- *Corresponding Author:
- S. Joshi
Department of Pharmaceutical Sciences, Devsthali Vidyapeeth College of Pharmacy, Udhamsingh Nagar, Uttarakhand 263148, India
E-mail: swetajoshi668@gmail.com
| Date of Received | 30 March 2021 |
| Date of Revision | 23 December 2022 |
| Date of Acceptance | 03 March 2023 |
| Indian J Pharm Sci 2023;85(2):273-287 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This review compiles green synthetic methods intended for the synthesis of oxazole derivatives. Oxazoles are a class of compounds with a wide range of biological activities. The application and biological activity of oxazoles is highly dependent on their structure. Now-a-days, scientists have been performing the synthesis of oxazole derivatives. High demands in synthesis often result in the production of various hazardous chemical substances. So, to minimize the production of toxic chemical substances, green synthetic approaches are used in this manner. Green synthesis covers different synthetic approaches, including the application of microwave techniques, ultrasound, ionic liquids, deep-eutectic solvents, the use of catalysts and continuous flow synthesis. In this review, the authors mentioned that not only these green synthetic approaches reduce the formation and utilisation of toxic chemicals, but there is an increase in the reaction performance that enhances the product yields, purity, post-synthetic processes and energy consumption when compared to conventional methods. Due to the biological and pharmacological significance of oxazoles and the demands of decreasing toxic solvents, catalysts, and energy consumption, this article gives a full literature survey on the significance of green synthetic methods in oxazole synthesis. It contains a literature survey over the period from 2010-2020. The emphasis of this article is its comprehensive literature survey on oxazole, which is helpful for the researchers working on the oxazole scaffold and gaining information on the green synthetic approaches to their synthesis.
Keywords
Oxazole, microwave assisted synthesis, green synthesis, ultrasound, ionic liquids
Heterocyclic compounds are extensively used for therapeutic purpose, research areas and industries. Heterocycle containing nitrogen and oxygen atoms are a vital class of compounds in the medicinal chemistry. The chemistry and biological activity of heterocyclic compounds has shown interest for researchers from decades and oxazole moiety has become popular in last few years considering its increasing relevance in the area of medicinal chemistry[1]. Oxazole contains two unsaturations in five membered ring including 1 oxygen atom at position 1 and a nitrogen at position 3 supported by carbons in between[2]. The structure of oxazole derivatives exerts weak interactions like hydrophobic effect, vanderwaals force, hydrogen bonds, coordination bonds, ion-dipole, pi-pi bond and so on, hence the derivatives exhibit potential application in agricultural, biotechnology, medicinal, chemical and material sciences. This review article shows the novel methods and changes in earlier studies for the preparation of oxazole. These novel methods have prepared many substituted oxazoles and thus involved in the innovation of oxazole chemistry[3].
Chemistry of Oxazole
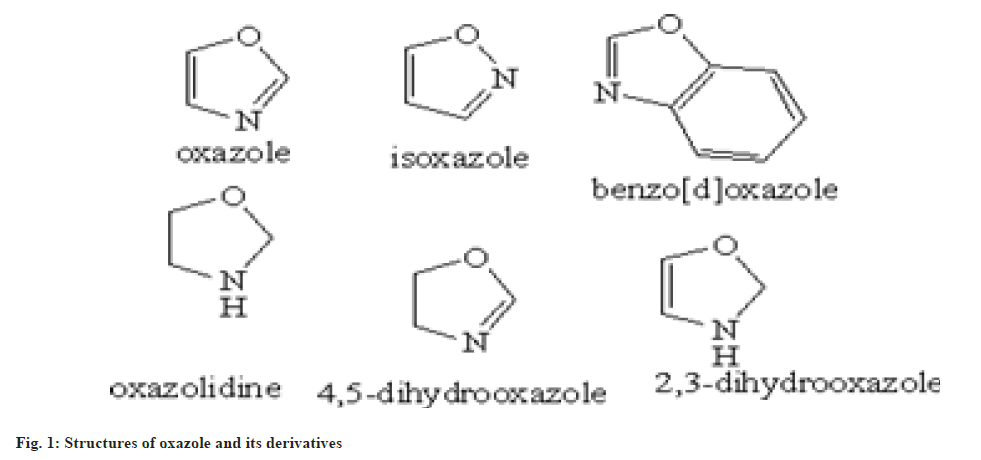
In 1962 oxazole entity was firstly synthesized, but the chemistry of oxazole established in past 1876 by synthesizing 2-methyl oxazole. In the beginning of 1st world war oxazole entity came into prominence, when penicillin antibiotic was invented. During the invention of dienes in the Diels Alder reaction there will be new beginning of oxazole chemistry will occur[4]. Oxazole has 3 carbon, 1 nitrogen and 1 oxygen atom. These all are sp2 hybridized and planar. The atoms also contain unhybridized p orbital which is perpendicular to the plane of σ bonds[5]. Total six non-bonding electrons are present in which 3 of carbon 1 from nitrogen and 2 of oxygen. So oxygen atom is highly electronegative, thus the delocalization is not overly effective[6]. Some basic structure derivatives of oxazole are shown in fig. 1.
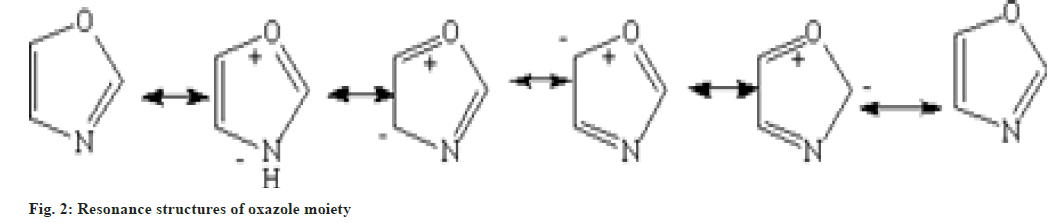
Oxazole heteroatom is structurally identical to the oxygen present in furan and the nitrogen in pyridine. The studies shows that the nature of oxazole also intervene as furan and pyridine. Oxazole is basic in nature and shows similarity to those of pyridine in some aspects[7]. It shows various resonating structures shown in fig. 2.
Pharmacological Activities of Oxazole
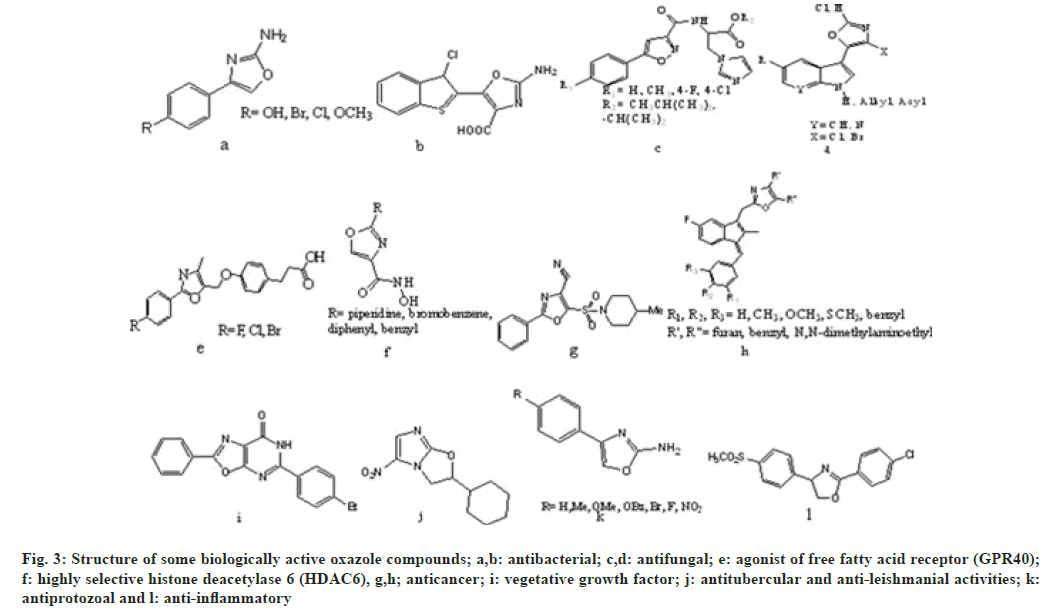
Oxazole and its derivatives are used in treatment of various types of illness[8]. Oxazole shows numerous biological activities such as anti-bacterial[9,10], antifungal[11,12], agonist of free fatty acid receptor (GPR40)[13], highly selective histone deacetylase 6 (HDAC6)[14], anticancer[15,16], vegetative growth factor[17], anti-tubercular, anti-leishmanial[18], antiprotozoal[ 19] and anti-inflammatory[20]. Apart from these pharmacological activities, oxazole derivatives can also be used as anti-corrosive agent for stainless steel, dyes[21], anti-ageing[22], antioxidant[23], metabolism enhancing agent[24], pesticides and herbicides[6]. These compounds also involves as chelating agents for binding[25] shown in fig. 3.
Fig. 3: Structure of some biologically active oxazole compounds; a,b: antibacterial; c,d: antifungal; e: agonist of free fatty acid receptor (GPR40); f: highly selective histone deacetylase 6 (HDAC6), g,h; anticancer; i: vegetative growth factor; j: antitubercular and anti-leishmanial activities; k: antiprotozoal and l: anti-inflammatory
Oxazoles are weak base that are employed in various chemical reactions to produce numerous biologically and therapeutically active species of organic entities[26]. Various synthetic approaches are presented in the literature owing therapeutic and physicochemical properties of oxazole derivatives. In this review, we have summarized different green approaches towards synthesis of oxazole.
Conventional Methods for Synthesis of Oxazole Derivatives
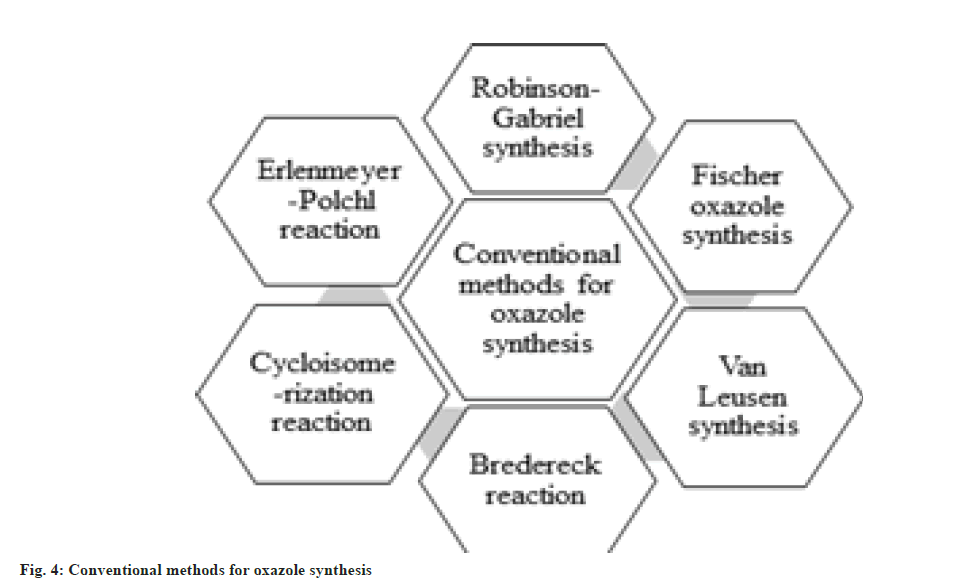
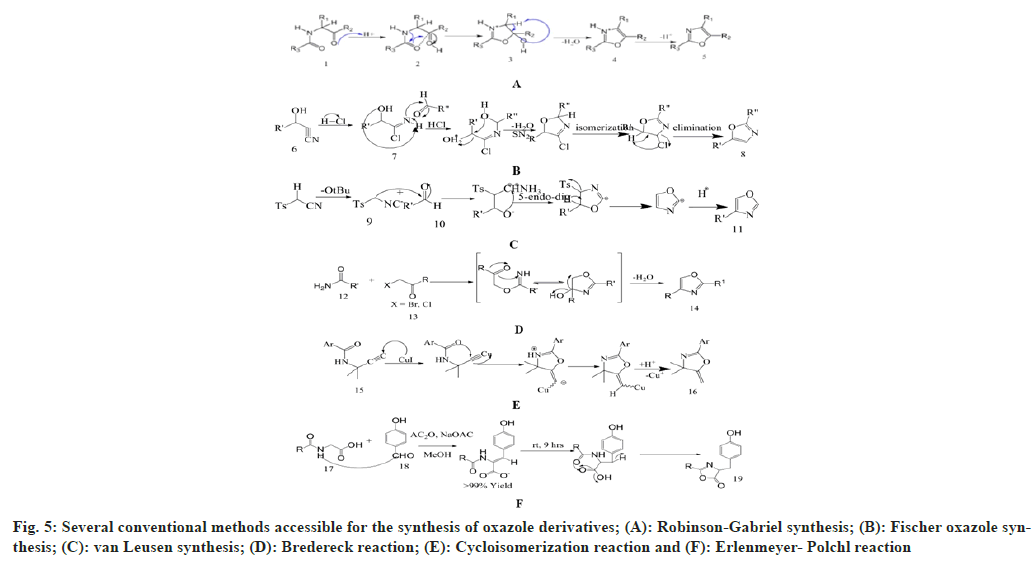
It has been disclosed by the literature survey that there are several conventional methods available for the synthesis of oxazole derivatives, namely, Robinson-Gabriel synthesis, Fischer oxazole synthesis, van Leusen synthesis, Bredereck reaction, Cycloisomerization reaction and Erlenmeyer-Polchl reaction shown in fig. 4 and fig. 5.
Robinson Gabriel synthesis: This method synthesizes 2, 5-diaryloxazole derivatives. This is termed after the names of Sir Robert Robinson and Siegmund Gabriel, who described this reaction in 1909 and 1910 respectively[27]. The reaction mechanism shows protonation of acylamino keto moiety, into cyclization, then dehydration in presence of mineral acid, which form 2,5-disubstituted oxazole moiety. They produce low yield if cyclo-dehydrating agents are PCl5, H2SO4 and POCl3 etc. The yield can be increased by using polyphosphoric acid to 50-60 %[28].
Fischer oxazole synthesis: Emil fisher discovered the first synthesis for 2,5-disubstituted oxazole in 1896. This chemical synthesis occurs between equimolar amount of cyanohydrin and aromatic aldehyde in presence of dry ether and anhydrous hydrochloric acid. This is basically a dehydration reaction which occurs in mild environment due to the rearrangement of the groups[29,30].
van Leusen synthesis: van Leusen et al. in 1972 invented a novel chemical synthesis for oxazole ring system. He synthesizes 5-substituted oxazoles by reacting aldehyde and tosylmethyl isocyanide (TosMIC) as a precursor in a one-step reaction that takes place in mild and basic state. This process is called van Leusen synthesis. In this process, TosMIC has reactive isocyanide carbons, an active methylene group, and a leaving group. A bond will form between the hydroxyl group and the isocyano group when TosMIC is added to the aldehyde, and an intermediate will be formed as oxazoline. After the elimination of TosH, this intermediate is converted into the 5-substituted oxazole derivative[31].
Bredereck reaction: Bredereck synthesize oxazole derivatives by reacting α-haloketones with amides. 2, 4-disubstituted oxazoles can be easily synthesized by this method[32]. This is an efficient, clean and economical process for oxazole synthesis. Pei et al. has improved this method by using α-hydroxyketone as a starting material[33].
Cycloisomerization reaction: A versatile silica gel supported cycloisomerization reaction of easily available propargylic amide which produces polysubstituted oxazoles. This method synthesizes oxazole derivatives with high efficiency and under mild conditions. It starts with cyclization into oxazolines, then isomerization to make the oxazoles[34].
Erlenmeyer-Polchl reaction: This reaction is named after Friedrich Gustav Carl Emil Erlenmeyer, who is credited with discovering this reaction, which is used for the synthesis of oxazoles. This reaction occurs due to condensation between hippuric acid and aldehyde in presence of dry acetic anhydride and acetate ions. This prepares 2, 5-disubstituted oxazolone derivatives[35].
Modern Synthetic Methods for Oxazoles
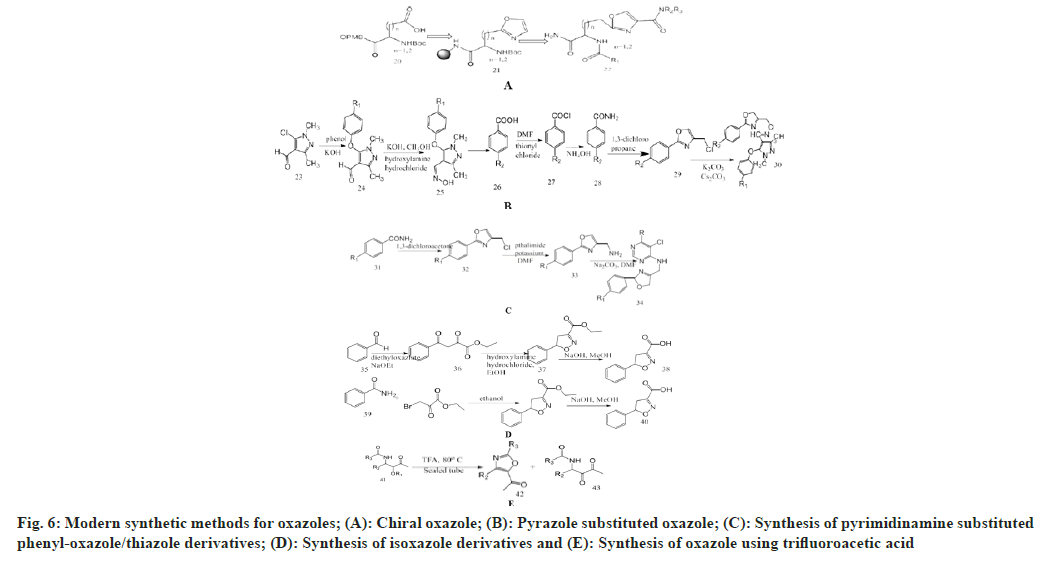
Murru et al.[36] synthesize novel chiral oxazolyl alanine derivatives which finally used for preparing novel trifunctional oxazole. Oxazole ester (Boc- L-Asp-Oxz-OBn) was mixed with ethanol and ethylacetate (1:1) and treated with 10 % palladium metal on carbon support as a catalyst, then passed under hydrogen gas for 2 h. By removing Boc group with 10 ml of 55 % TFA/CH2Cl2 for 30 min with neutralization and coupling with free amino group and carboxylic acid, resulting in formation of new amide functions. Then washed with Dimethylformamide (DMF) and CH2Cl2, and cleaved in presence of HF and washed with nitrogen gas stream to remove HF and extracted with 95 % acetic acid for 1 h, transferred to a vial and lyophilized upon freezing.
Wang et al.[37] prepared pyrazole substituted oxazole derivatives from pyrazole aldehyde into intermediates in presence of DMF, hydroxylamine hydrochloride under basic conditions, 4-chloromethyl-2- aryloxazole was prepared from substituted benzoic acid. In presence of thionyl chloride, it is converted into substituted benzoyl chloride using DMF as catalyst. This intermediate with ammonium hydroxide give substituted benzamide which on reacting with 1,3-dichloropropanone produced 4-chloromethyl- 2-aryloxazole this on reacting with oxime and Cs2CO3 produces pyrazole oxazole derivatives.
Zhong et al.[38] designed pyrimidinamine substituted phenyl-oxazole/thiazole derivatives as potent antifungal agents. Substituted pyrimidine when reacted with substituted oxazole rings and anhydrous potassium carbonate, ethanol, and reflux for 2-4 h. Completion of the reaction was monitored in the Thin Layer Chromatography (TLC) chamber and extracted with ethyl acetate (3×80 ml).
Zhao et al.[39] synthesize aromatic heterocycles by use of acetophenone as starting material and treating it with diethyl oxalate in the presence of NaOEt through Claisen condensation to obtain an intermediate and performing a ring closure reaction with hydroxylamine hydrochloride to form isoxazole. By replacing oxygen from benzamide with sulphur to yield thiobenzamide and treating it with ethyl bromopyruvate by intermolecular cyclization to prepare oxazole and thiazole.
Yasaei et al.[40] synthesize oxazole derivatives by heating trifluoroacetic acid-ketoneamides with acid labile substituted alkoxy molecules which undergo formation of a 5-acetyl-substituted oxazole derivative. Phalke[41] added acetyl glycine, various aldehydes, anhydrous sodium acetate and acetic anhydride to the flask. Continuously stir with warming until the completion of the reaction. Heat the solution for 1 h. After completion of the reaction, cool it and leave the container overnight. The yellow crystals of oxazolone derivatives are formed (fig. 6).
Microwave Assisted Synthesis of Oxazole Derivatives
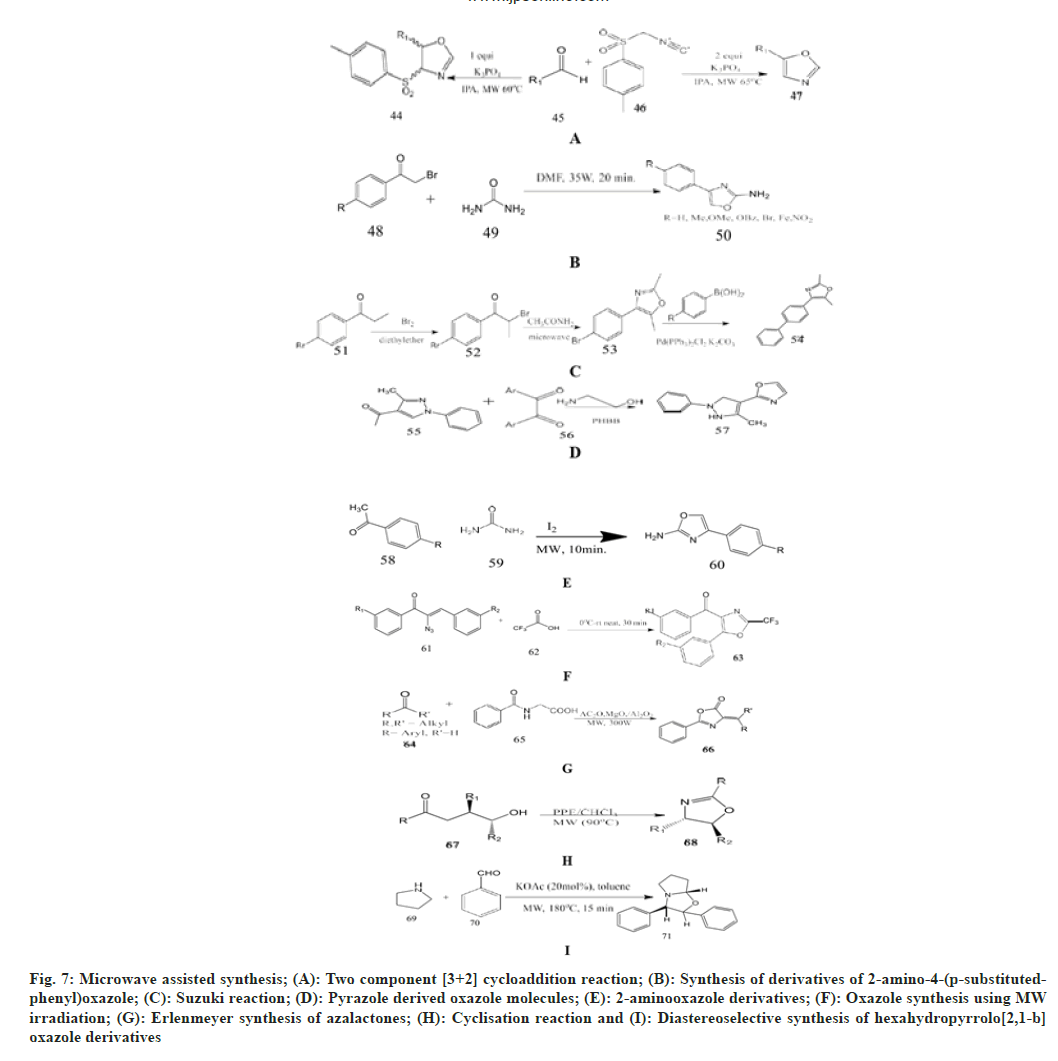
Mukku et al.[42] selected substituted aryl aldehyde, TosMIC as a starting material and added 10 ml of isopropyl alcohol in a round-bottom flask, then charged with potassium phosphate as a catalyst. Then it was irradiated in an open vessel at 800 rpm at 65° and 350 W for 8 min. The completion of the reaction is checked by TLC and cooled to room temperature. This gives a very good chance of making 4, 5-disusbtituted oxazolines and 5-substituted oxazoles.
Carballo et al.[19] synthesised and tested seven derivatives of 2-amino-4-(p-substituted phenyl)- oxazole as an antiprotozoal agent in vitro against Giardia lamblia and Trichomonas vaginalis. These oxazole derivatives were synthesized under microwave irradiation of a mixture of p-substituted 2-bromoacetophenone and urea in the presence of DMF.
Singh et al.[43] described the Suzuki reaction of 4-(4-bromophenyl)-2,5-dimethyloxazole obtained by the bromination reaction of p-bromo phenyl ethanone and further cyclisation with acetamide under microwave. This is reacted with substituted phenylboronic acid in the presence of bis (triphenylphosphine) palladium, chloride, potassium and DMF, which produce novel 2,5-dimethyl-4- substituted biphenyl-1,3-oxazole derivatives in a good yield and are evaluated for in vitro antimicrobial activity.
Hafez et al.[44] synthesized novel pyrazole derivatives having pyran, pyridine, pyrazole, imidazole, 1,3-oxazole and 1,3,4-thiadiazole moiety. 4-disubstitutted imidazolyl-3-methyl- 1-phenyl1Hpyrazole derivatives has been synthesized under microwave irradiation by reacting 1,2-diaryldiketones and 3-methyl-1-phenyl-1H-pyrazole-4- carboxaldehyde in the presence of 2-aminoethanol with pyridinium hydrobromide perbromide as a catalyst in water afforded 2-(3- methyl-1 phenyl-1Hpyrazol- 4-yl)-4,5 dihydro-1,3-oxazole.
Kumar et al.[45] react aromatic ketone, thiourea/urea and iodine in an open vessel, place it in a microwave cavity and irradiated it at 50 W (140°) for 10 min. After the completion of reaction, the reaction mixture is cooled to 70° and affords the 2-aminooxazole derivatives.
Rajaguru et al.[46] reported synthesis of oxazole derivatives by reacting α-azidochalcone with trifluoroacetic acid in dry two-neck round bottom vessel fitted with a calcium guard tube and a magnetic bar and then gradually heated to a higher temperature in the microwave for 30 min. The reaction is monitored by TLC using petroleum ether/ethyl acetate (8:2) as a solvent to yield oxazole derivatives.
Rostamizadeh et al.[47] synthesized azalactones using microwave assisted Erlenmeyer synthesis. Reaction of hippuric acid and substituted aldehyde or ketone with acetic anhydride and MgO/Al2O3 as a catalyst in the microwave oven. The progress of the reaction is checked by TLC and cooled to room temperature in hot ethanol (2:1) added to it and stirred for 15 min. Then the catalyst is separated by decantation to afford the final product as an azalactone.
Mollo et al.[48] examined the cyclization reaction of N-benzoylaminoethanol with PPE/CHCl3 in an openvessel microwave reactor for 8 min at 70° in reflux, which led to the synthesis of 2-phenyl-2-oxazolines with an excellent yield of 88 %. On the other hand, using a closed vessel, the yields increased to 95 %. Rahman et al.[49] synthesised diastereoselective hexahydropyrrolo[2,1-b]-oxazoles. On reacting pyrrolidine and 4-chlorobenzaldehyde as substrates under microwave irradiation in toluene for 15 min, the final diastereomer oxazole with two phenyl groups in trans form.
Kadagathur et al.[50] establish the construction of a novel series of 2-aminobenzoxazole derivatives. This includes reactions between o-amino phenol and phenyl isothiocynante using various oxidants under different reaction conditions such as changing temperature and reaction time. To begin, the reaction is carried out in a microwave with ethanol as the solvent and no oxidant to produce the final product of N- phenylbenzo[d]oxazol-2-amine.
Ziarani et al.[51] investigate the synthesis of novel benzoxazole derivatives by performing a condensation reaction between 2-amino-3- hydroxypyridine and benzoyl chloride using Santa Barbara Amorphous-15 (SBA-15) as a basic nanocatalyst in microwave irradiation and solvent free conditions. This reaction proceeds in two ways by using DMF as solvent at optimum temperature in the presence of SBA-15, resulting in the formation of N-(3-hydroxy-2-pyridyl) Benzamide and substituted benzoic acid as an intermediate. Then, using the same reagents under microwave at room temperature, this gives 2-aryloxazolo-[4,5-b] pyridine as a final product (fig. 7).
Fig. 7: Microwave assisted synthesis; (A): Two component [3+2] cycloaddition reaction; (B): Synthesis of derivatives of 2-amino-4-(p-substitutedphenyl) oxazole; (C): Suzuki reaction; (D): Pyrazole derived oxazole molecules; (E): 2-aminooxazole derivatives; (F): Oxazole synthesis using MW irradiation; (G): Erlenmeyer synthesis of azalactones; (H): Cyclisation reaction and (I): Diastereoselective synthesis of hexahydropyrrolo[2,1-b] oxazole derivatives
Synthesis of Oxazoles Using Various Catalyst
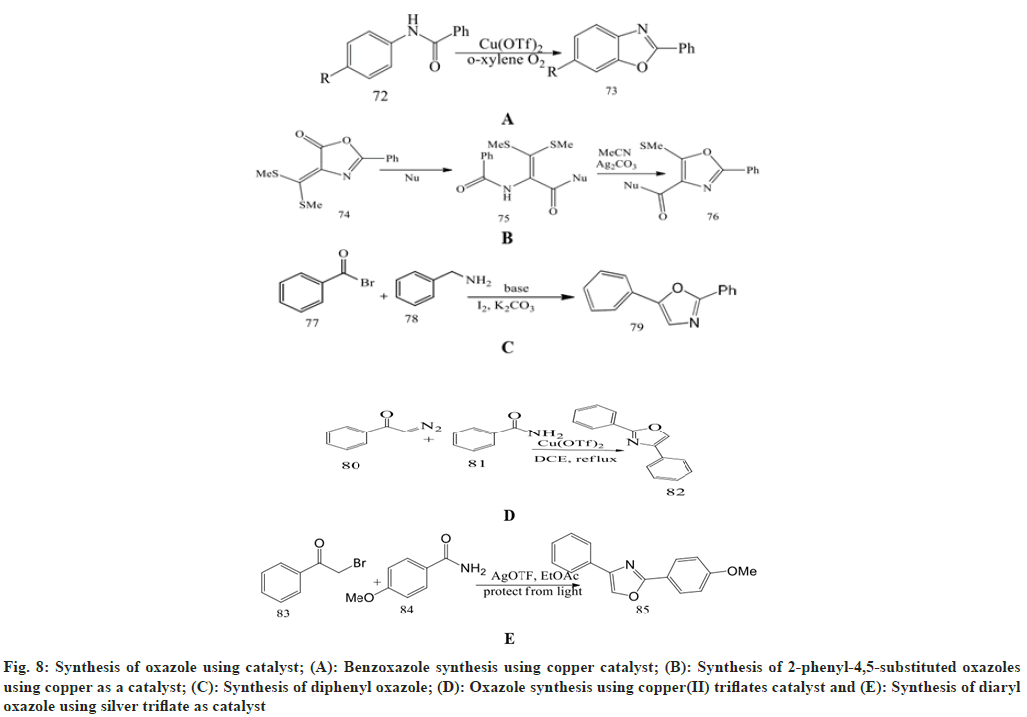
Ueda et al.[52] described an efficient, safe, and novel benzoxazole synthesis using a copper catalyst [Cu(OTf)2]/O2. The reaction of substituted benzanilides in the meta or ortho position with electron donating alkyl or alkoxy groups yielded the corresponding benzoxazole with a high yield.
Tomi et al.[53] have developed a novel multistep synthesis for oxazole and benzoxazole derivatives. Firstly, glycine is treated with 4-methoxy benzoyl chloride in a NaOH solution to give 4-methoxy hippuric acid. Then it is reacted with an equal quantity of ethyl chloroformate in the presence of N-methylmorpholine in CH2Cl2 to produce 2-(4-methoxyphenyl)-5-oxazolones. This azlactone with excess toluene and anhydrous AlCl3 at room temperature gives 2-aza-1-(4-methoxyphenyl)-4- toluel-1,4-butandiones. Then reflux with phosphorus oxychloride for 48 h to give 2-(4-methylphenyl)-5-(4- methoxyphenyl)-1,3-oxazole.
Kumar et al.[54] performed a two-step synthesis for 2-phenyl-4,5-substituted oxazoles using copper as a catalyst. The reaction involves the nucleophilic ring opening of the 4- bis(methylthio)methylene-2- phenyloxazole-5-one template to reduce N-benzoyl-- bis(methylthio)enamide. These enamide derivatives are successively changed into 2-phenyl-5- (methylthio)-4-alkoxycarbonyl/amido/acyloxazoles by using silver carbonate as a catalyst, forming the 5-endo cyclization.
Gao et al.[55] synthesized oxazole in the presence of iodine and potassium carbonate in DMF at 80° using bromoacetophenone and benzylamine as starting reagents to give 2,5-diphenyloxazole in the 46 % yield. Reddy et al.[56] describe an easy and convenient synthesis of 2,4-disubstituted oxazoles by coupling substituted α-diazoketones with substituted amides in 1,2- dichloroethane in the presence of copper(II) triflate [Cu(OTf)2].The desired product, 2,4-disubstituted oxazole, is obtained at an 87 % yield after increasing the temperature from 25° to 80°. Bailey et al.[57] identified the optimal reaction conditions for the synthesis of 2,4-disubstituted and 2,4,5-trisubstituted oxazoles by using haloketones and substituted arylamides in the presence of ethyl acetate and silver triflate (AgOTF) as a catalyst used for promotion of cyclization reaction, observing the effect of temperature, stoichiometry, and optimum light on the process.
Hu et al.[58] explored several cyclization reactions of propargylic amides, because of their rapid association of structural complexity and functional group similarity, in the recent years this method has gained consideration. The reaction is effectively attained with the help of various transition metals, Bronsted acid, Lewis acid, halogens, strong base, nontransition metals and catalyst mediated cyclization.
Yamada et al.[59] developed a novel synthetic method for the synthesis of trisubstituted oxazoles through a one-pot oxazole synthesis/Suzuki-Miyaura coupling. In this reaction, carboxylic acid, dehydrative condensing agent, amino acids and (4-(4,6-dimethoxy- 1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride) are used. In the presence of a Ni catalyst, Suzuki- Miyaura coupling with boronic acids produces a resultant 2,4,5-trisubstituted oxazole with excellent yield. Regalla et al.[60] aimed at the synthesis of 2,4-disubstituted oxazole using palladium/copper as a catalyst by direct arylation. This has been synthesised by reacting 4-substituted oxazole with aryl bromide in the presence of potassium hydroxide, CuI and Pd (PPh3)4 in dimethoxyethane (fig. 8).
Fig. 8: Synthesis of oxazole using catalyst; (A): Benzoxazole synthesis using copper catalyst; (B): Synthesis of 2-phenyl-4,5-substituted oxazoles using copper as a catalyst; (C): Synthesis of diphenyl oxazole; (D): Oxazole synthesis using copper(II) triflates catalyst and (E): Synthesis of diaryl oxazole using silver triflate as catalyst
Ultraosund Assisted Synthesis Of Oxazoles
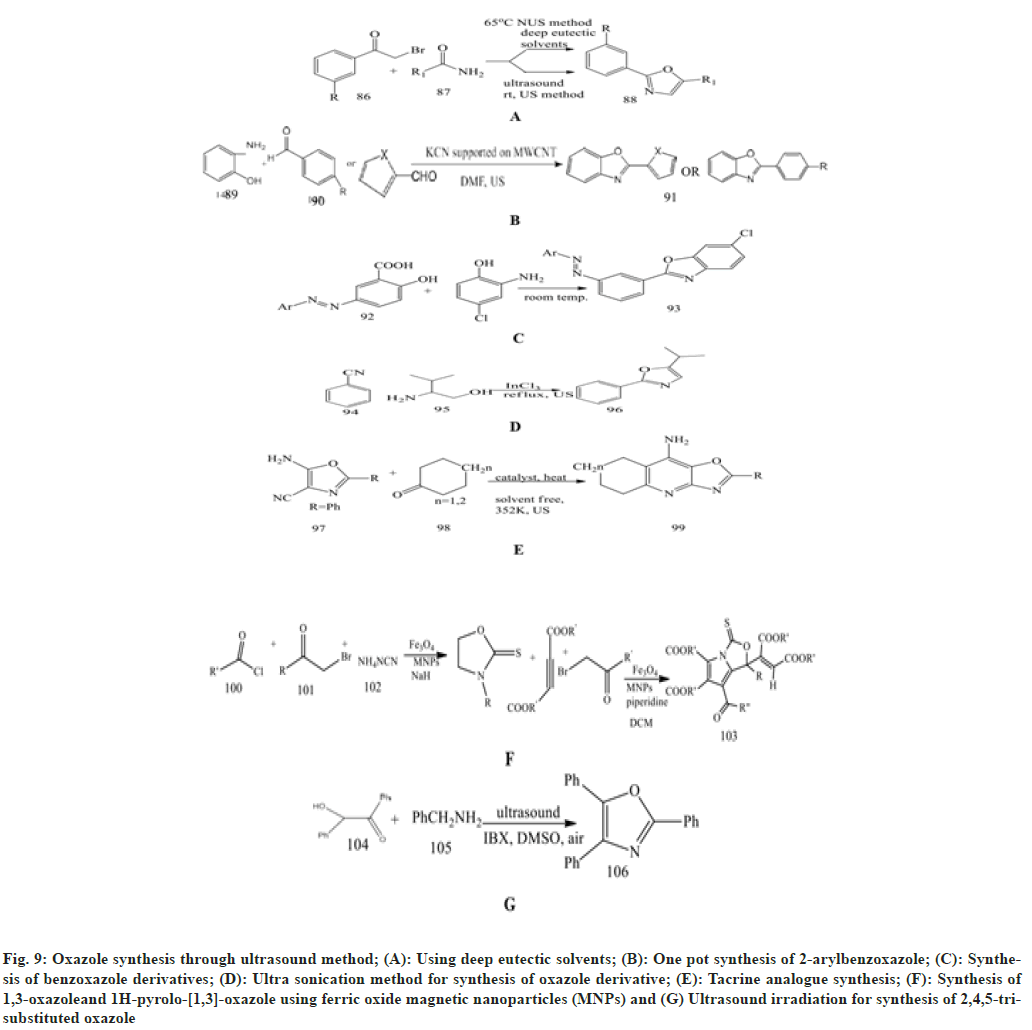
Singh et al.[61] described a novel synthesis of oxazole derivatives through Ultrasound (US) and Deep Eutectic Solvent (DES). As the reaction between 4-substituted phenacylbromide and amide derivatives goes on, DES are added and stirred for 3-5 min. Then TLC is used to check the reaction at 65°. This is then extracted with dichloromethane (DCM).
Naeimi et al.[62] presented an easy and effective technique for one-pot synthesis of 2- arylbenzoxazoles, which are promptly synthesised from a mixture of o-aminophenol and aromatic aldehyde or heteroaromatic aldehyde using MWCN held KCN in DMF using ultrasound irradiation with a power of 60 W at 55 % amplitude and at a frequency of 20 kHz for a range of 12-650 s. For the completion of a reaction, TLC is used. The product benzoxazole was characterised by the spectroscopic data.
Nikpassand et al.[63] investigated a green approach by using ultrasound for the environmentally friendly and safer synthesis of benzoxazole derivatives. The mixture of azo-linked substituted salicylic acid with different quantities, 1.14 gm of 2-amino- 4-chlorophenol and 10 ml of ethanol is added to a Pyrex glass open vessel and irradiated with ultrasound in a water bath under silent conditions at room temperature.
Kaur et al.[64] illustrate the synthesis of various heterocyclic compounds using the ultrasonication method due to its short reaction time, high yield and mild reaction environment. After 30 min of ultrasonic irradiation, a reaction occurs between benzonitrile and 2-aminoethanol and is mixed with InCl3 to synthesise 2-phenyloxazoline with excellent yield.
Nikseresht et al.[65] performed reaction between 5 - a m i n o - 4 - c y a n o - 2 - p h e n y l - 1 , 3 - o x a z o l e , cyclohexanone and activated [Cu3(BTC)2] and heated for 353 K for 2 h. The mixture is placed in an ultrasonic bath and irradiated at room temperature (20-25°). Completion of the reaction is monitored by TLC 1:2 (EtOAc/n-hexane as eluent). This method is easy, efficient, and environmentally friendly for synthesising tacrine analogues in the presence of [Cu3(BTC)2] as a catalyst.
Abdolmohammadi et al.[66] synthesis of 1,3-oxazole and 1H-pyrolo-[1,3]-oxazole, both of which are effective antioxidants with high yield. To the solution of alpha bromoketone, add acid chloride by magnetically stirring, ammonium thiocyanate, NaH, and Fe3O4 Magnetic Nanoparticles (MNPs) in 5ml of water at 50° for 30 min from the start of the reaction. Afterwards, the 1,3-oxazole solution is magnetically stirred, then activated acetylenic compound and piperidine in 5 ml of DCM are added. Then a solution of alpha-bromoketone and activated acetylenic compound by magnetically stirring with Fe3O4 MNPs for about 10-20 min with the stirring.
Kandula et al.[67] used 2-Iodoxybenzoic acid as an oxidant in dimethyl sulfoxide at 30° in the presence of air under ultrasound irradiation to perform the domino reaction of benzoin and substituted benzylamine due to its advantages over conventional methods. It produces a compound with an excellent yield. This 2,4,5-trisubstituted oxazole is synthesized by this method (fig. 9).
Fig. 9: Oxazole synthesis through ultrasound method; (A): Using deep eutectic solvents; (B): One pot synthesis of 2-arylbenzoxazole; (C): Synthesis of benzoxazole derivatives; (D): Ultra sonication method for synthesis of oxazole derivative; (E): Tacrine analogue synthesis; (F): Synthesis of 1,3-oxazoleand 1H-pyrolo-[1,3]-oxazole using ferric oxide magnetic nanoparticles (MNPs) and (G) Ultrasound irradiation for synthesis of 2,4,5-trisubstituted oxazole
Continuous Flow Synthesis of Oxazoles
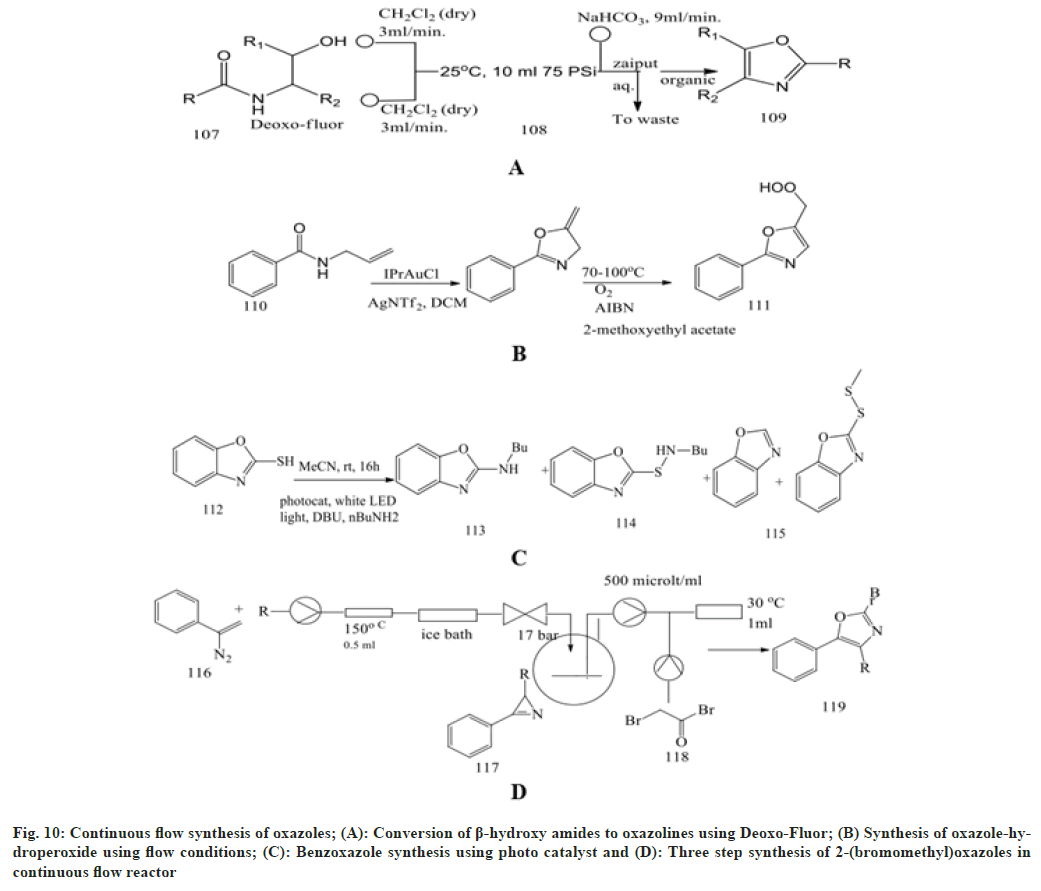
Glockner et al.[68] focused on continuous flow synthesis using Deoxo-Fluor, resulting in more than 99 % conversion of β-hydroxy amides to the oxazolines. These fluorinated compounds resulted in the synthesis of a larger number of unwanted by-products. A solution of β-hydroxy amides (0.25 M) and deoxo-fluor at a flow rate of 3.00 ml/min is combined at a T-piece before passing into a reactor coil at 25° Then sodium bicarbonate solution is added at a T-mixer to quench any residual hydrogen fluoride and then directed in to a Zaiput liquid-liquid membrane separator.
Bay et al.[69] performed the reaction of alkylideneoxazoles with molecular oxygen in a micro structured reactor to enhance safety and provide a significant application for synthesis. The synthesis of oxazole-hydroperoxide is carried out using flow conditions using starting compounds like oxazolines at temperatures ranging from 70° to 100° at two varying pressures (1 and 18 bar). This oxazoline is mixed with reagent AIBN, n-dodeacne and the solvent 2-methoxy ethylacetate in a microstructured reactor containing the PTFE-T-mixer.
Rattanangkool et al.[70] screened the photoredox catalysis through an amination reaction between 2-mercaptobenoxazole and n-butylamine through irradiation using white light-emitting diode. The reaction is carried out in the presence of a transition metal photoredox catalyst, Ru(byp)3Cl2, which results in a lower yield of benzoxazole, sulfenamide and disulphide product. So, to enhance the yield, we can use both Rose Bengal and EosinY, which give an excellent yield. In recent years, continuous flow synthesis has gained attention in organic synthesis as it is safer, more efficient, waste reduction and requires fewer solvents.
Rossa et al.[71] developed a three-step synthesis of vinyl azides in a continuous flow reactor containing a Perfluoroalkoxy coil immersed in a silicon bath at 150°. The vinyl azide solution in acetone is delivered into the reactor using a syringe pump to generate azirines under nitrogen gas generation, which is then reacted with bromoacetyl bromide in the reaction vessel placed between the reaction zones to remove excess nitrogen to yield 2-(bromomethyl) oxazoles.
Bracken et al.[72] performed a two-step synthesis of claisen condensation between acetophenones and diethyl oxalate to form 1,3-dicarbonyls, which were treated with hydroxylamine hydrochloride in a thermal cyclocondensation process to produce isoxazole with excellent yield. This compound undergoes further reaction under the vapourtec E-series module at a flow rate of 0.5 ml/min. Different solvents have been used, like DCM, acetone, tetrahydrofuran and ethanol. Temperatures should be kept between 25- 45° and nitrogen gas should be used to produce oxazole.
Ramanjaneyulu et al.[73] created a flow synthesis by reacting benzo[d]oxazole-thiol and benzhydryl bromide as starting materials in reactor vessels with litium thiolate species. This forms a reacting intermediate which, on reaction with n-BuLi and using two T-micromixers M1 and M2, forms a desired product with good yield (fig. 10).
Fig. 10: Continuous flow synthesis of oxazoles; (A): Conversion of β-hydroxy amides to oxazolines using Deoxo-Fluor; (B) Synthesis of oxazole-hydroperoxide using flow conditions; (C): Benzoxazole synthesis using photo catalyst and (D): Three step synthesis of 2-(bromomethyl)oxazoles in continuous flow reactor
Synthesis of Oxazoles Using Ionic Liquids
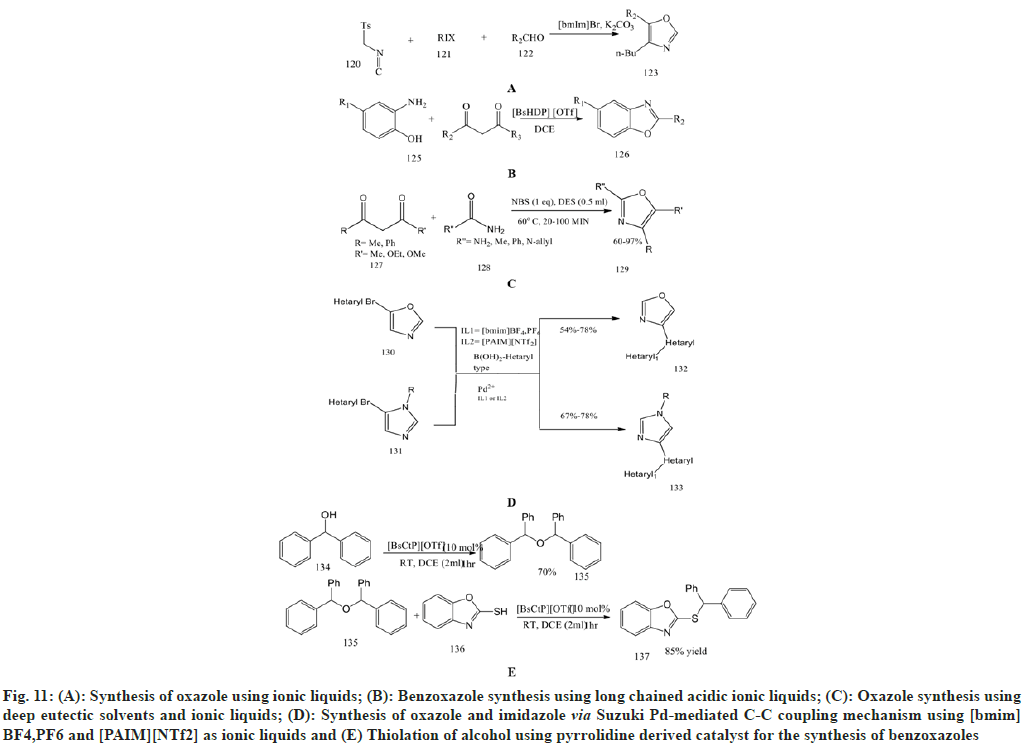
Wu et al.[74] prepared novel oxazoles using advanced one-pot van Leusen synthesis in ionic liquids. The oxazoles were synthesized from TosMIC with aliphatic halides, [bmim] Br as solvents and aldehydes, which produced a high yield of products. This ionic liquid can also be reused up to six times without any loss of yield.
Miao C et al.[75] synthesized novel benzoxazole derivatives using metal-free and efficient long-chained acidic ionic liquids. It involved intermolecular cyclisation of 2-amino phenol with 2, 4-pentanedione in the presence of ionic liquids to synthesise benzoxazole. The long-chained ionic liquids such as [BsMeP] [OTf] and [BsHDP] [OTf] are better than simple ionic liquids showing micellar action and surfactivity and could be recycled at least six times.
Azizi N et al.[76] performed one-pot synthesis using DES as an ionic liquid to synthesise 2-aminooxazole and 2-aminothiazole derivatives. This includes a one-pot three-component reaction between urea or thiourea, active methylene compounds and N-Bromosuccinamide (NBS) using DES as an effective catalyst to produce 2-aminooxazole and 2-aminothizole with excellent yield in mild reaction conditions.
Abonia R et al.[77] synthesized oxazole derivatives using imidazolium as an ionic liquid in the Suzuki Pd-mediated C-C coupling mechanism along with Piperidine-Appended Imidazolium [PAIM] [NTf2] ion as a specific ionic liquid Previously synthesised oxazoles and imidazoles are obtained via van Leusen synthesis and subjected to Suzuki Pd-catalyzed C-C coupling reactions. This mechanism is highly effective for synthesising imidazole and oxazole compounds with good yield.
Miao C et al.[78] synthesis of oxazole derivatives using pyrrolidine-derived long-chain ionic liquids. This synthesis involves the thiolation of alcohols to prepare variouscompounds containing a thioether moiety. The ionic liquids are more effective than common ionic liquids containing imidazole, providing 99 % yield with [BsCtP] [OTf] as a catalyst. This catalyst is widely applicable for the reaction of aromatic alcohol with aliphatic thiols and aromatic thiols such as benzothiazole-2-thiols and benzoxazole-2- thiols.
Cheng et al.[79] investigated one-pot synthesis for the preparation of 4-aryl2-phenyloxazoles by performing cyclocondensation of benzamide with [(2,4-dinitrobenzene) sulfonyl]oxy molecule in the presence of [Bmin] [PF6] at 80°. As shown in scheme 58, the ionic liquid in this reaction plays a very important role both as solvent and promoter and can be easily recovered and reused without any loss of activity.
Zhou et al.[80] focused on direct oxidative amination using ionic liquids as catalysts for benzoxazoles at room temperature and metal-free conditions. This oxidative amination reaction is carried out under mild catalytic states between several derivatives of benzoxazoles and secondary amines to give substituted benzoxazole derivatives. The use of ionic liquid [BPy] is inexpensive and eco-friendly and can be recycled and reused more than four times without any loss of catalytic activity.
Muthyala et al.[81] performed a novel and highly effective one-pot synthesis for 2,4- disubstituted thiazoles and oxazoles from substituted ketone derivatives and reacting with amides/urea and thiamides/thiourea using phenyltrimethylammonium bromide (PTT) as an in situ brominating agent under [bmim] [BF4] as an ionic liquid. This procedure has great advantages over other methods due to the avoidance of hazardous organic chemicals, the handling of lacrymetric solvents, and the toxic catalyst (fig. 11).
Fig. 11: (A): Synthesis of oxazole using ionic liquids; (B): Benzoxazole synthesis using long chained acidic ionic liquids; (C): Oxazole synthesis using deep eutectic solvents and ionic liquids; (D): Synthesis of oxazole and imidazole via Suzuki Pd-mediated C-C coupling mechanism using [bmim] BF4,PF6 and [PAIM][NTf2] as ionic liquids and (E) Thiolation of alcohol using pyrrolidine derived catalyst for the synthesis of benzoxazoles
Conclusion
This pioneer literature reveals the importance of oxazole as a conversant nucleus, showing immense potential for the advancement of potent novel chemical entities retaining anti-inflammatory, antidiabetic, antiviral, antibacterial, anticancer, analgesic, and antihypertensive activity. This article also studied various synthetic pathways and reactions undergoing oxazole and its derivatives. Finally, different synthetic methods have been studied for the synthesis of di-substituted and tri-substituted oxazole derivatives through multicomponent reactions shown in different literature sites, which have been presented in this review article. Through various literature surveys, we have summarized all the multicomponent synthesis of the oxazole moiety using different methods, which include conventional methods, microwave assisted techniques, catalytic synthesis, and ultrasound-assisted synthesis. Overall, this review summarizes various green methods for the synthesis of oxazole and its derivatives.
Conflict of interests:
Authors declare that there is no conflict of interest.
References
- Al-Mulla A. A review: Biological importance of heterocyclic compounds. Der Pharma Chemica 2017;9(13):141-7. [Crossref]
[Google scholar] [PubMed]
- Zhang HZ, Zhao ZL, Zhou CH. Recent advance in oxazole-based medicinal chemistry. European journal of medicinal chemistry. 2018;144:444-92.
[Crossref] [Google scholar] [PubMed]
- Shriram P, Kumar D, Sudhir I, Asif K. Study on synthesis and different biological activities of oxazole based derivatives. Uni Res J Chem 2013;1:16-29. [Crossref]
[Google scholar] [PubMed]
- Kharb R, Sharma J, Sharma AK. Vistas of novel oxazole derivatives as potent antimicrobial agents. Int J Adv Pharm Bio Chem 2013;2(2):390-404.
- Kakkar S, Narasimhan B. A comprehensive review on biological activities of oxazole derivatives. BMC Chem 2019;13(1):1-24.
[Crossref] [Google scholar] [PubMed]
- Rymbai EM, Chakraborty A, Choudhury R, Verma N, De B. Review on chemistry and therapeutic activity of the derivatives of furan and oxazole: The oxygen containing heterocycles. Der Pharma Chemica. 2019;11(1):20-41.
- Joshi S, Bisht AS, Juyal D. Systematic scientific study of 1, 3-oxazole derivatives as a useful lead for pharmaceuticals: A review. Pharm Innov 2017;6(1, Part B):109.
- Shankar M, Srividya BK, Hemachandar K, Sivanna P, Babu MN. Importance of structural activity relationship in computer aided drug design: A review. Eur J Pharm Sci Res 2014;1:13-6.
- Babar SP, Kamble VM. Synthesis of thiazole and oxazole derivatives by organic clay as a novel method and their biological evaluation. Int J Res App Sci Eng Technol 2019;7(8),602-11. [Crossref]
[Google scholar] [PubMed]
- Hashim ZB, Atia AJ, Al-Bayti RI, Al-Marjani MF, Salih RH. Synthesis of new 1, 3-thiazole and 1, 3 oxazole from 3-chlorobenzo [b] thiophene and evaluation of anti-bacterial activity. J Pharm Sci Res 2018;10(6):1629-34. [Crossref]
[Google scholar] [PubMed]
- Zhao S, Zhang X, Wei P, Su X, Zhao L, Wu M, et al. Design, synthesis and evaluation of aromatic heterocyclic derivatives as potent antifungal agents. EurJ Med Chem 2017;137:96- 107.
[Crossref] [Google scholar] [PubMed]
- Zhang MZ, Chen Q, Xie CH, Mulholland N, Turner S, Irwin D et al. Synthesis and antifungal activity of novel streptochlorin analogues. EurJ Med Chem 2015;92:776-83.
[Crossref] [Google scholar] [PubMed]
- Zahanich I, Kondratov I, Naumchyk V, Kheylik Y, Platonov M, Zozulya S, et al. Phenoxymethyl 1, 3-oxazoles and 1, 2, 4-oxadiazoles as potent and selective agonists of free fatty acid receptor 1 (GPR40). Bioorg Med Chem Lett 2015;25(16):3105-11.
[Crossref ] [Google scholar] [PubMed]
- Senger J, Melesina J, Marek M, Romier C, Oehme I, Witt O, et al. Synthesis and biological investigation of oxazole hydroxamates as highly selective histone deacetylase 6 (HDAC6) inhibitors. J Med Chem 2016;59(4):1545-55.
[Crossref] [Google scholar] [PubMed]
- Kachaeva MV, Pilyo SG, Zhirnov BV. Oxazole-5-sulfonamides as Novel Promising Anticancer lead compounds. Int J Curr Res 2018;10(5):69410-25.
- Mathew B, Hobrath JV, Connelly MC, Guy RK, Reynolds RC. Oxazole and thiazole analogs of sulindac for cancer prevention. Fut Med Chem 2018;10(7):743-53.
[Crossref ] [Google scholar] [PubMed]
- Hurenko AO, Solomyanny RM, Mrug GP, Frasinyuk MS, Pilyo SG, Kornienko AM, et al. Auxin-like effect of derivatives of pyrimidine, pyrazole, isoflavones, pyridine, oxazolopyrimidine and oxazole on acceleration of vegetative growth of flax. Int J Pharm Tech Res 2018;11(3):274-86.
- Nagarajan K, Rao SK, Shridhara K, Nayak SP, Thomas SP, Row GT, et al. Structural studies on nitroimidazooxazoles with antitubercular and antileishmanial activities. Ind J Chem 2017;56B:145-51.
- Carballo RM, Patrón-Vázquez J, Cáceres-Castillo D, Quijano-Quiñones R, Herrera-España A, Moo-Puc RE, et al. Synthesis and in vitro antiprotozoal activity of some 2-amino-4-phenyloxazole derivatives. Tropical Journal of Pharmaceutical Research. 2017 Sep 7;16(8):1951-6.
- Abdellatif KR, Amin NH, Mohammed AA. Synthesis of some benzoxazole derivatives and their anti-inflammatory evaluation. J Chem Pharm Res 2016;8(4):1253-61.
- Ehsani A. Inhibitory effect of new oxazol derivative on corrosion of stainless steel in acidic medium: An electrochemical investigation. Ind J Chem Tech 2016;23:289-295.
- Koufaki M, Fotopoulou T, Kapetanou M, Heropoulos GA, Gonos ES, Chondrogianni N. Microwave-assisted synthesis of 3, 5-disubstituted isoxazoles and evaluation of their anti- ageing activity. Eur J Med Chem 2014;83:508-15.
[Crossref] [Google scholar] [PubMed]
- Manfrin A, Borduas-Dedekind N, Lau K, McNeill K. Singlet oxygen photooxidation of peptidic oxazoles and thiazoles. J Org Chem 2019;84(5):2439-47.
- Averina EB, Vasilenko DA, Gracheva YA, Grishin YK, Radchenko EV, Burmistrov VV, et al. Synthesis and biological evaluation of novel 5-hydroxylaminoisoxazole derivatives as lipoxygenase inhibitors and metabolism enhancing agents. Bioorg Med Chem 2016;24(4):712-20.
[Crossref] [Google scholar] [PubMed]
- Ke S, Zhang Z, Shi L, Liu M, Fang W, Zhang Y, et al. An efficient synthesis and bioactivity evaluation of oxazole-containing natural hinduchelins A–D and their derivatives. Org Biomol Chem 2019;17(14):3635-9.
[Crossref] [Google scholar] [PubMed]
- Swellmeen L. 1, 3-Oxazole derivatives: A review of biological activities as antipathogenic. Der Pharma Chemica. 2016;8(13):269-86.
- Li JJ, Li JJ. Robinson–Gabriel synthesis. Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications. 2009:472-3.
- Turchi IJ, Dewar MJ. Chemistry of oxazoles. Chem Reviews 1975;75(4):389-437.
- Joshi S, Bisht AS, Juyal D. Systematic scientific study of 1, 3-oxazole derivatives as a useful lead for pharmaceuticals: A review. Pharm Innov 2017;6(1, Part B):109.
- Li JJ, Li JJ. Fischer oxazole synthesis. Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications. 2009:229-30.
- Zheng X, Ma Z, Zhang D. Synthesis of imidazole-based medicinal molecules utilizing the van leusen imidazole synthesis. Pharmaceuticals 2020;13(3):37.
[Crossref ] [Google scholar] [PubMed]
- Erian AW, Sherif SM, Gaber HM. The chemistry of α-haloketones and their utility in heterocyclic synthesis. Molecules 2003;8(11):793-865.
[Crossref ] [Google scholar] [PubMed]
- Pei W, Li S, Nie X, Li Y, Pei J, Chen B, et al. Convenient syntheses of 2-alkyl (aryl)-4, 5-diphenyloxazoles and 2-alkyl (aryl)-4-phenyloxazoles. Synthesis 1998;1998(09):1298-304.
- Hu Y, Xin X, Wan B. Cyclization reactions of propargylic amides: Mild access to N-heterocycles. Tetrahedron Lett 2015;56(1):32-52.
- Al-hazazm HA, Rhadi HA. Synthesis, characterization and biological study of some new 1, 3-oxazolone-5 (4h)-one derivatives. Synthesis 2015;7(2):40-5.
- Murru S, Bista R, Nefzi A. Synthesis of novel oxazolyl amino acids and their use in the parallel synthesis of disubstituted oxazole libraries. Synthesis 2018;50(7):1546-54.
- Wang SL, Shi YJ, He HB, Li Y, Li Y, Dai H. Synthesis and bioactivity of novel pyrazole oxime derivatives containing oxazole ring. Chin Chem Lett 2015;26(6):672-4.
- Yan Z, Liu A, Ou Y, Li J, Yi H, Zhang N, et al. Design, synthesis and fungicidal activity evaluation of novel pyrimidinamine derivatives containing phenyl-thiazole/oxazole moiety. Bioorg Med Chem 2019;27(15):3218-28.
- Zhao S, Zhang X, Wei P, Su X, Zhao L, Wu M, et al. Design, synthesis and evaluation of aromatic heterocyclic derivatives as potent antifungal agents. Eur J Med Chem 2017;137:96-107.
[Crossref] [Google scholar] [PubMed]
- Yasaei Z, Mohammadpour Z, Shiri M, Tanbakouchian Z, Fazelzadeh S. Isocyanide reactions toward the synthesis of 3-(oxazol-5-yl) quinoline-2-carboxamides and 5-(2-tosylquinolin-3-yl) oxazole. Frontiers Chem 2019;7:433.
[Crossref ] [Google scholar] [PubMed]
- Phalke PL. Synthesis of different α, β-unsaturated oxazolone derivatives. J Drug Del Therap 2019;9(1):124-7.
- Mukku N, MadivalappaDavanagere P, Chanda K, Maiti B. A facile microwave-assisted synthesis of oxazoles and diastereoselective oxazolines using aryl-aldehydes, p- toluenesulfonylmethylisocyanide under controlled basic conditions. ACS omega 2020;5(43):28239-48.
[Crossref ] [Google scholar] [PubMed]
- Singh RK, Bhatt A, Chauhan PK, Kant R. Design, Synthesis and Biological evaluation of some novel biphenyl substituted oxazole derivatives. Chem Biol Interface 2017;7(1):32-9.
- Hafez HN. Microwave-assisted synthesis and cytotoxicity evaluation of some novel pyrazole containing imidiazole, pyrazole, oxazole, thiadiazole and benzochromene derivatives. Egypt J Chem 2017;60(6):1015-28.
- Kumar R, Subban R, Sundaram K, Venkatachalapthi S, Ali M. Conventional and microwave assisted synthesis of 2-aminothiazoles and oxazoles and their anti cancer activity. Indo Am J Pharm Res. 2015;5:555-61.
- Rajaguru K, Mariappan A, Suresh R, Manivannan P, Muthusubramanian S. Investigation on the reactivity of α-azidochalcones with carboxylic acids: Formation of α-amido-1, 3-diketones and highly substituted 2-(trifluoromethyl) oxazoles. Beilstein J Org Chem 2015;11(1):2021-8.
- Rostamizadeh N, Khajeh-Amiri A, Moghanian H. Microwave-assisted Erlenmeyer synthesis of azlactones catalyzed by MgO/Al2O3 under solvent-free conditions. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chem 2016;46(5):631-4.
- Mollo MC, Orelli LR. Microwave-assisted synthesis of 2-aryl-2-oxazolines, 5, 6-dihydro-4 h-1, 3-oxazines, and 4, 5, 6, 7-tetrahydro-1, 3-oxazepines. Org lett 2016;18(23):6116-9.
- Rahman M, Bagdi AK, Mishra S, Hajra A. Functionalization of an sp 3 C–H bond via a redox-neutral domino reaction: diastereoselective synthesis of hexahydropyrrolo [2, 1-b] oxazoles. Chem Commun 2014;50(22):2951-3.
[Crossref ] [Google scholar]
- Kadagathur M, Sigalapalli DK, Patra S, Tangellamudi ND. Microwave-assisted hydrogen peroxide-mediated synthesis of benzoxazoles and related heterocycles via cyclodesulfurization. Syn Commun 2021;51(14):2213-24.
- Ziarani GM, Nahad MS, Lashgari N, Badiei A. Facile microwave-assisted synthesis of 2- aryloxazolo [4, 5-b] pyridines using SBA-Pr-NH2. J Nanostruct Chem 2015;5(1):39-44.
- Ueda S, Nagasawa H. Synthesis of 2‐arylbenzoxazoles by copper‐catalyzed intramolecular oxidative c-o coupling of benzanilides. Angew Chem Int Ed Engl 2008;47(34):6411-3.
[Crossref] [Google scholar] [PubMed]
- Tomi IH, Tomma JH, Al-Daraji AH, Al-Dujaili AH. Synthesis, characterization and comparative study the microbial activity of some heterocyclic compounds containing oxazole and benzothiazolemoieties. J Saudi Chem Soc 2015;19(4):392-8.
- Vijay Kumar S, Saraiah B, Misra NC, Ila H. Synthesis of 2-phenyl-4, 5-substituted oxazoles by copper-catalyzed intramolecular cyclization of functionalized enamides. J Org Chem 2012;77(23):10752-63.
- Gao WC, Wang RL, Zhang C. Practical oxazole synthesis mediated by iodine from α- bromoketones and benzylamine derivatives. Org Biomol Chem 2013;11(41):7123-8.
- Reddy MR, Reddy GN, Mehmood U, Hussein IA, Rahman SU, Harrabi K, et al. Copper (II) Triflate Catalyzed Synthesis of 2, 4-Disubstituted Oxazoles from α-Diazoketones. Synthesis 2015;47(21):3315-20.
- Bailey JL, Sudini RR. Synthesis of 2, 4-and 2, 4, 5-substituted oxazoles via a silver triflate mediated cyclization. Tetrahedron Lett 2014;55(27):3674-7.
- Hu Y, Xin X, Wan B. Cyclization reactions of propargylic amides: Mild access to N- heterocycles. Tetrahed Lett 2015;56(1):32-52.
[Crossref ] [Google scholar]
- Yamada K, Kamimura N, Kunishima M. Development of a method for the synthesis of 2, 4, 5-trisubstituted oxazoles composed of carboxylic acid, amino acid, and boronic acid. Beilstein J Org Chem 2017;13(1):1478-85.
[Crossref ] [ Google scholar] [PubMed]
- Regalla VR, Addada RK, Puli VS, Saxena AS, Chatterjee A. Highly efficient synthesis of 2, 4-disubstituted oxazoles through palladium/copper comediated direct arylation reaction. Asian J Pharm Clin Res. 2018;11(8):511-4.
- Singh BS, Lobo HR, Pinjari DV, Jarag KJ, Pandit AB, Shankarling GS. Ultrasound and deep eutectic solvent (DES): A novel blend of techniques for rapid and energy efficient synthesis of oxazoles. Ultrason Sonochem 2013;20(1):287-93.
[Crossref] [Google scholar] [PubMed]
- Naeimi H, Rahmatinejad S, Nazifi ZS. A mild convenient ultrasound assisted synthesis of 2-aryl benzoxazoles catalyzed by KCN/MWCNT as an efficient heterogeneous nanocatalyst. J Taiwan Inst Chem Eng 2016;58:1-7.
- Nikpassand M, Fekri LZ, Farokhian P. An efficient and green synthesis of novel benzoxazoleunder ultrasound irradiation. Ultrason Sonochem 2016;28:341-5.
[Crossref] [Google scholar] [PubMed]
- Kaur N. Ultrasound-assisted green synthesis of five-membered O-and S-heterocycles. Synth Commun 2018;48(14):1715-38.
- Nikseresht A, Ghasemi S, Parak S. [Cu3 (BTC)2]: A metal–organic framework as an environment-friendly and economically catalyst for the synthesis of tacrine analogues by Friedländer reaction under conventional and ultrasound irradiation. Polyhedron 2018;151:112-7.
[Crossref ] [ Google scholar]
- Abdolmohammadi S, Hossaini Z. Fe3O4 MNPs as a green catalyst for syntheses of functionalized [1, 3]-oxazole and 1 H-pyrrolo-[1, 3]-oxazole derivatives and evaluation of their antioxidant activity. Mol Diversity 2019;23(4):885-96.
- Kandula VR, Pothireddy M, Babu KS, Kapavarapu R, Dandela R, Pal M. Sonochemical synthesis of polyarylated oxazoles as potential cytotoxic agents. Tetrahedron Lett 2021;70:153011. [Crossref ]
- Glöckner S, Tran DN, Ingham RJ, Fenner S, Wilson ZE, Battilocchio C, et al. The rapid synthesis of oxazolines and their heterogeneous oxidation to oxazoles under flow conditions. Org Biomol Chem 2015;13(1):207-14.
- Bay S, Baumeister T, Hashmi AS, Rö der T. Safe and fast flow synthesis of functionalized oxazoles with molecular oxygen in a microstructured reactor. Org Process Res Dev 2016;20(7):1297-304.
- Rattanangkool E, Sukwattanasinitt M, Wacharasindhu S. Organocatalytic visible light enabled SNAr of heterocyclic thiols: A metal-free approach to 2-aminobenzoxazoles and 4-aminoquinazolines. J Org Chem 2017;82(24):13256-62.
- Rossa TA, Suveges NS, Sá MM, Cantillo D, Kappe CO. Continuous multistep synthesis of 2- (azidomethyl) oxazoles. Beilstein J Org Chem 2018;14(1):506-14.
[Crossref ] [Google scholar]
- Bracken C, Baumann M. Development of a continuous flow photoisomerization reaction converting isoxazoles into diverse oxazole products. J Org Chem 2020;85(4):2607-17.
- Ramanjaneyulu BT, Vidyacharan S, Ahn GN, Kim DP. Ultrafast synthesis of 2-(benzhydrylthio) benzo [d] oxazole, an antimalarial drug, via an unstable lithium thiolate intermediate in a capillary microreactor. React Chem Eng 2020;5(5):849-52.
[Crossref ] [ Google scholar] [PubMed]
- Wu B, Wen J, Zhang J, Li J, Xiang YZ, Yu XQ. One-pot van Leusen synthesis of 4, 5-disubstituted oxazoles in ionic liquids. Synlett 2009;2009(03):500-4.
- Miao C, Hou Q, Wen Y, Han F, Li Z, Yang L, et al. Long-chained acidic ionic liquids- catalyzed cyclization of 2-substituted aminoaromatics with β-diketones: A metal-free strategy to construct benzoazoles. ACS Sustainable Chem Eng 2019;7(14):12008-13. [Crossref ]
- Azizi N, Rahimi Z, Alipour M. Deep eutectic solvent-assisted one-pot synthesis of 2-aminothiazole and 2-aminoxazole derivatives. Comptes Rendus Chimie 2015;18(6):626-9.
- Abonia R, Laali KK. Ionic liquid-mediated synthesis and functionalization of heterocyclic compounds. Adv Heterocyclic Chem 2019;128:333-431.
- Miao C, Zhuang H, Wen Y, Han F, Yang QF, Yang L, Li Z, Xia C. Efficient thiolation of alcohols catalyzed by long chained acid‐functionalized ionic liquids under mild conditions. Eur J Org Chem 2019;2019(19):3012-21.
- Cheng HT, Hou RS, Wang HM, Chen LC. Hypervalent iodine (iii) sulfonate reagent mediated synthesis of 4‐aryl‐2‐phenyloxazoles in ionic liquid. J Chin Chem Soc 2008;55(4):919-22.
- Zhou Y, Liu Z, Yuan T, Huang J, Liu C. The synthesis of 2-aminobenzoxazoles using reusable ionic liquid as a green catalyst under mild conditions. Molecules 2017;22(4):576.
[Crossref] [Google scholar] [PubMed]
- Muthyala MK, Kumar A. A novel and efficient one pot synthesis of 2, 4‐disubstituted thiazoles and oxazoles using phenyltrimethylammoniumtribromide in ionic liquid. J Heterocycl Chem 2012;49(4):959-64.