- *Corresponding Author:
- S. Ashida
Department of Botany, Mahatma Gandhi (MG) College, Thiruvananthapuram, Kerala 695004, India
E-mail: ashidashanavas@gmail.com
| Date of Received | 21 May 2020 |
| Date of Revision | 29 March 2022 |
| Date of Acceptance | 09 November 2022 |
| Indian J Pharm Sci 2022;84(6):1380-1388 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study focuses on the efficiency of high-performance thin layer chromatography and gas chromatography-mass spectrometry analysis in qualitative and quantitative analysis of triterpenoid from the terpene fraction of hexane extract of Simarouba glauca leaves. The total terpenoid content of the hexane extract was assessed which shows that 73.69 % of the extract content is terpenoid. The terpene fraction extracted in petroleum ether from the hexane extract of Simarouba glauca leaves was subjected to gas chromatography-mass spectrometry and high-performance thin layer chromatography analysis. Gas chromatography-mass spectrometry analysis results indicated seventeen compounds out of which only one was triterpene (Squalene an important bioactive triterpenoid). In high-performance thin layer chromatography the separation was achieved by using hexane:ethyl acetate in a 5:5 proportion as mobile phase and anisaldehyde sulphuric acid reagent was used for derivatization which imparted violet color to the triterpenoid containing bands. Detection and quantification of betulinic acid and other triterpenes was done by densitometric scanning at 525 nm. Betulinic acid produced compact spots at Rf 0.66. Linear range of betulinic acid was prepared using concentrations 1-7 μg/spot with a correlation coefficient R2 and standard deviation of 0.96004 %±19.68 % respectively. The results indicated the presence of 10 triterpenoids showing different Rf values including betulinic acid. This study confirmed the presence of betulinic acid in the terpene fraction of the hexane extract of Simarouba glauca leaves.
Keywords
Simarouba glauca, betulinic acid, high-performance thin layer chromatography, gas chromatography-mass spectrometry, squalene
Simarouba glauca (S. glauca) DC. also known as paradise tree or bitter wood, is an evergreen small to medium sized and shade tolerant tree. It is well known for its medicinal properties and is used as a traditional medicine in different parts of the world. The leaves and bark are used in the treatment of various diseases. Lakshmi et al.[1] reported the in vitro antibacterial, anti-oxidant, hemolytic and thrombolytic activities of S. glauca. Researchers have discovered a range of medicinally active four alkaloid derivatives from S. glauca having cytotoxic activity against human colon cancer, human oral epidermoid cancer, human hormone-dependent prostate cancer and human lung cancer cells. But the major groups of compounds which contribute to the anticancer properties of S. glauca were identified as triterpenes and their derivatives known as quassinoids. Tricaproin isolated from this plant inhibits the growth of human colorectal carcinoma cell lines by targeting class I histone compound in the plant. The leaf extracts have shown anticancer properties against different cancer cell lines like T lymphoblast (MOLT-3), human immortalised myelogenous leukemia (K-562), erythroleukemia (KG-1) and human urinary bladder (T-24) cell lines[2,3]. Rivero-Cruz et al.[4] isolated four alkaloid derivatives from S. glauca having cytotoxic activity against human colon cancer, human oral epidermoid cancer, human hormone-dependent prostate cancer and human lung cancer cells. But the major groups of compounds which contribute to the anticancer properties of S. glauca were identified as triterpenes and their derivatives known as quassinoids. Tricaproin isolated from this plant inhibits the growth of human colorectal carcinoma cell lines by targeting class I histone deacetylase[5].
The quassinoids shows strong anticancer property. The identified phytochemicals include glaucarubol, glaucarubinone, scopoletin, canthin-6-one, squalene, tricaproin etc. which possess various biological activities and many of which have been patented[6-10]. Recent works have reported and isolated many different triterpenes from various parts of S. glauca but the presence of betulinic acid which is a well-known triterpene having various biological activities is not yet reported from this plant. Betulinic acid is a pentacyclic triterpene acid which is found in the bark of various species of plants. As a natural compound it shows wide range of pharmacological activities because of its low toxicity which includes inhibition of Human Immunodeficiency Virus (HIV), antibacterial, antimalarial, anti-inflammatory and antioxidant properties. Betulinic acid shows potent anti-maturation activity against HIV-I[11]. Fulda et al.[12] identified betulinic acid as a new cytotoxic agent against neuroectodermal tumor cells including neuroblastoma, medulloblastoma, glioblastoma and Ewing’s sarcoma cells, which represent the most common solid tumors of childhood. Park et al.[13] reported that the anticancer effect of betulinic acid is mediated through reactive oxygen species dependent cell cycle arrest and apoptosis. Betulinic acid extracted from the leaves of Vitex negundo demonstrated antibacterial activity against Bacillus subtilis at a concentration of 1000 ug/disc with a zone of inhibition of 18.8 mm2[14]. In vitro antiplasmodial activity (half maximal inhibitory concentration (IC50)) of the betulinic acid was studied and isolated from the root bark of the Tanzanian tree against chloroquine-resistant (KI) and sensitive (T9-96) Plasmodium falciparam were found to be 19.6 ug/ml and 25.9 ug/ml respectively[15]. Recio et al.[16] reported the analgesic and anti-inflammatory activity on betulinic acid isolated from Diospyros leucomelas. Zuco et al.[17] studied the anticancer activity of betulinic acid for selective tumor growth inhibition without damaging the normal cells. The present study was aimed to find out the qualitative and quantitative detection of various classes of triterpenes by using Gas Chromatography-Mass Spectrometry (GC-MS) and High-Performance Thin Layer Chromatography (HPTLC) with special emphasize on betulinic acid.
Materials and Methods
Plant collection:
The leaves of S. glauca were collected from College junction, Kollam district (8°52’43’’ N latitude and 76°36’10’’ E longitude) in the month of September 2018 as shown in fig. 1.
Extraction in n-hexane:
All chemicals and solvents used were of analytical grade and obtained from Nice® Chemicals Ltd. 30 g of dried leaf powder was subjected to Soxhlet extraction with 250 ml of hexane. Extraction was carried out for 9 cycles and temperature was maintained at 65°. The color of the extract was dark green. The extract was collected and cooled at room temperature filtered through filter paper and poured in glass petri dishes and then evaporated at 40° using hot air oven. Dried extract was kept in desiccator for 2 d and stored at 5° in airtight containers until further use.
Preparation of standard solution:
Betulinic acid was used as a standard both for qualitative detection of triterpenes and for the quantitative detection of the betulinic acid in the plant extract using in HPTLC. For this purpose, Betulinic acid ≥98 % high-performance liquid chromatography grade was purchased from sigma and a 100 µg/ml solution was prepared by transferring 0.1 mg of accurately weighed betulinic acid into 1 ml ethyl acetate and mixing it thoroughly.
Extraction and quantification of terpenes:
The total terpenoid was extracted using standard protocol[18]. 100 mg of dried hexane extract was taken and soaked in 9 ml of ethanol for 24 h. The extract after filtration, was extracted with 10 ml of petroleum ether using separating funnel. The ether extract was separated in pre-weighed glass vials and waited for its complete drying. Ether was evaporated and the yield (%) of total terpenoids contents was measured by the formula
Percentage yield=(wti-wtf/wti)×100
Where,
wti represents initial weight of the dried plant extract (100 mg) and wtf weight of the terpene fraction left after complete drying of ether. The dried terpenoid fraction (0.24 g) was dissolved in 10 ml hexane and used for GC-MS and HPTLC analysis.
Anisaldehyde-sulphuric acid reagent:
Place 170 ml of methanol in 200 ml glass bottle and cooled it down in ice cube water bath. To the ice-cold methanol, 20 ml of acetic acid and 10 ml of sulphuric acid were added slowly and carefully and mixed well. Allow the mixture to cool to room temperature, then added 1 ml of anisaldehyde.
GC-MS analysis of terpene extract:
The GC-MS analysis was carried out using a 7890A Gas chromatograph equipped and coupled to a triple axis mass detector, DB-5MS 30 m×0.250 mm×0.25 mm thickness capillary column. The instrument was set to an initial temperature (oven temperature) of 40° for 5 m. At the end of this period, the rate of increase of temperature was 5°/min. Final temperature maintained was 280° for 10 m, 3 ul of sample was injected and the injector temperature was maintained at 280°. For GC-MS detection an electron ionization system with ionization energy 80 eV was used and helium was used as a carrier gas at a constant flow rate of 1 ml/m and pressure 7.0699 psi. The quantification of the components was based on the total number of fragments of the metabolites as detected by the mass spectrometer. The identification of the chemical components was carried out based on the Retention time (Rt) of each component compared with those of the National Institute of Standards and Technology 08 mass spectral libraries.
HPTLC analysis of terpene extract:
Chromatography conditions: Chromatography was performed on a 10×10 cm reactivated HPTLC Silica gel 60 F254 plates (Merck, Darmstadt, Germany). 10 μl, 30 μl, 50 μl and 70 μl of the samples and standard were separately applied to the plate by spotting on HPTLC plate using automatic TLC applicator Linomat-V with N2 flow (CAMAG, Switzerland), with a band width of 8 mm and 8 mm from the bottom. Scanning was performed with CAMAG scanner III at 525 nm with a speed of 20 mm/s. A slit dimension of 5×0.45 was employed. Linear ascending development was carried out in a 10×10 cm CAMAG twin glass tank pre-saturated with the mobile phase at room temperature. 10 ml of hexane:ethyl acetate in a 5:5 proportion was used for chromatographic development.
Detection and quantification: After development, plates were dried with a hair dryer and then derivatized in 200 ml of anisaldehyde sulphuric acid reagent in immersion device CAMAG and heated at 105° for 5 min. The concentration of betulinic acid present per spot of the plant extract applied was calculated from the standard curve obtained from the HPTLC analysis.
Results and Discussion
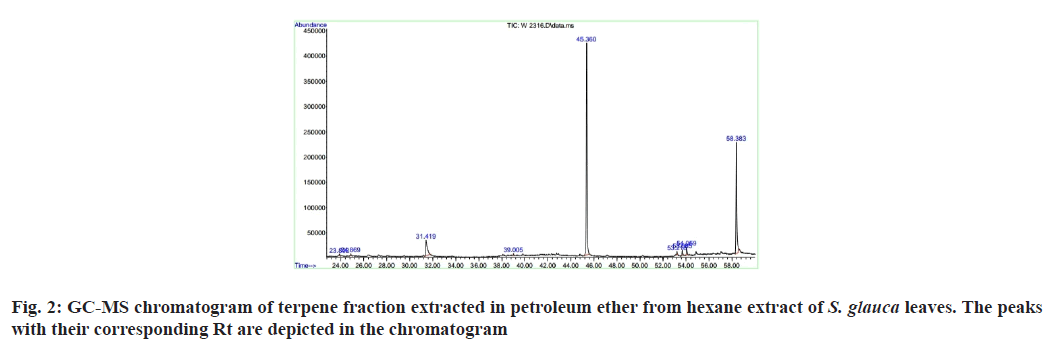
The results indicated that terpenes are the most dominant class of phytochemicals present in the leaves of S. glauca. The terpene content determined was 73.69 mg/100 mg which accounts for about 73.69 % of the total phytochemical content. The GC-MS analysis results indicated the presence of seventeen compounds out of which seven were terpenes. The chromatogram represents the peaks of detected compounds shown in fig. 2. Out of the seven terpenes three sesquiterpenes, two monoterpenes, one diterpene and one triterpene were identified (Table 1). The Rt, compound name and molecular formula are presented in Table 2[19-23]. The triterpenoid identified in GC-MS analysis was squalene. Squalene is a very important bioactive triterpenoid. Many steroids like cholesterol are squalene derivatives. An entire class of triterpenoids is represented as squalene group many of which have important bioactivities. Studies have suggested that squalene has a potential to decrease fibrosis induced as a result of atherosclerotic diet have been reversed by hydroxytyrosol and squalene, natural products from the minor fraction of virgin olive oil[24]. Squalene has been shown to have anti-oxidant activity in mammary epithelial tumor cells[25]. Studies have reported the anticancer property of squalene[26]. Ganbold et al.[27] reported a new amphiphilic squalene derivative improves metabolism of adipocytes differentiated from diabetic adipose-derived stem cells and prevents excessive lipogenesis.
| Sl. no | Name of the compound | Rt | Molecular formula | CAS No |
|---|---|---|---|---|
| 1 | Octane,2,7-dimethyl- | 23.891 | C10H22 | 1072-16-8 |
| 2 | Decane,2,9-dimethyl- | C12H26 | 1002-17-1 | |
| 3 | Octane, 3,5-dimethyl- | 24.873 | C10H22 | 15869-93-9 |
| 4 | Undecane,4,7-dimethyl- | C13H28 | 17301-32-5 | |
| 5 | Dibutyl phthalate | 31.415 | C16H22O4 | 84-74-2 |
| 6 | 1,2-Benzenedicarboxylic acid, butyl 2-methylpropyl ester | C16H22O4 | 17851-53-5 | |
| 7 | Hexadecane,2,6,11,15-tetramethyl- | 39.009 | C20H42 | 504-44-9 |
| 8 | Dodecane,2,6,11-trimethyl- | C15H32 | 31295-56-4 | |
| 9 | 1,2-Benzenedicarboxylic acid, diisooctyl ester | 45.362 | C24H38O4 | 27554-26-3 |
| 10 | 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester | C16H22O4 | 4376-20-9 | |
| 11 | Squalene | 53.214 | C30H50 | 7683-64-9 |
| 12 | 2,6,10,14,18,22-Tetracosahexane,2,6,10,15,19,23-hexamethyl-(all-E)- | C30H50 | 111-02-4 | |
| 13 | 1-pyrrolidinebutanoic acid,2-[(1,1-dimethylethoxy) carbonyl]-α nitro-,2,6-bis(1,1-dimethylethyl)-4-methoxyphenyl ester, [S-(R*,R*)]- | 53.684 | C28H44N2O7 | 124201-86-1 |
| 14 | 3-furancarboxylic acid, 2-(ethoxymethyl)-5-methyl-methyl ester | C10H14O4 | 35339-98-1 | |
| 15 | Acetamide, N-(4-methylphenyl)-N-[4-methyl-2-[[2-(phenyl amino) phenyl] methyl] phenyl]- | 54.06 | C29H28N2O | 52812-78-9 |
| 16 | Vitamin E | 58.38 | C29H50O2 | 59-02-9 |
| 17 | dl-α-Tocopherol | C29H50O2 | 10191-41-0 |
Table 1: The Physical Properties of The Compounds Detected in The Gc-Ms Analysis of The Terpene Fraction of S. glauca Leaves
| Name of the compound | Structure | M.W | Activity |
|---|---|---|---|
| Octane,2,7-dimethyl-(monoterpene) | 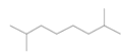 |
142 | No biological activity reported |
| Hexadecane,2,6,11,15-tetramethyl-(diterpene) | 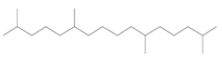 |
282 | Molecular indicator for the anaerobic oxidation of methane[19]. |
| Dodecane,2,6,11-trimethyl-(sesquiterpene) | 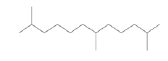 |
212 | No biological activity reported |
| Squalene 2,6,10,14,18,22-Tetracosahexane,2,6,10,15,19,23-hexamethyl-(all-E)-(triterpene) |  |
410 | Antioxidant and antitumor |
| 3-furancarboxylic acid, 2-(ethoxymethyl)-5-methyl-methyl ester (monoterpenoid) |  |
198 | No biological activity reported |
| Vitamin E (sesquiterpene) |  |
430 | Dietary supplement important antioxidant protection of vision and health of blood, brain and skin[20-22]. |
| dl-α-Tocopherol (sesquiterpene) |  |
430 | Antioxidant and anticarcinogenic[23]. |
Table 2: The Name of The Iupac Name, Structure, Molecular Weight and Bio-Activity of The Terpenes Detected in Terpene Fraction Obtained From Hexane Extract of S. glauca Leaves in GC-MS Analysis
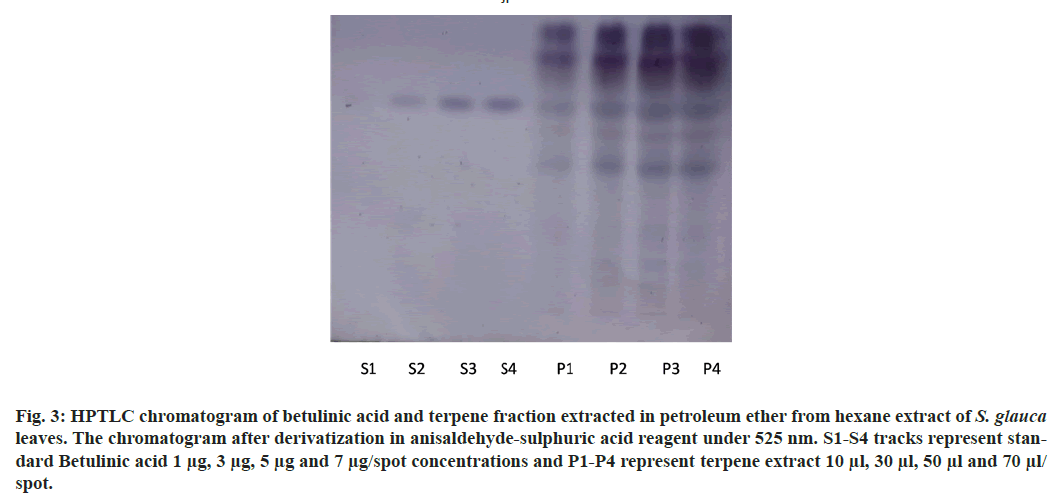
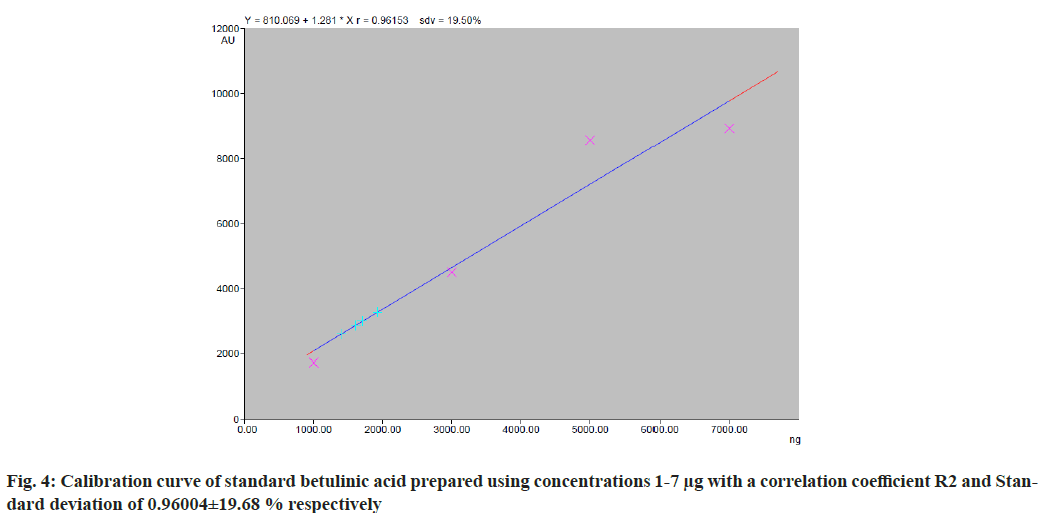
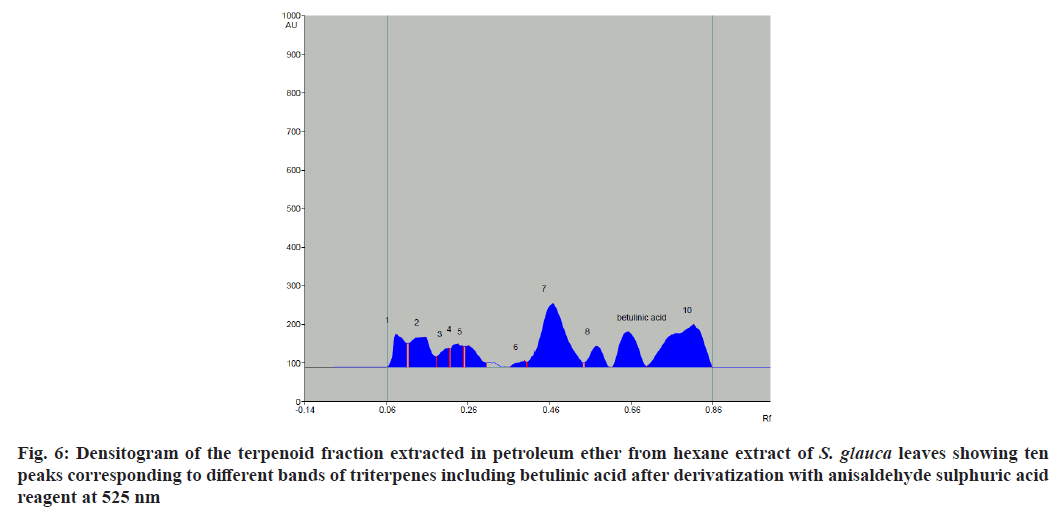
The chromatographic analysis after derivatization with anisaldehyde sulfuric acid reagent showed the presence of 10 bands in violet color with different Retention factor (Rf) values indicating the presence of ten different compounds (fig. 3). Triterpenes produce violet and blue color when treated with anisaldehyde sulphuric acid reagent and heated at 105°[28]. The standard triterpene used here for validation was betulinic acid which also developed a violet color. Thus, ten different triterpenes were detected. Quantitative analysis of the betulinic acid was done by scanning the plates at 525 nm using CAMAG TLC scanner III equipped with win-CATS-V 1.2.3 software (CAMAG). The identification of betulinic acid was confirmed by superimposing the Ultraviolet (UV) spectra of the samples and standards within same Rf 0.66 window (Table 3). A calibration curve for betulinic acid was plotted with four known standard concentrations 1 µg, 3 µg, 5 µg and 7 µg by spotting 10 µl, 30 µl, 50 µl and 70 µl of the standard solution on HPTLC plate respectively with a band width of 8 mm. Each concentration peak area in the plant extract was plotted against the concentration of betulinic acid spotted or injected. The linear regression of standard curve was determined with R2=0.96004 %±19.68 % as shown in fig. 4.
| Peaks | Rf value | Inference |
|---|---|---|
| 1 | 0.1 | unknown |
| 2 | 0.13 | unknown |
| 3 | 0.14 | unknown |
| 4 | 0.24 | unknown |
| 5 | 0.26 | unknown |
| 6 | 0.47 | unknown |
| 7 | 0.58 | unknown |
| 8 | 0.66 | Betulinic acid |
| 9 | 0.77 | unknown |
| 10 | 0.82 | unknown |
Table 3: The Rf Value, Absorbance Area and % Area of Ten Peaks Corresponding To Triterpenes Obtained From The Terpene Fraction of S. glauca Leaves After Derivatization With Anisaldehyde Sulphuric Acid Reagent At 525 nm
Fig. 3: HPTLC chromatogram of betulinic acid and terpene fraction extracted in petroleum ether from hexane extract of S. glauca leaves. The chromatogram after derivatization in anisaldehyde-sulphuric acid reagent under 525 nm. S1-S4 tracks represent standard Betulinic acid 1 μg, 3 μg, 5 μg and 7 μg/spot concentrations and P1-P4 represent terpene extract 10 μl, 30 μl, 50 μl and 70 μl/ spot.
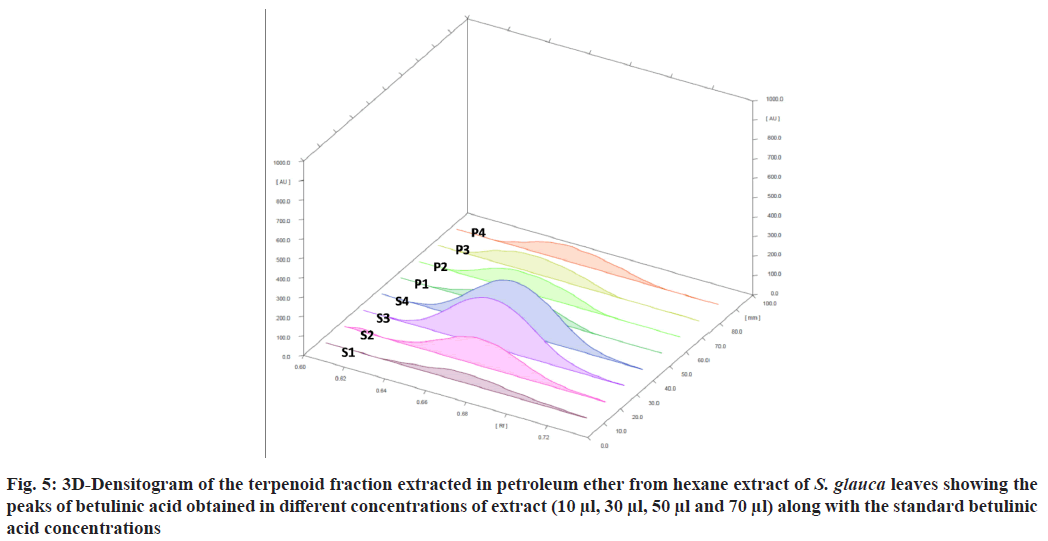
The standard triterpene betulinic acid produced a violet-colored band Rf (0.66). All four concentrations of plant extract showed the presence of spot at Rf value 0.66 corresponding to betulinic acid. The results clearly indicate the presence of betulinic acid in the hexane extract of S. glauca leaves. The concentration of betulinic acid obtained in 10-70 µl of the fraction is provided in Table 4. The concentration of betulinic acid did not show a steady increase with the increasing concentration of the terpene fraction applied but the presence was confirmed by the presence of the same Rf value 0.66 band corresponding to betulinic acid in all the sample concentrations (fig. 5). As described, triterpenes produce violet color after derivatization with anisaldehyde sulphuric acid reagent followed by heating so the other nine bands which produced violet colour and showed absorbance at 525 nm similar to betulinic acid were identified as triterpenes as shown in fig. 6[28].
| Plant extract (µl) | Concentration of Betulinic acid obtained from peak height (µg) | Concentration of Betulinic acid obtained from peak area (µg) |
|---|---|---|
| 10 | 1.833 | 1.608 |
| 30 | 1.983 | 1.925 |
| 50 | 1.722 | 1.706 |
| 70 | 1.503 | 1.403 |
Note: The concentration of betulinic acid obtained upon loding 10-70 μl of the terpene fraction obtained from hexane extract of S. glauca leaves, calculated according to the peak height and peak area
Table 4: The Concentration of Betulinic Acid Obtained From The Densitogram
The present study was intended to detect different triterpenoids present in the terpene fraction extracted from hexane extract of S. glauca leaves using GC-MS and HPTLC. HPTLC method was used to qualitatively and quantitatively detect the presence of betulinic acid from the leaves extract of S. glauca. Although many triterpenoids and quassinoids have been detected from different parts of S. glauca, no study has concentrated on qualitative and quantitative detection of betulinic acid from leaves of S. glauca. In the present study the GC-MS analysis was not found to be an effective tool to detect triterpenoids as only one triterpene squalene was detected in GC-MS. This may due to heat stable nature of most of the triterpenoids, in contrast to monoterpenes and diterpenes which are more heat liable. But HPTLC results clearly indicate the presence of ten triterpenoid including betulinic acid which was not detected in GC-MS analysis. The betulinic acid was confirmed by the presence of corresponding Rf value similar to the standard betulinic acid. The densitometric chromatogram did not show an increase in betulinic acid concentration with the increasing concentration of the terpene fraction.
To conclude, the present study indicates that GC-MS is not an effective tool for detection of triterpenes when compared to HPTLC as HPTLC showed the presence of ten triterpenes while GC-MS could detect only one triterpene (Squalene). The identification of these triterpenes requires further analysis using either Mass Spectroscopy (MS) in combination with HPTLC or other techniques like Liquid Chromatography Mass Spectroscopy (LCMS). The study for the best of our knowledge newly reports the presence of betulinic acid in the terpene fraction extracted using petroleum ether from hexane extracts of S. glauca leaves. Thus, leaves of S. glauca can be used as a source for the extraction of betulinic acid which is a potent anti-cancer agent. Future works can focus on the extraction and identification of different triterpenes from the hexane extracts of S. glauca which will help to find out the other biologically active triterpenes and similar or modified versions of betulinic acid which can be exploited for the therapeutic purpose.
Acknowledgements:
The authors are thankful to M.G Collage, Thiruvananthapuram and CEPCI, Laboratory and Research institute, Kollam, India for providing the research facility. The author is also thankful to CSIR for providing the fellowship for this work and the File number is 08/0538(16053)/2022-EMR-I.
Conflict of interests:
The authors declared no conflict of interests.
References
- Lakshmi KS, Sangeetha D, Sivamani S, Tamilarasan M, Rajesh TP, Anandraj B. In vitro antibacterial, antioxidant, haemolytic, thrombolytic activities and phytochemical analysis of Simarouba glauca leaves extracts. Int J Pharm Sci Res 2014;5(2):432-7.
- Prajapati CK, Reddy MN, Bhatt MH. Evaluation of anticancer activity using leaf extract of Simarouba glauca on leukemic cancer cell lines. Int J Bot Stud 2018;3(2):52-6.
- Puranik SI, Ghagane SC, Nerli RB, Jalalpure SS, Hiremath MB. Evaluation of in vitro antioxidant and anticancer activity of Simarouba glauca leaf extracts on T-24 bladder cancer cell line. Pharmacog J 2017;9(6).
- Rivero-Cruz JF, Lezutekong R, Lobo-Echeverri T, Ito A, Mi Q, Chai HB, et al. Cytotoxic constituents of the twigs of Simarouba glauca collected from a plot in Southern Florida. Phytother Res 2005;19(2):136-40.
[Crossref] [Google Scholar] [PubMed]
- Jose A, Chaitanya MV, Kannan E, Madhunapantula SV. Tricaproin isolated from Simarouba glauca inhibits the growth of human colorectal carcinoma cell lines by targeting class-1 histone deacetylases. Front Pharmacol 2018;9:127.
[Crossref] [Google Scholar] [PubMed]
- Cronquist A. Studies in the simaroubaceae-II. The genus Simarouba. Bull Torrey Bot Club 1944:226-34.
- Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z. Medicinal plants in therapy. Bull World Health Organ 1985;63(6):965-81.
[Google Scholar] [PubMed]
- Govindaraju K, Darukeshwara J, Srivastava AK. Studies on protein characteristics and toxic constituents of Simarouba glauca oilseed meal. Food Chem Toxicol 2009;47(6):1327-32.
[Crossref] [Google Scholar] [PubMed]
- Ham EA, Schafer HM, Denkewalter RG, Brink NG. Structural studies on glaucarubin from Simarouba glauca. J Am Chem Soc 1954;76(23):6066-8.
- Kupchan SM, Lacadie JA, Howie GA, Sickles BR. Structural requirements for biological activity among antileukemic glaucarubolone ester quassinoids. J Med Chem 1976;19(9):1130-3.
[Crossref] [Google Scholar] [PubMed]
- Gerrish D, Kim IC, Kumar DV, Austin H, Garrus JE, Baichwal V, et al. Triterpene based compounds with potent anti-maturation activity against HIV-1. Bioorg Med Chem Lett 2008;18(24):6377-80.
[Crossref] [Google Scholar] [PubMed]
- Fulda S, Jeremias I, Pietsch T, Debatin KM. Betulinic acid: A new chemotherapeutic agent in the treatment of neuroectodermal tumors. Klin Pädiatr 1999;211(04):319-22.
[Crossref] [Google Scholar] [PubMed]
- Park C, Jeong JW, Han MH, Lee H, Kim GY, Jin S, et al. The anti-cancer effect of betulinic acid in u937 human leukemia cells is mediated through ROS-dependent cell cycle arrest and apoptosis. Anim Cells Syst 2021;25(2):119-27.
[Crossref] [Google Scholar] [PubMed]
- Chandramu C, Manohar RD, Krupadanam DG, Dashavantha RV. Isolation, characterization and biological activity of betulinic acid and ursolic acid from Vitex negundo L. Phytother Res 2003;17(2):129-34.
[Crossref] [Google Scholar] [PubMed]
- Steele JC, Warhurst DC, Kirby GC, Simmonds MS. In vitro and in vivo evaluation of betulinic acid as an antimalarial. Phytother Res 1999;13(2):115-9.
[Crossref] [Google Scholar] [PubMed]
- del Carmen Recio M, Giner RM, Manez S, Gueho J, Julien HR, Hostettmann K, et al. Investigations on the steroidal anti-inflammatory activity of triterpenoids from Diospyros leucomelas. Planta Med 1995;61(1):9-12.
[Crossref] [Google Scholar] [PubMed]
- Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Gambacorti-Passerini C, et al. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett 2002;175(1):17-25.
[Crossref] [Google Scholar] [PubMed]
- Ferguson NM. A textbook of pharmacognosy. Macmillan; 1956.
- Greenwood PF, Summons RE. GC-MS detection and significance of crocetane and pentamethylicosane in sediments and crude oils. Organic Geochem 2003;34(8):1211-22.
- Burton GW, Joyce A, Ingold KU. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys 1983;221(1):281-90.
[Crossref] [Google Scholar] [PubMed]
- Erol FS, Topsakal C, Ozveren MF, Kaplan M, Ilhan N, Ozercan IH, et al. Protective effects of melatonin and vitamin E in brain damage due to gamma radiation. Neurosurg Rev 2004;27(1):65-9.
[Crossref] [Google Scholar] [PubMed]
- Nachbar F, Korting HC. The role of vitamin E in normal and damaged skin. J Mol Med 1995;73(1):7-17.
[Crossref] [Google Scholar] [PubMed]
- Das Gupta S, Suh N. Tocopherols in cancer: An update. Mol Nutr Food Res 2016;60(6):1354-63.
[Crossref] [Google Scholar] [PubMed]
- Bullon P, Quiles JL, Morillo JM, Rubini C, Goteri G, Granados-Principal S, et al. Gingival vascular damage in atherosclerotic rabbits: Hydroxytyrosol and squalene benefits. Food Chem Toxicol 2009;47(9):2327-31.
[Crossref] [Google Scholar] [PubMed]
- Finotti E, D'ambrosio M, Paoletti F, Vivanti V, Quaglia G. Synergistic effects of α-tocopherol, β-sitosterol and squalene on antioxidant activity assayed by crocin bleaching method. Nahrung 2000;44(5):373-4.
[Crossref] [Google Scholar] [PubMed]
- Nakagawa M, Yamaguchi T, Fukawa H, Ogata J, Komiyama S, Akiyama SI, et al. Potentiation by squalene of the cytotoxicity of anticancer agents against cultured mammalian cells and murine tumor. Jpn J Cancer Res 1985;76(4):315-20.
[Crossref] [Google Scholar] [PubMed]
- Ganbold M, Ferdousi F, Arimura T, Tominaga K, Isoda H. New amphiphilic squalene derivative improves metabolism of adipocytes differentiated from diabetic adipose-derived stem cells and prevents excessive lipogenesis. Front Cell Dev Biol 2020;8:577259.
[Crossref] [Google Scholar] [PubMed]
- Hotettmann K, Marston A. Saponins: Part of Chemistry and Pharmacology of Natural Products. Cambridge University Press, Switzerland; 2005.