- *Corresponding Author:
- S. V. Suresh kumar
Department of Pharmacognosy, Sri Padmavathi School of Pharmacy, Tirupati-517 501,India
E-mail: sureshsolleti@yahoo.co.in
| Date of Submission | 24 November 2004 |
| Date of Revision | 26 May 2005 |
| Date of Acceptance | 6 February 2006 |
| Indian J Pharm Sci, 2006, 68 (1): 32-35 |
Abstract
The ethanolic extract obtained from roots of Operculina turpethum (Convolvulaceae) were evaluated for hepatoprotective activity in rats by inducing liver damage by paracetamol. The ethanol extract at an oral dose of 200 mg/kg exhibited a significant protective effect by lowering serum levels of glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, alkaline phosphatase and total bilirubin. These biochemical observations were supplemented by histopathological examination of liver sections. Silymarin was used as positive control.
Operculina turpethum, which is commonly known as trivit, is a large stout perennial twinner with milky juice and fleshy branched roots [1]. It is one of the plants mentioned in the literature having claims of activity against liver disorders [2]. It also has anthelmintic expectorant, antipyretic, anti-inflammatory and purgative properties [2]. It contains a wide variety of phyto constituents, which are useful in treatment of different ailments and includes glycosidic resin, coumarins, beta-sitosterol, and essential oils [1-4]. The present study was undertaken to evaluate the hepatoprotective activity of root extract of this plant in experimental animals against paracetamol-induced hepatotoxicity and is reported here.
Materials and Methods
Roots of Operculina turpethum were obtained from Srinivasa Ayurvedic Pharmacy, Tirupati, Andhra Pradesh, as a gift sample and their identity was confirmed by specimen species preserved at the Department of botany herbarium, S. V. University, Tirupati. The roots are airdried, powdered and used for further studies. All the chemicals used for glutamic oxaloacetic transaminase (SGOT), glutamic pyruvic transaminase (SGPT), alkaline phosphatase (ALKP) and total bilirubin (TBL) determinations are analytical grade of E. Merck, Mumbai.
Preparation of extract
The powdered roots are extracted with petroleum ether (40-60°) to remove lipids and then again extracted with ethanol in Soxhlet extractor. The extract was concentrated under vacuum to get the residue. The residue was dried in vacuum desiccator. The extractive yield of ethanol was found to be more, and it was selected for hepatoprotective screening. All the test suspensions (100 mg/ml) were prepared in the vehicle, i.e., 5% w/v acacia mucilage and were administered in the dose of 200 mg/kg orally.
Toxicity studies
Wistar rats weighing 150-175 g of either sex, maintained under standard husbandry conditions, were used for all sets of experiments in groups of six animals. Animals were allowed to take standard laboratory feed and tap water. The ethanolic extract was administered to different groups of rats in doses ranging from 100-2000 mg/kg. There is no lethality in any of the groups. One tenth of the maximum dose of the extract, tested for acute toxicity, was selected for evaluation of hepatoprotective activity, i.e., 200 mg/kg [5]. The experiments were performed after the experimental protocols had been approved by the Institutional Animal Ethics Committee, M. S. University of Baroda, Vadodara.
Effect on normal liver functions
The ethanolic extract was evaluated for its effect on normal liver functions by studying its effect on normal serum biochemical parameters. The rats were divided into control and test groups, each comprising of six animals. The control group received vehicle (5% acacia mucilage, 1 ml/kg orally) at 0, 24 and 48 h intervals, and the test group received ethanolic extract (200 mg/kg orally) at 0, 24 and 48 h intervals. After 72 h of first dose administration, blood was collected by puncturing the retro-orbital plexus and was allowed to clot at room temperature. Serum was separated by centrifuging at 2500 rpm. The serum obtained was used for the determination of SGOT [6], SGPT [6]. Serum ALKP was assayed by phenyl phosphate method [7] and TBL assay was carried out according to the method of Michaelssohons modification of Jendrassik and Grof [8].
Paracetamol-induced hepatotoxicity [9]
Rats were divided into four groups of six each: control, hepatotoxin, test and positive-control groups. The control group received vehicle at 0, 24 and 48 h orally. The animals in the hepatotoxin group received the vehicle at 0, 24 and 48 h, followed by paracetamol at a dose of 3 g/ kg orally. The test group received the first dose of extract at 0 h; the second dose of extract at 24 h; and at 48 h, the third dose of extract followed by a dose of paracetamol. The animals in the positive-control group received the first dose of silymarin (200 mg/kg orally)10 at 0 h; the second dose of silymarin at 24 h; and at 48 h, the third dose of silymarin followed by a dose of paracetamol. After 96 h, blood was collected from all the groups and was allowed to clot, for the separation of serum. Serum was utilized for estimation of SGOT, SGPT, ALKP and TBL by reported methods to assess liver functions.
Histopathological studies
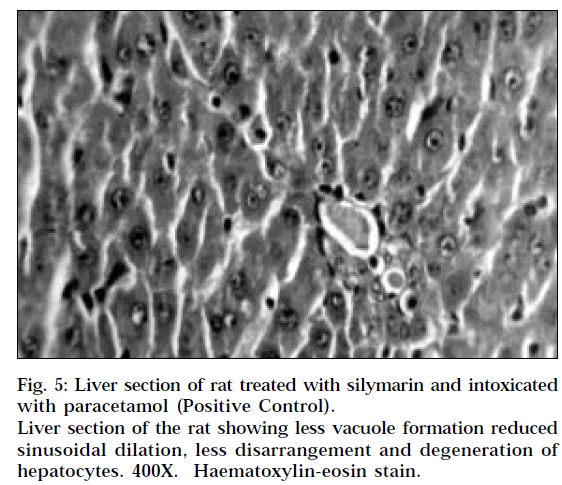
One animal from the treated groups showing maximal activity as indicated by improved biochemical parameters from each test, positive control, hepatotoxin and control groups were utilized for this purpose. The animals were sacrificed, and the abdomen was cut open to remove the liver. Then, 5 mm thick pieces of the liver were fixed in Bouin’s solution (mixture of 75 ml of saturated picric acid, 25 ml of 40% formaldehyde and 5 ml of glacial acetic acid) for 12 h and then embedded in paraffin, using conventional methods [11], and cut into 5 μm thick sections and stained, using haematoxylin-eosin dye, and finally mounted in diphenylxylene. Then the sections were observed under microscope for histopathological changes in liver architecture, and their photomicrographs were taken.
Statistical analysis
The mean values±SEM were calculated for each parameter. Percentage reductions against the hepatotoxin by test samples were calculated by considering enzyme level difference between the hepatotoxin treated and the control group as 100% levels of reduction. For determining the significant inter-group difference, each parameter was analyzed separately, and one-way analysis of variance (ANOVA) [12] was carried out .Then the individual comparisons of the group mean values were done using Dunnet’s Procedure [12].
Results
In the study of the effect of the ethanol extract of Operculina turpethum on normal liver functions, it was found to be non-toxic at the selected dose (200 mg/kg) since the parameters SGOT, SGPT, ALKP and TBL were within the limits like that of control (Table 1). Paracetamol intoxication in normal rats elevated the levels of SGOT, SGPT, ALKP and TBL significantly, indicating acute centrilobular necrosis. The rats treated with ethanolic extract of Operculina turpethum showed a significant reduction in all the four biochemical parameters elevated by paracetamol (Table 2). This reduction in biochemical parameters exhibited by ethanol extract is similar when compared with that of silymarin. The percentage reductions of all four biochemical parameters against hepatotoxin by ethanol extract and positive control are given in Table 3.
| Group | Biochemical parameters mean±SEM | |||
|---|---|---|---|---|
| SGOT (U/ml) | SGPT (U/ml) | ALKP (KA units/100ml) | TBL (mg/dl) | |
| Control | 53.3±4.3 | 80.7±10.9 | 67.5±7.1 | 0.9±0.1 |
| Ethanolic extract | 60.3±3.7 | 78.2±9.3 | 70.8±5.4 | 1.0±0.1 |
| F calculated | 1.5 | 0.03 | 0.1 | 1.3 |
| 5% Allowance | 14.7 | 37.0 | 22.9 | 0.3 |
F critical = 4.96 (P<0.05). Mean values are average of six determinations.
Table 1: Effect Of Ethanolic Extract On Liver Functions
| Group | Biochemical Parameters mean ± SEM | |||
|---|---|---|---|---|
| SGOT (U/ml) | SGPT (U/ml) | ALKP (KA Units/100ml) | TBL (mg/dl) | |
| Control | 53.3±4.4 | 89.3±9.3 | 73.7±5.8 | 1.0±0.1 |
| Paracetamol | 149.0±13.6 | 188.7±21.5 | 174.6±12.4 | 1.94±0.1 |
| Ethanolic extract | 94.8±8.5* | 77.3±5.8* | 84.7±7.1* | 1.6±0.1* |
| Silymarin | 50.7±6.2* | 72.0±6.4* | 80.5±7.0* | 0.95±0.1* |
| F Calculated | 27.0 | 19.5 | 32.0 | 93.7 |
| 5% Allowance | 31.7 | 44.6 | 30.2 | 0.2 |
F critical = 3.10 (P<0.05). *Significant reduction compared to paracetamol. Mean values are average of six determinations.
Table 2: Effect Of Operculina Turpethum Roots On Paracetamol-Induced Hepatotoxicity
| GROUP | SGOT | SGPT | ALKP | TBL |
|---|---|---|---|---|
| Ethanolic extract | 56.6 | 112.1 | 89.1 | 82.1 |
| Silymarin | 102.7 | 117.4 | 93.2 | 104.2 |
Table 3: Percent Reduction Of Biochemical Parameters By Ethanolic Extract And Silymarin
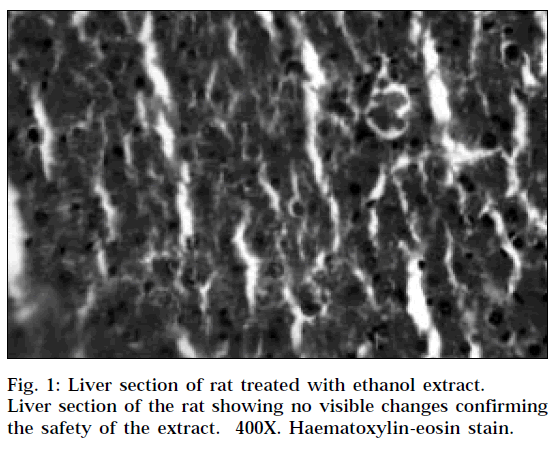
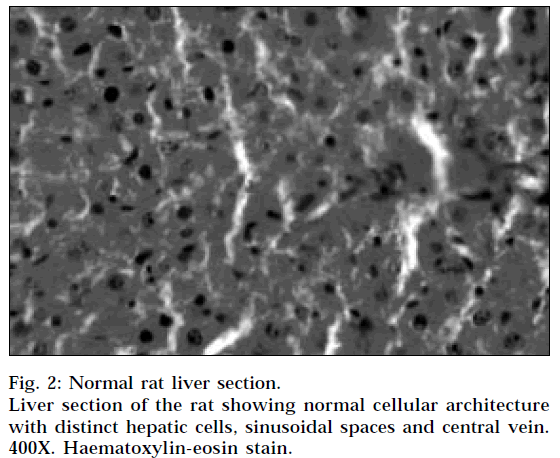
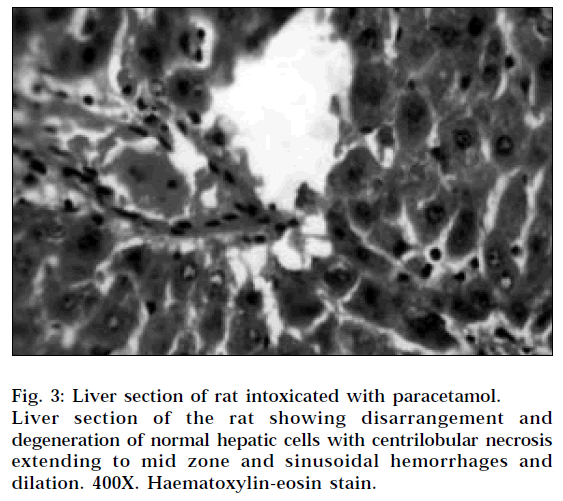
The histopathological profile of the rat treated with ethanolic extract (Fig. 1) showed no visible changes confirming the safety of the extract at selected dose regimen. Histopathological examination of liver sections of control group (Fig. 2) showed normal cellular architecture with distinct hepatic cells, sinusoidal spaces and central vein. In the liver sections of the rats intoxicated with paracetamol (Fig. 3), there is disarrangement and degeneration of normal hepatic cells with intense centrilobular necrosis extending to mid-zone and sinusoidal haemorrhages and dilatation. There was chronic inflammatory cell infiltrate in the portal tracts.
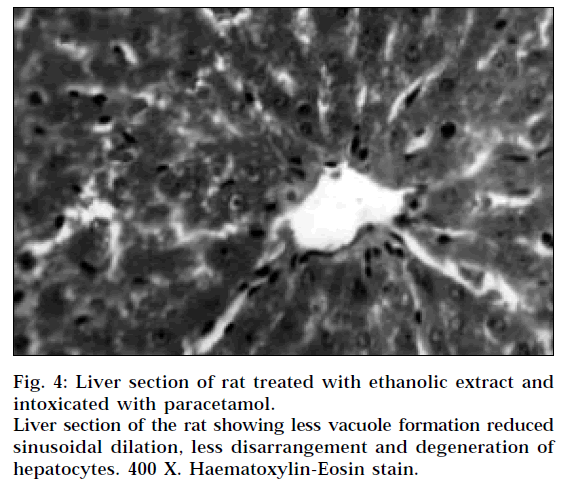
The liver sections of the rats treated with ethanolic extract and intoxicated with paracetamol (Fig. 4) and rats treated with silymarin and intoxicated with paracetamol (Fig. 5) showed less vacuole formation, reduced sinusoidal dilation, and less disarrangement and degeneration of hepatocytes, indicating marked regenerative activity. The intensity of centrilobular necrosis was less.
Discussion
Paracetamol, an analgesic and antipyretic, is assumed to be safe in recommended doses; overdoses, however, produce hepatic necrosis. Small doses are eliminated by conjugation followed by excretion, but when the conjugation enzymes are saturated, the drug is diverted to an alternative metabolic pathway, resulting in the formation of a hydroxylamine derivative by cytochrome P450 enzyme. The hydroxylamine derivative, a reactive electrophillic agent, reacts non-enzymatically with glutathione and detoxifies. When the hepatic reserves of glutathione depletes, the hydroxylamine reacts with macromolecules and disrupts their structure and function. Extensive liver damage by paracetamol itself decreases its rate of metabolism and other substrates for hepatic microsomal enzymes. Induction of cytochrome P450 or depletion of hepatic glutathione is a prerequisite for paracetamol-induced toxicity [9]. The ethanol extract of Operculina turpethum reduced the elevated levels of all the four biochemical parameters by paracetamol.
Paracetamol-induced liver necrosis was inhibited significantly by Operculina turpethum root extract, which confirms the protective action of the ethanolic extract of Operculina turpethum against experimentally induced liver damage in rats. SGOT, SGPT, ALKP and TBL are the most sensitive tests employed in the diagnosis of hepatic disease [13]. The elevated levels of these parameters were significantly reduced by the treatment of Operculina turpethum root extract. It can be concluded from this investigation that roots of Operculina turpethum possess hepatoprotective activity. Further detailed studies may, however, confirm the utility profile of this drug.
Acknowledgements
The authors thank the authorities of Sri Padmavathi School of Pharmacy, Tirupati; and M. S. University of Baroda, Vadodara for providing necessary facilities.
References
- The Wealth of India, A dictionary of Indian raw materials and Industrial products, Vol. VII, CSIR, Delhi, 2001, 96.
- Vasudesan, N,V., In; Indian Medicinal Plants, Vol. IV, Orient Longman Ltd, Chennai, 1995, 172.
- Jain, N.K. and Saxena, V.K., Acta Gene. Indica. Chem., 1987, 13, 171.
- Deeaph, G. and Malti, G., Indian Drugs, 1994, 31, 294.
- Handa, S.S. and Anupama, S., Indian J. Med. Res., 1990, 92, 276.
- Reitman, S. and Frankel, S., Amer. J. Clin. Pathol., 1957,28, 56.
- Wooton, I.D.P., In; Microanalysis in Medical Biochemistry, 4th Edn., J and A Church Hill Ltd, London, 1964, 172.
- Harold, V, Alan, H.G. and Maurice, B., In; Practical Clinical Biochemistry, Vol. I, 5th Edn., CBS Publishers and Distributers, Delhi, 1991, 360.
- Kurma, S.R. and Mishra, S.H., Indian Drugs., 1996, 33, 458.
- Morazzoni, P. and Bombardelli. E., Fitoterapia, 1995, LXIV 39.
- Galighor, A.E and Kozloff, E.N., In; Essentials of Practical Micro technique, 2nd Edn., Lea and Febiger, New York, 1976, 210.
- Sanford, B., In; Gennaro, A.R., Eds., Remington; The Science and Practice of Pharmacy, 19th Edn., Vol. I, Mack Publishing Company, Easton, PA, 1995, 111.
- Harper, H.A, In; Review of Physiological Chemistry, Lange Medical Publishers, Los Atlos, California, 1961, 271