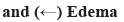- *Corresponding Author:
- Korotkova Irina Pavlovna
Department of Animal and Veterinary Sciences, Primorskaya State Academy of Agriculture, Ussuriisk 692500, Russia
E-mail: korotkovaira@mail.ru
| This article was originally published in a special issue, “Role of Biomedicine in Pharmaceutical Sciences” |
| Sciences” Indian J Pharm Sci 2023:85(2) Spl Issue “112-117” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the preventive and therapeutic effects of total flavonoids of willow buds on acute pancreatitis is the objective of the study. The willow buds of Salix babylonica L. (~1 cm) were harvested from Shenyang Institute of Technology between March 2020 to April 2020. Ultrasound-assisted extraction of total flavonoids of willow buds was conducted and an L-arginine-induced acute pancreatitis mouse model was established. The content of naringenin was determined by Agilent 1100 high-performance liquid chromatography. Serum biochemical test, serum oxidation test and plasma leukocyte content was determined and HE staining was performed. The protective and therapeutic effects of total flavonoids of willow buds on acute pancreatitis in mice were investigated. Total flavonoids of willow buds effectively decreased the activity of serum amylase, lipase and malondialdehyde, reduced plasma white blood cells and increased serum calcium content and superoxide dismutase activity in acute pancreatitis mice; total flavonoids of willow buds alleviated the pathological injury of the pancreas in acute pancreatitis mice. In conclusion, total flavonoids of willow buds may reduce pancreatic injury via reducing the inflammatory response and increasing the activity of antioxidant enzymes, which is a promising natural anti-acute pancreatitis drug.
Keywords
Total flavonoids of willow sprouts, acute pancreatitis, L-arginine, antioxidant enzymes
Willow sprouts refer to the new buds of Salix babylonica L. having high nutritional and medicinal value. As we have been evidenced previously, willow sprouts contain abundant flavonoids, such as luteolin, luteoloside, rutin, naringenin, kaempferol and baicalin[1,2]. These compounds have been proven to mediate various mechanisms to activate cell apoptosis or inhibit necrosis for alleviating Acute Pancreatitis (AP)[3-6].
AP is an unpredictable and potentially fatal disease and a common digestive system disease, with an ever-increasing incidence rate worldwide[7]. AP progression can be complicated with various systemic diseases, including systemic inflammatory response syndrome, multiple organ dysfunction syndromes and multiple organ failure.
In a previous study, Total Flavonoids of Willow Buds (TFW) has been successfully extracted and verified for the abundant flavonoids and potent antioxidant effect[2]. In a previous study, L-arginine is used to induce the stable and reliable mouse AP model and can cause damage to pancreatic cells[8]. Therefore, in the present study, the mouse AP model was induced by intraperitoneal injection of 20 % L-arginine. Mice were treated with TFW (intragastric administration) to explore the preventive and therapeutic effects of TFW on AP in mice.
Materials and Methods
Plant collection and extract preparation:
The willow buds of Salix babylonica L. (~1 cm) were harvested from Shenyang Institute of Technology between March 2020 to April 2020 and verified by Professor Junfan Fu of College of Life Engineering, Shenyang Institute of Technology. Then, the collected willow buds were washed, naturally dried and ground into fine powders for the following experiments.
The dried willow bud powder was subjected to ultrasonic extraction for 35 min under the condition of the liquid-solid ratio of 70:1 and the ethanol concentration of 50 %. The extracted liquid was concentrated by rotary evaporation, dried to powder and refrigerated for use.
Determination of contents of four flavonoids in TFW:
The content of naringenin was determined by Agilent 1100 High-Performance Liquid Chromatography (HPLC) using TFPNCD18 column (250 mm×4.6 mm; 5 μm, United States of America (USA)). This tool was equipped with Ultraviolet (UV) detection system with mobile phase consisted of methanol:0.1 % phosphorus water (50:50), iso-degree elution flow rate of 1.0 ml/min, temperature at 35°, detection wavelength of 283 nm and sample size of 10 µl. The contents of rutin, isoquercitrin and astragaloside were determined by Waterse2695 HPLC using the TFPNCD18 column (250 mm×4.6 mm; 5 μm, USA) with UV detection system at 35°. Mobile phase is composed of a mixture of methanol:0.1 % phosphorus water (42:58) at flow rate of 1.0 ml/min. Wavelength was set at 280 nm and 360 nm. The sample size was set at 10 µl. All test solutions were spectroscopic grade and filtered through a 0.45 μm filter membrane before use. The standard products of naringenin, rutin, isoquercitrin and astragaloside are purchased from National Institutes for Food and Drug Control (Beijing).
Animal grouping:
Kunming male mouse of Specific-Pathogen- Free (SPF) grade (weighing 18~22 g, Liaoning Changsheng Biotechnology Co., Ltd., Experimental animal license No.: SCXK (Liao) 2020-0001) was acclimated for at least 1 w under controlled condition (24°, pathogen free, 12/12 h light-dark cycle). The mice were fed with standard laboratory food and given free access to water. The animal experiments were conducted in accordance with the international regulations on the use and welfare of laboratory animals and were approved by the Ethics Committee of Shenyang Institute of Technology (SITLLBA2021002, Approval date: October 1, 2021).
For prevention experiment, the mice were randomly divided into 5 groups: Blank group, model group and TFW groups of different doses (0.25 g/kg, 0.5 g/kg, 1 g/kg of body weight (bw)). Mice in blank group and model group were given the same volume of normal saline and the intragastric volume was 0.5 ml with continuous gavage of 7 d. 1 h after the last intragastric administration, mice in model group and TFW groups of different doses were given intraperitoneal injection of 20 % L-arginine with the administration dosage of 4 g/(kg·bw), and the operation was repeated at an interval of 1 h. After 24 h, plasma and serum were taken from each group for the determination of White Blood Cells (WBC), biochemical and oxidation indexes, respectively. Pancreases of mice in each group were randomly selected for pathological sections and Hematoxylin- Eosin (HE) staining. For treatment experiment, mice were assigned into 5 groups: Blank group, model group and TFW groups of different doses (0.25, 0.5, 1 g/(kg·bw)). Mice in model group and TFW groups of different doses were given intraperitoneal injection of 20 % L-arginine with the administration dosage of 4 g/(kg·bw) and the operation was repeated at an interval of 1 h. The next day, mice were given intragastric administration of TFW for twice a day, with a single intragastric dosage of 0.5 ml. Besides, equal volume of normal saline was given to mice in blank and model groups. Relevant indexes were detected 72 h after treatment.
Serum biochemical test:
Serum sample was collected for determination of amylase (C016-1-1), calcium ion (C004-1-1) and lipase (A054-2-1) activities using commercial kits (Nanjing Jiancheng Bioengineering Institute).
Serum oxidation test:
Serum sample was collected for determination of Malondialdehyde (MDA) (A003-1) and Superoxide Dismutase (SOD) (A001-3) activity using commercial kits (Nanjing Jiancheng Bioengineering Institute).
Determination of plasma leukocyte content:
Blood of mice from each group was collected and placed into an anticoagulation tube containing Ethylenediamine Tetraacetic Acid (EDTA). Then, an appropriate amount of plasma was harvested after the Quantitative Buffy Coat (QBC) tube was inserted into the pipette. After centrifugation at 12 000 g for 5 min, the blood leucocytes were analyzed through IDEXX VetAutoread.
HE staining:
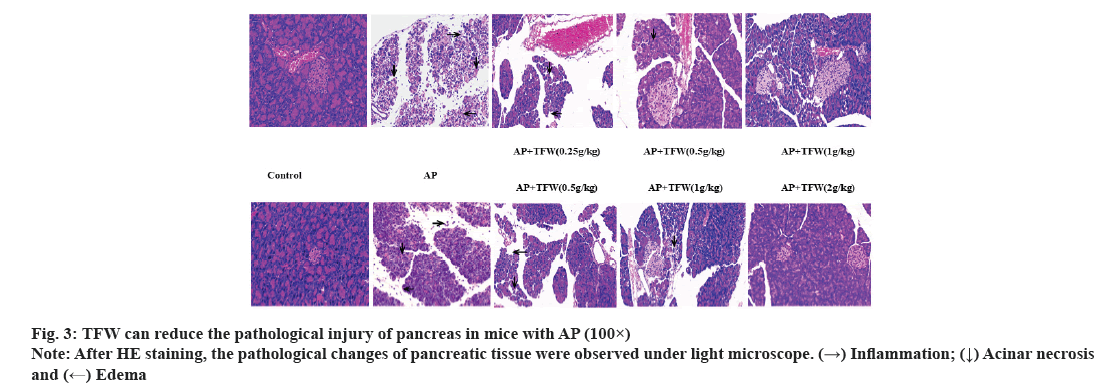
Pancreatic tissues of mice were fixed in 10 % formaldehyde for 48 h, paraffin-embedded and sectioned. The sections were stained with hematoxylin and with eosin, dehydrated and blocked. Pathological characteristics of pancreatic tissues of each group were observed under a microscope and images were collected and analyzed.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 17.0 software was used for statistical analysis and data analysis was performed using univariate analysis of variance and Least Significant Difference (LSD) post hoc test, p<0.05 was considered statistically significant. The histogram was drawn using GraphPad Prism 6 software.
Results and Discussion
TFW contents were analyzed and it was shown in fig. 1. In the previous study, the flavonoid compounds (such as astragaloside, rutin, isoquercitrin and naringenin) in TFW were verified using Q-Orbitrap high-resolution Liquid Chromatograph-Mass Spectrometer (LC-MS). In this study, TFW contents were detected using Ultra-Performance Liquid Chromatography (UPLC) (fig. 1). According to single standard quantification results, it was detected that rutin (2.41 mg/g) content accounted for the highest in TFW, followed by naringenin (2.32 mg/g), isoquercetin (1.18 mg/g) and astragaloside (1.32 mg/g).
Effect of TFW on the biochemical indexes of AP mice after treatment were analyzed and shown in Table 1. As shown in Table 1, L-arginine-induced mice showed noticeably increased lipase and amylase activities and decreased serum calcium ions. After 7 h of TFW treatment (intragastric administration) with different dosages (0.5 g, 1 g and 2 g/(kg·bw)), the activities of lipase were decreased by 27.37 %, 46.92 % and 69.98 %, respectively and amylase was decreased by 31.83 %, 48.48 % and 66.57 %, respectively, were remarkably decreased in a dose- dependent manner and serum calcium content was dramatically increased (increased by 28.46 %, 51.22 % and 80.49 %, respectively) in each group of AP mice. The above results suggested that TFW can alleviate pancreatic injury by affecting lipase and amylase activities and serum calcium.
| Group | Calcium ion (Ca2+) content (mmol/l) | Lipase activity (U/l) | Alpha (α)-amylase activity (U/dl) |
|---|---|---|---|
| Control | 2.29±0.13 | 29.61±1.91 | 202.47±9.02 |
| AP | 1.23±0.07## | 114.53±3.88## | 904.62±18.881## |
| 0.5 g/kg | 1.58±0.06** | 83.18±4.81** | 616.67±22.31** |
| 1 g/kg | 1.86±0.09** | 60.79±5.43** | 466.05±35.53** |
| 2 g/kg | 2.22±0.05** | 34.38±3.03** | 302.41±7.315** |
Note: ##p<0.01, show extremely significant differences compared with the control group and **p<0.01, show significant differences from the AP model group
Table 1: Effect of TFW on the Biochemical Indexes of AP MICE after Treatment (n=6)
As shown in Table 2, the pretreatment with TFW (0.25 g/kg, 0.5 g/kg and 1 g/kg) for 7 d caused a significant difference in serum amylase and lipase activities and calcium ion content in a dose-dependent manner in mice between the TFW group and the model group. Specifically, the activity of serum amylase (decreased by 25.49 %, 45.46 % and 68.43 %, respectively) and lipase was decreased (decreased by 24.52 %, 40.52 % and 57.16 %, respectively) dose-dependently; the calcium ion content was increased (increased by 25.0 %, 42.65 % and 69.85 %, respectively) dose- dependently. From all the above results, TFW can effectively improve the biochemical indexes of AP mice.
| Group | Ca2+ content (mmol/l) | Lipase activity (U/l) | α-amylase activity (U/dl) |
|---|---|---|---|
| Control | 2.53±0.19 | 28.70±2.86 | 239.01±19.34 |
| AP | 1.36±0.10## | 127.39±12.20## | 970.26±67.50## |
| 0.25 g/kg | 1.70±0.05** | 96.16±3.66** | 722.96±28.10** |
| 0.5 g/kg | 1.94±0.05** | 75.77±3.98** | 529.22±42.36** |
| 1 g/kg | 2.31±0.59** | 54.57±4.80** | 306.34±11.90** |
Note: ##p<0.01, show extremely significant differences compared with the control group and **p<0.01, show significant differences from the AP model group
Table 2: Results of Biochemical Indexes in AP MICE Prevented by TFW (n=6)
Effect of TFW on the oxidative indexes in AP mice after treatment was shown in Table 3. As shown in Table 3, L-arginine-induced AP mice showed remarkably increased MDA activity and decreased SOD activity. TFW treatment could noticeably decrease MDA activity (decreased by 27.52 %, 49.0 % and 74.79 %, respectively) and increase SOD activity (increased by 14.43 %, 44.93 % and 64.13 %, respectively). Moreover, pretreatment with different dosages of TFW (0.25 g/kg, 0.5 g/kg and 1 g/kg) can significantly improve the oxidation indexes of AP mice (Table 4). Namely, with the increase in TFW dosage, serum MDA activity was notably decreased (decreased by 36.55 %, 49.92 % and 61.17 %, respectively) and serum SOD activity was increased (increased by 23.84 %, 56.67 % and 88.16 %, respectively) in a dose-dependent manner. According to the above findings, TFW can effectively alleviate L-arginine-caused pancreatic lipid accumulation and peroxidation, reduce the activity of MDA (lipid peroxidation metabolite), and improve SOD activity; TFW showed a potent antioxidant capacity.
| Group | MDA content (nmol/l) | SOD activity (U/ml) |
|---|---|---|
| Control | 6.88±1.65 | 96.46±4.19 |
| AP | 38.12±3.69## | 45.80±2.67## |
| 0.5 g/kg | 27.63±3.17** | 52.41±1.82** |
| 1 g/kg | 19.44±2.13** | 66.38±3.12** |
| 2 g/kg | 9.61±3.12** | 75.17±3.03** |
Note: ##p<0.01, show extremely significant differences compared with the control group and **p<0.01, show significant differences from the AP model group
Table 3: Effect of TFW on the Oxidative Indexes in AP MICE after Treatment (n=6)
| Group | MDA content (nmol/l) | SOD activity (U/ml) |
|---|---|---|
| Control | 7.62±0.66 | 86.49±3.89 |
| AP | 31.57±1.69## | 40.80±2.67## |
| 0.25 g/kg | 20.03±1.07** | 50.53±3.42** |
| 0.5 g/kg | 15.81±0.73** | 63.92±4.03** |
| 1 g/kg | 12.26±0.62** | 76.77±2.43** |
Note: ##p<0.01, show extremely significant differences compared with the control group and **p<0.01, show significant differences from the AP model group
Table 4: Results of Oxidative Indexes in AP MICE, Pretreated with Different Dosages of TFW (n=6)
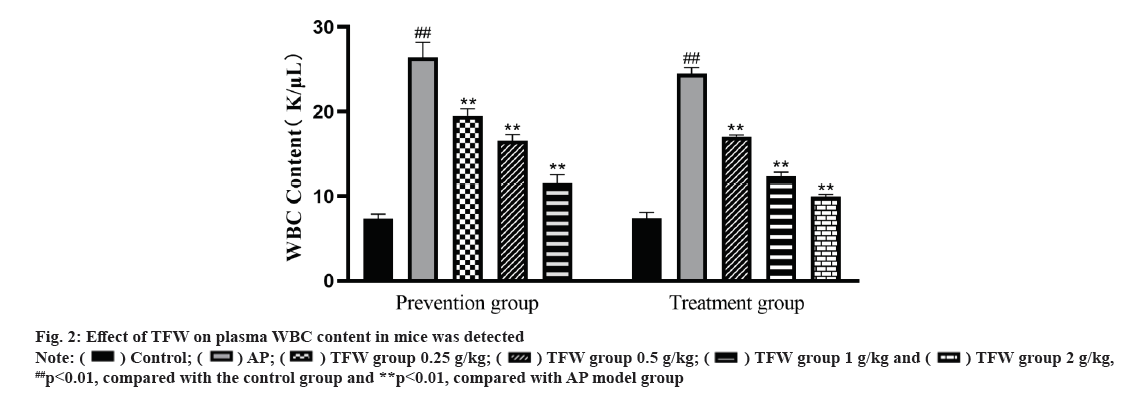
Effect of TFW on plasma WBC content was shown in fig. 2. L-arginine-induced AP mice showed an increase in plasma WBC. After 72 h treatment with TFW, the plasma WBC of mice in each group was dramatically decreased in a dose-dependent manner. Additionally, after 7 d of TFW pretreatment, the plasma WBC of mice in the TFW group was remarkably reduced when compared with that in the model group. Taken together, TFW could reduce the inflammatory response in AP mice.
Examination of pancreas morphology and histopathology was shown in fig. 3. The histopathological changes of the pancreas in each group are displayed in fig. 3. Treatment with different dosages of TFW effectively improved acinar necrosis, edema, hyperemia and inflammatory cell infiltration in the pancreas of mice; 2 g/(kg·bw) TWF most remarkably improved the pathological changes of the pancreas relative to other positive control treatments.
TFW exerted preventive and therapeutic effects on L-arginine-induced AP model mice. TFW may exert therapeutic effects by mediating the mechanism of anti-oxidation, anti-inflammation and pancreatic cell repair. The specific mechanism of TFW needs further investigation in the future. This study provided evidence for the potent effects of TFW on AP, which is conducive to the further research of the plant drug willow sprouts.
Funding:
This study was supported by the grants from the Basic research project of Education Department of Liaoning Province (No. LJKQZ2021196); Project of Natural Science Foundation of Liaoning Province (No. 2022-NLTS-17-01) and Science and Technology Program of the Demonstration Area (No.2020JH14).
Acknowledgements:
All data and information were available and there were no conflicts of interest in the study. The experiments were designed by Peng Zhang, Korotkova Irina Pavlovna and Jiaqing Wang; Wenjie Guo and Shuang Hu were responsible for extraction and content determination of TFW; the prevention and treatment of AP was tested by Peng Zhang and Yufu Li. Peng Zhang, Jiaqing Wang and Korotkova Irina Pavlovna organized data and wrote manuscript.
Conflict of interests:
The authors declared no conflict of interest.
References
- González-Alamilla EN, Gonzalez-Cortazar M, Valladares-Carranza B, Rivas-Jacobo MA, Herrera-Corredor CA, Ojeda-Ramírez D, et al. Chemical constituents of Salix babylonica L. and their antibacterial activity against gram-positive and gram-negative animal bacteria. Molecules 2019;24(16):2992.
[Crossref] [Google scholar] [PubMed]
- Zhang P, Song Y, Wang H, Fu Y, Zhang Y, Pavlovna KI. Optimization of flavonoid extraction from Salix babylonica L. buds and the antioxidant and antibacterial activities of the extract. Molecules 2022;27(17):5695.
[Crossref] [Google scholar] [PubMed]
- Gaman L, Dragos D, Vlad A, Robu GC, Radoi MP, Stroica L, et al. Phytoceuticals in acute pancreatitis: Targeting the balance between apoptosis and necrosis. Evid Based Complement Alternat Med 2018;2018:1-27.
[Crossref] [Google scholar] [PubMed]
- Carvalho KM, Morais TC, de Melo TS, de Castro Brito GA, de Andrade GM, Rao VS, et al. The natural flavonoid quercetin ameliorates cerulein-induced acute pancreatitis in mice. Biol Pharm Bull 2010;33(9):1534-9.
[Crossref] [Google scholar] [PubMed]
- Zhen J, Chen W, Liu Y, Zang X. Baicalin protects against acute pancreatitis involving JNK signaling pathway via regulating miR-15a. Am J Chin Med 2021;49(01):147-61.
[Crossref] [Google scholar] [PubMed]
- Kim SH, Park JG, Sung GH, Yang S, Yang WS, Kim E, et al. Kaempferol, a dietary flavonoid, ameliorates acute inflammatory and nociceptive symptoms in gastritis, pancreatitis and abdominal pain. Mol Nutr Food Res 2015;59(7):1400-5.
[Crossref] [Google scholar] [PubMed]
- Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet 2020;396(10252):726-34.
- Zhang P, Yin X, Wang X, Wang J, Na G. Paeonol protects against acute pancreatitis by Nrf2 and NF-κB pathways in mice. J Pharm Pharmacol 2022;74(11):1618-28. [ Crossref]
[Google scholar] [PubMed]
 ##p<0.01, compared with the control group and **p<0.01, compared with AP model group
##p<0.01, compared with the control group and **p<0.01, compared with AP model group