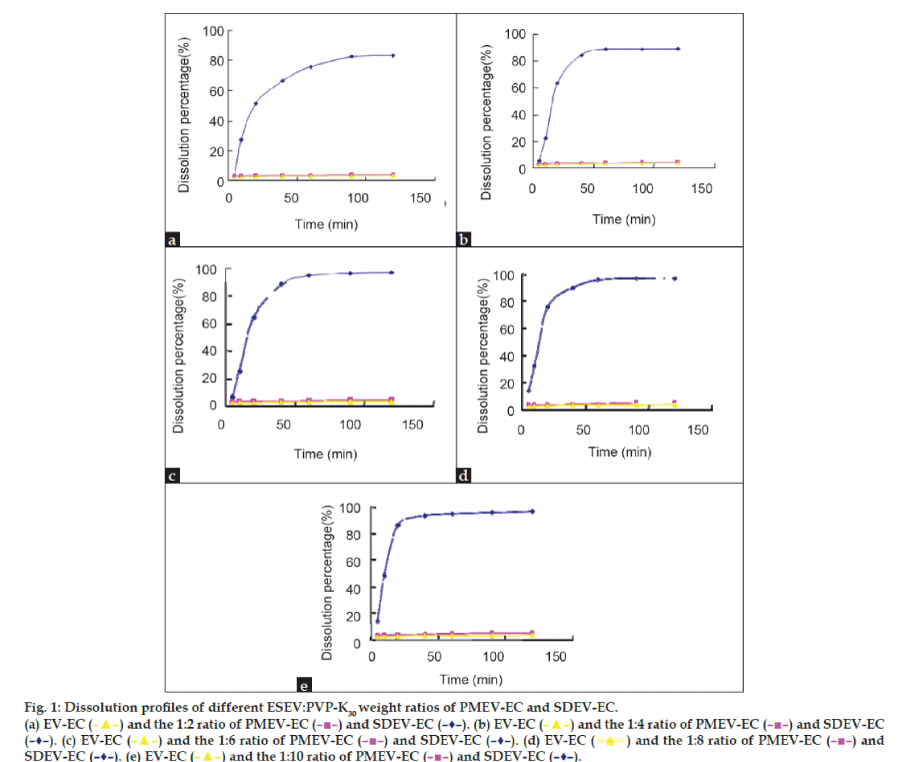
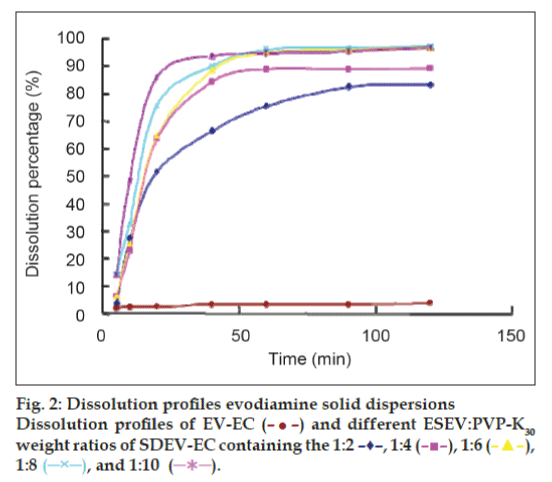
Dissolution studies were performed under sink conditions in a pH 6.8 phosphate buffer. PMEV-HC and SDEV-HC were tested for dissolution properties and compared with that of EV-HC. The results of the dissolution profiles are shown in figs. 1a-e and 2. Evidently, the release rate of EV from all the physical mixtures was very little improved compared with that from EV-HC. This showed that the solubilizing effect of PVP K30 to EV was very small. SDEV-HC resulted in a remarkable dissolution increase of EV compared with the PMEV-HC and EV-HC. The release rate of EV from SDEV-HC varied with the ESEV:PVP K30 ratios and reached the maximum at the ESEV:PVP K30 ratio of 1:6 (fig. 2). Pandit and Khakurel [29] suggested that the decrease in dissolution rate of the solid dispersion containing higher proportions of the polymer might be caused by the leaching out of the carrier during dissolution which could form a concentrated layer of solution around the drug particles, therefore, the migration of the released drug particles to the bulk of the dissolution medium was slowed down.
Figure 1: Dissolution profiles of different ESEV:PVP-K30 weight ratios of PMEV-EC and SDEV-EC.
(a) EV-EC (![]() ) and the 1:2 ratio of PMEV-EC (
) and the 1:2 ratio of PMEV-EC ( ![]() ) and SDEV-EC (–♦–). (b) EV-EC (
) and SDEV-EC (–♦–). (b) EV-EC ( ![]() ) and the 1:4 ratio of PMEV-EC (
) and the 1:4 ratio of PMEV-EC (![]() ) and SDEV-EC (–♦–). (c) EV-EC (
) and SDEV-EC (–♦–). (c) EV-EC (![]() ) and the 1:6 ratio of PMEV-EC (
) and the 1:6 ratio of PMEV-EC ( ![]() ) and SDEV-EC (–♦–). (d) EV-EC (
) and SDEV-EC (–♦–). (d) EV-EC ( ![]() ) and the 1:8 ratio of PMEV-EC (
) and the 1:8 ratio of PMEV-EC (![]() ) and SDEV-EC (–♦–). (e) EV-EC (
) and SDEV-EC (–♦–). (e) EV-EC ( ![]() ) and the 1:10 ratio of PMEV-EC (
) and the 1:10 ratio of PMEV-EC ( ![]() ) and SDEV-EC (–♦–).
) and SDEV-EC (–♦–).
After 20 min, almost 64.6% of drugs released from SDEV-HC (ESEV/PVP K30=1/6), while the PMEV-HC (ESEV/PVP K30=1/10) and EV-HC resulted in only 3.3% and 2.72% dissolution, respectively. At 40 min, the dissolution percentage of SDEV-HC (ESEV/PVP K30=1/6), PMEV-HC (EV/PVP K30=1/10) and EV-HC were characterized by 88.6%, 3.6% and 3.2% of drug release, respectively. The dissolution percentage of SDEZ-EC (ESEV/PVP K30=1/6) was about 27.7-fold increase compared with EV-HC. The improvement in the drug release is probably resulted from the absence of crystal structure and the drug particles’ improved ability to become moistened during the formation of solid dispersions [30,31].
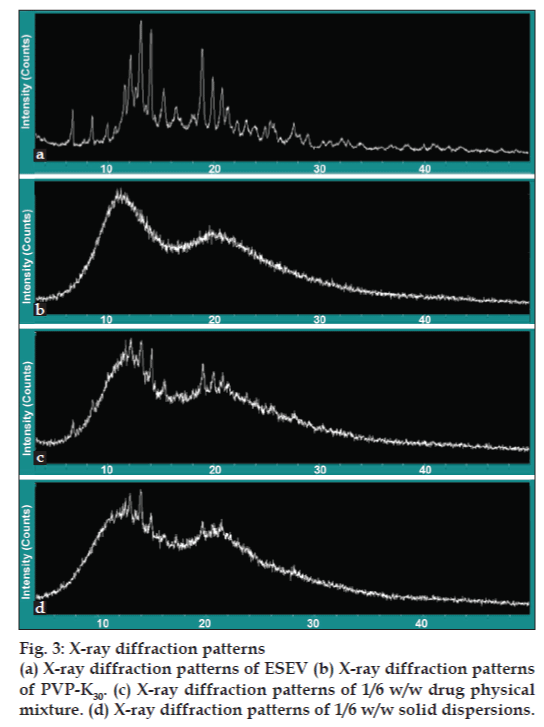
Powder X-ray diffraction patterns taken from the different samples provided us with enough information concerning the lack of solid-state interaction between ESEV and PVP K30. The X-ray diffraction patterns corresponding to ESEV, PVP K30, solid dispersion (ESEV/PVP K30=1/6) and physical mixture (ESEV/PVP K30=1/6) are shown in fig. 3a-d. X-ray diffractograms displayed the presence of peaks corresponding to ESEV in freshly prepared solid dispersions and a perfect concordance between the diffraction spectra obtained for physical mixtures and solid dispersions. These observations proved that ESEV remained unalterable after it’s manufacturing as solid dispersions and that crystallization of PVP K30 does not modify the crystalline structure of the drug. Based on the fact that there is no evidence of interaction between the drug and the PVP K30, it can be concluded that ESEV presents a total chemical stability after preparation as solid dispersions.
As shown in fig. 4 and Table. 1, after a single dose of evodiamine 57.5 mg, the geometric mean Cmax values of SDEV-HC, 27.85±13.78 mg/l, was obviously higher than that of PMEV-HC (P<0.05), which was 10.48±7.28 mg/l. The Tmax values of the former, 0.57±0.19 h, were significantly advanced compared with those of the latter, which were 2.18±0.88 h. This might cause a shorter T1/2 of the former (P<0.05). The AUC(0-24h) values of the former were obviously higher than those of the latter. These results suggest that the absorption rate of SDEV-HC is notably faster and greater than that of PMEV-HC and the bioavailability of the former is notably faster and greater than that of the latter. A cross-over test was used to avoid the influences of individual differences in the beagles. It was revealed in the in vitro study that the dissolution rate of SDEV-HC was obviously higher than that of PMEV-HC. We argue that the difference in absorption rates between SDEV-HC and PMEV-HC is derived from the difference between the procedures for their preparation.
| Drugs | C max (ng/ml) (means ± SD) | T max (h) (means ± SD) | AUC (0-24h) (ng/ml) (means ± SD) | T 1/2 (h) (means ± SD) |
|---|---|---|---|---|
| SDEV-EC | 27.85 ± 13.78 | 0.57 ± 0.19 | 51.22 ± 38.01 | 0.25 ± 0.25 |
| PMEV-EC | 10.48 ± 7.28 | 2.18 ± 0.88 | 34.31 ± 28.07 | 2.18 ± 0.88 |
Table 1: Pharmacokinetic Parameters of evodiamine in sdev-ec and in pmev-ec after a single oral Administration in beagles. N=8
In this study, SDEV was prepared using the solvent method. PVP K30 was used as the carrier in the preparation. The dissolution rate of SDEV-HC was markedly faster and greater than that of EV-HC and PMEV-HC. The PXRD studies showed that ESEV presented a total chemical stability after its preparation as solid dispersions (ESEv/PVPK30=1/6, w/w). This meant that the solid dispersion form was an effective way to increase the dissolution of EV.
The in vitro study also showed that the dissolution rate of SDEV-HC was faster and greater than that of EV-HC and PMEV-HC. Using a cross-over test design, it was found that the absorption rate of SDEV-HC was faster and greater than that of PMEV-HC in the oral administration study corresponded with the in vitro dissolution. These results showed that evodiamine solid dispersions improved the solubility in vitro and the oral bioavailability in vivo. Solid dispersions are suitable for evodiamine preparations of anticancer to induce an anticancer effect in vivo.
Acknowledgements
This project was supported by National Key Technologies R and D Program of China in the 11th Five year Plan under Grant No.2007BAI41B06 and National Basic Research Program of China, No. 2006CB933303.
References
- Pharmacopoeia of People′s Republic of China, (in Chinese). Vol. 1. Beijing: Chemical Industry Press; 2002.
- Lin H, Tsai SC, Chen JJ, Chiao YC, Wang SW, Wang GJ, et al. Effect of evodiamine on the secretion of testosterone in rat testicular interstitial cells. Metabolism 1999;48:1532-5.
- Yoshizumi M, Houchi H, Ishimura Y, Hirose M, Kitagawa T, Tsuchiya K, et al. Effect of evodiamine on catecholamine secretion from bovine adrenal medulla. J Med Invest 1997;44:79-82.
- Kobayashi Y. The nociceptive and anti-nociceptive effects of evodiamine from fruits of Evodiarutaecarpa in mice. Planta Med 2003;69:425-8.
- Chiou WF, Sung YJ, Liao JF, Shum AY, Chen CF. Inhibitory effect of dehydroevodiamine and evodiamine on nitric oxide production in cultured murine macrophages. J Nat Prod 1997;60:708-11.
- Kobayashi Y, Nakano Y, Kizaki M, Hoshikuma K, Yokoo Y, Kamiya T. Capsaicin-like anti-obese activities of evodiamine from fruits of Evodiarutaecarpa, a vanilloid receptor agonist. Planta Med 2001;67:628-33.
- Chiou WF, Chou CJ, Shum AY, Chen CF. The vasorelaxant effect of evodiamine in rat isolated mesenteric arteries: Mode of action. Eur J Pharmacol 1992;215:277-83.
- Tsai TH, Lee TF, Chen CF, Wang LC. Thermoregulatory effects of alkaloids isolated from Wu-chu-yu in afebrile and febrile rats. Pharmacol Biochem Behav 1995;50:293-8.
- King CL, Kong YC, Wong NS, Yeung HW, Fong HH, Sankawa U. Uterotonic effect of Evodiarutaecarpa alkaloids. J Nat Prod
- 1980;43:577-82.
- Ogasawara M, Matsubara T, Suzuki H. Screening of natural compounds for inhibitory activity on colon cancer cell migration. Biol Pharm Bull 2001;24:720-3.
- Liao CH, Pan SL, Guh JH, Chang YL, Pai HC, Lin CH, et al. Antitumor mechanism of evodiamine, a constituent from Chinese herb Evodiaefructus, in human multiple-drug resistant breast cancer NCI/ADR-RES cells in vitro and in vivo. Carcinogenesis 2005;26:968-75.
- Kan SF, Yu CH, Pu HF, Hsu JM, Chen MJ, Wang PS. Anti-proliferative effects of evodiamine on human prostate cancer cell lines DU145 and PC3. J Cell Biochem 2007;101:44-56.
- Huang DM, Guh JH, Huang YT, Chueh SC, Chiang PC, Teng CM. Induction of mitotic arrest and apoptosis in human prostate cancer PC-3 cells by evodiamine. J Urol 2005;173:256-61.
- Kang SF, Huang WJ, Lin LC, Wang PS. Inhibitory effects of evodiamine on the growth of human prostate cancer cell line LNCaP. Int J Cancer 2004;110:641-51.
- Lee TJ, Kim EJ, Kim S, Jung EM, Park JW, Jeong SH, et al. Caspase-dependent and caspase-independent apoptosis induced by evodiamine in human eukemic U937 cells. Mol Cancer Ther 2006;5:2398-407.
- Huang YC, Guh JH, Teng CM. Induction of mitotic arrest and apoptosis by evodiamine in human leukemic T-lymphocytes. Life Sci 2004;75:35-49.
- Wang C, Wang MW, Tashiro S, Onodera S, Ikejima T. Evodiamine induced human melanoma A375-S2 cell death partially through interleukin 1 mediated pathway. Biol Pharm Bull 2005;28:984-9.
- Fei XF, Wang BX, Li TJ, Tashiro S, Minami M, Xing DJ, et al. Evodiamine, a constituent of Evodiaefructus, induces anti-proliferating effects in tumor cells. Cancer Sci 2003;94:92-8.
- Ogasawara M, Matsubara T, Suzuki H. Inhibitory effects of evodiamine on in vitro invasion and experimental lung metastasis of murine colon cancer cells. Biol Pharm Bull 2001;24:917
- Ogasawara M, Matsubara T, Takahashi S, Saiki I, Suzuki H. Anti-invasive and metastatic activities of evodiamine. Biol Pharm Bull 2002;25:1491-3.
- Shyr MH, Lin LC, Lin TY, Tsai TY. Determination and pharmacokinetics of evodiamine in the plasma and feces of conscious rats. Anal ChimActa 2006;3:16-21.
- Yamashita K, Nakate T, Okimoto K, Ohike A, Tokunaga Y, Ibuki R, et al. Establishment of new preparation method for solid, dispersionformulation of tacrolimus. Int J Pharm 2003;267:79-91.
- Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersions. J Pharm Sci 1971;60:1281-302.
- Ford JL. The current status of solid dispersions. Pharm ActaHelv 1986;61:69-88.
- Craig DQ. Polyethylene glycols and drug release. DrugDevInd Pharm 1990;16:2501-27.
- Serajuddin AT. Solid dispersions of poorly water-soluble drugs: Early promises, subsequent problems, and recent breakthroughs. J Pharm Sci 1999;88:1058-66.
- Craig DQ. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm 2002;231:131-44.
- Craig C, Duncan QM. The mechanisms of drug release from solid dispersions in water soluble polymers. Int J Pharm 2002;32:213-6.
- Pandit JK, Khakurel BK. In vitro and in vivo evaluation of some fast release dosage forms of hydrochlorothiazide. Drug DevInd Pharm 1984;10:1709-24.
- Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm 2000;50:47-60.
- Chauhan B, Shimpi S, Paradkar A. Preparation and evaluation of libenclamide-polyglycolized glycerides solid dispersions with silicon dioxide by spray drying technique. Eur J Pharm Sci 2005;26:219-30.
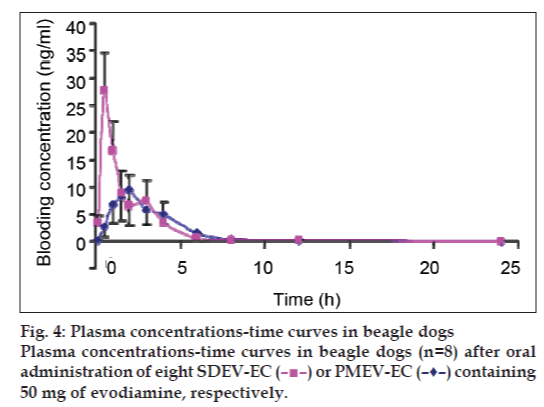
 ) or PMEV-EC (–♦–) containing 50 mg of evodiamine, respectively.
) or PMEV-EC (–♦–) containing 50 mg of evodiamine, respectively.