- *Corresponding Author:
- D. Yu
Department of Digestive Disease, Huangshi Central Hospital, Edong Healthcare Group, No.141 Tianjin Road, Huangshi City, 435000, China
E-mail: yudelindl_1997@163.com
| This article was originally published in a special issue, “Trends in Therapeutic Management of Various Clinical Conditions-II” |
| Indian J Pharm Sci 2021:83(2)Spl issue;46-50 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aims to discuss the relationship between gastric perfusion and gastric mucosal injury induced by acute cerebrovascular disease in rats. Healthy adult male Wistar rats weighing 280-320 g are randomly divided into the following 4 groups: middle cerebral artery occlusion-reperfusion group, middle cerebral artery occlusion -sham group, gastric ischemia-reperfusion group and gastric ischemia-sham group, with 12 in each group. In gastric ischemia-reperfusion group, the similar changes occur to the mucous membrane of the rats, such as hyperaemia, edema, spotty or patchy hemorrhage and partial mucous exfoliation. There is no significant difference between middle cerebral artery occlusion-reperfusion group and gastric ischemia-reperfusion group in the number of gastrin cells and the expression of gas, but the number of gastrin cells and the expression of gas are significantly higher than those of their corresponding sham operation group, resulting in statistically significant difference. The gastric mucosal blood flow is significantly increased in the middle and high dose groups, which has significant difference compared with normal saline group (p<0.05). One of the important mechanisms of Calcitonin gene-related peptide protecting ischemic gastric mucosal injury is to increase gastric mucosal blood perfusion and improve microcirculation.
Keywords
Gastric blood perfusion, Acute cerebrovascular disease in rats, Correlation with gastric mucosal injury
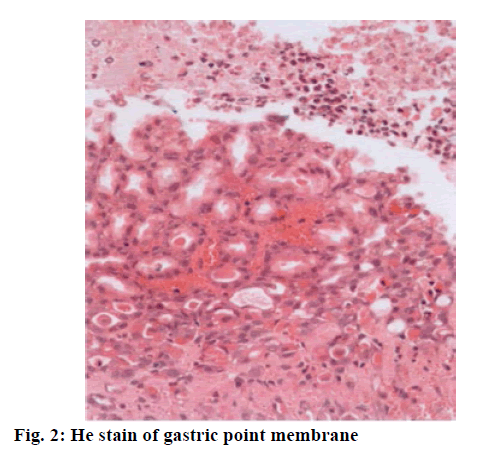
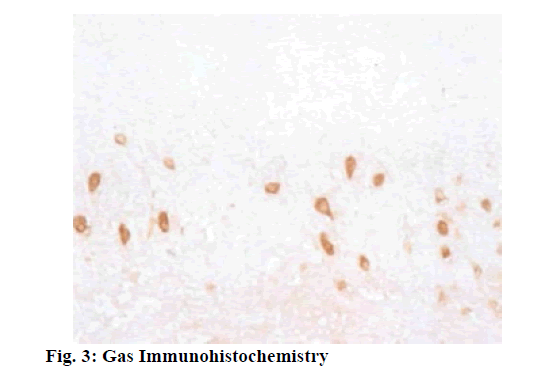
At present, it is believed that the occurrence of stress carcinoid cancer is due to the weakened protective mechanism of gastric mucosa, the relative intensified effect of injury factors, and the imbalance of neuroendocrine function of the body. The ultrastructure of gastric mucosa membrane capillary is characterized by thin blood vessel wall, with many pore-like structures with high permeability on its endothelial cells. The permeability facilitates the delivery of bicarbonate, plasma protein, and effective clearance of H+ and damaging irritants. Microcirculation can provide oxygen, various nutrients and gastrointestinal peptide hormones to mucosal cells to maintain their normal functions, including synthesis and secretion of mucus, surface-active phospholipids and bicarbonate, secretion of gastric acid, and renewal, proliferation and repair of cells, and can also timely and effectively remove cellular metabolites, reverse osmosis into mucosal H+ and injury factors, so as to maintain the relative stability of local microcirculation[1]. After mucosal epithelial damage, the mucosal microcirculation can still create a stable microenvironment in the injured area, which is beneficial to the rapid repair of injury. Prolonged hyperglycemia can reduce the regulatory effect of nitric oxide (NO) on gastric mucosal blood flow and reduce the protective effect of blood flow on cells, thus leading to gastric injury. Large dose injection of anticancer drugs can cause a significant reduction in gastric mucosal blood flow (GMBF). The reduction is insignificant at an injection dose of 50 mg/kg, and the GMBF is decreased with an increase in dose at an injection dose of 100 mg/kg or 200 mg/kg, indicating that GMBF is an important protective factor against gastric mucosal injury during chemotherapy[2]. In hypothermic cardiopulmonary shunts, dopamine can increase the microcirculatory perfusion of the stomach and without increasing oxygen delivery, oxygen consumption, and mean arterial pressure. Gastric mucosal lesion (fig. 1) is a serious complication of endotoxemia, wherein a decrease in endothelium derived relaxing factor leads to a reduction in GMBF. Compared with normal people, GMBF in patients with portal hypertension decreases significantly, and the vasomotor reflex of gastric vascular bed decreases. Sandostatin can decrease gastric mucosal blood flow in both normal and portal hypertension patients. Gastric mucosal ischemia complicated with Helicobacter pylori infection promotes formation of gastrohelcosis[3]. Intravenous administration of the trace amineassociated receptor-1 (TAR-1) agonist trifluoroacetate salt (TFLLR-NH2) in combination with inhibitors of amimycin can prevent gastric mucosal injury by hydrochloric ethanol or absolute alcohol. This protective effect can be blocked by rosiformin or a Cyclooxygenase-1 (COX-1) inhibitor, but capsaicin blocks sensory neurons from inhibiting this protective effect. Gastric flow-dependent increase in gastric mucosal produced by TFLLR-NH2 can be inhibited by blocking the endothelium-derived overexpression factor pathway and indomethacin can enhance this blocking effect. TFLLR-NH2 inhibits choline carbonateinduced gastric acid secretion in a reversible manner induced by B-indomethacin. Immunoassay analysis shows that protease-activated receptors-1 (PAR-1) and COX-1 are highly expressed in the gastric mucosa and smooth muscle layer of rats, and precursor protein is detected in blood vessels[4]. PAR-2 plays a protective role by activating capsaicin-sensitive sensory neurons. Isolatidine maleate and famotidine can reduce the degree of reduction of gastric mucosal blood flow by non-steroidal anti-inflammatory drugs. This study establishes the middle cerebral artery occlusion (MCAO) reperfusion model and the gastric ischemiareperfusion model (GI-R) model are established by the suture-occluded method and celiac artery occlusion method, observes and compares the histological and histochemical changes in gastric mucosa of MCAO rats and GI-R rats, using light, electron microscopy, and immunohistochemical techniques, and detects changes in gastric mucosal blood flow using Laser Doppler flowmeter. Combining with the experimental observation of vasoactive peptide-reducing hormone gene-related peptide on gastric mucosal injury, this study also comprehensively explores the role of gastric blood perfusion in gastric mucosal injury caused by acute cerebrovascular disease. Healthy adult male Wistar rats weighing 280-320 g are randomly divided into the following 4 groups: middle cerebral artery occlusion-reperfusion (MCAO-R) group, middle cerebral artery occlusion -sham (MCAO-sham) group, gastric ischemia-reperfusion (GI-R) group and gastric ischemia-sham (GI-sham) group, with 12 in each group. Establishment of MCAO-R model: Under the real-time detection of laser doppler flowmeter (LDF), with reference to the methods established by Koizumi et al. the healthy male Wistar rats weighing 280-320 g are improved with the following conditions, fasting for 12 h before surgery and free intake of water. Anesthetize the rats with an intraperitoneal injection of 3.5 % chloral hydrate (1 ml/100 g), and let the rats lie on their back on the operating table. Prepare the skin on the neck, fix the limbs and head, and sterilize the surgical field with 75 % alcohol. Take a midline incision to cut the skin of the neck, cut the superficial fascia, reveal the left sternocleidomastoid muscle, and bluntly separate the muscle space between the sternocleidomastoid muscle and the sternohyoid muscle, separate carotid sheath and drop a small amount of 5 g/L procaine hydrochloride solution to the surgical field in the process[5]. Expose the common carotid artery (CCA) and the vagus nerve, bluntly separate CCA, and separate the left external carotid artery (ECA) and the internal carotid artery (ICA). Ligate the proximal end of CCA, and thread the upper part of the ligature for backup. Attention shall be paid not to damage vagus nerve and ligate ECA. Temporarily clamp the CCA bifurcation with the arterial clamp, cut a “V” shaped small hole at the proximal end about 1 cm from the arterial clamp, and insert the suture. When the suture reaches the arterial clamp, tie it with a spare suture to avoid slipping. Gently pull the ECA ligature towards the outside and suture is inserted along the upper-inside angle in order to smoothly enter the ICA from the CCA and avoid mistakenly entering the pterygopalatine artery (PPA). Stop when the front end of the suture reaches the anterior cerebral artery (ACA) and encounters resistance, indicating that the suture has blocked the middle cerebral artery. As this point, the first marker reaches the ECA and ICA bifurcation points, and the second marker reaches the “V” shaped incision. The LDF shows that the blood flow drops below 30 % of the baseline value, which means that the embolization is successful. At this point, LDF sees a significant increase in blood flow, meaning reperfusion is successful. Adjust intraoperative and postoperative ambient temperature to around 25-3 (rC, paying close attention to vital signs)[6]. Use 3.5 % chloral hydrate to anesthetize rats in different groups, prepare skin in the neck and abdomen, and place them in a supine position on the operating table. Cut open the abdominal cavity along the ventral white line to expose the rat stomach. Insert a 20 ml syringe needle into the gastric cavity from the fundus, with special attention paid to avoiding damaging other parts of gastric mucosa. Insert the laser Doppler fiber probe into the gastric cavity from the needle hole, measure the gastric blood flow at each of 45 s in the order of lesser curvature, greater curvature, anterior gastric wall, and posterior gastric wall repeatedly for three times, and take the mean value to represent the basal gastric mucosal blood flow before cerebral infarction. Pull the needle out of the gastric cavity and soak it in saline. Wrap the exposed stomach with the gauze soaked in warm physiological saline, and the ligature was used to gently ligate the needle hole of the stomach so that no gastric fluid could leak out to prevent it from contaminating the abdominal cavity. Cover abdominal wound with saline gauze. Establish the model of focal cerebral ischemiareperfusion in rats by the same method as above. After embolizing the middle cerebral artery with a fishing line, insert the needle into the stomach cavity again from the needle hole, and continuously detect the gastric mucosal blood flow according to the same method[7]. After 1 hour, pull out the fish line a little, restore and reperfuse the cerebral blood flow, and detect the gastric blood flow for 30 min. The detection of the effects of exogenous Calcitonin gene-related peptide (CGRP) on gastric mucosal blood flow after cerebral ischemia-reperfusion in rats is as follows. The experimental animals are divided into 3 groups: Sham group, normal saline (NS) group and iCGRP group. The model is established with the same method as above. After 1 h after ischemia, the resume reperfusion, and NS (1 ml/100 g) and CGRP are administered. CGRP group is divided into three dose groups: Lowdose group (1.5 μg/ml, 1 ml/100 g), medium-dose group (3 μg/ml, 1 ml/100 g) and high-dose group (6 μg/ ml, 1 mV/100 g). After administration of CGRP, gastric mucosal blood flow is measured with the above method[8]. SPSS 17.0 statistical software and Microsoft Office Excel 2003 were used for statistical processing of relevant data. All data were expressed by mean±standard deviation soil±S). In the comparison of sample mean, the homogeneity test and the normality test are performed first, and the data conforming to the two tests are subjected to one-way analysis of variance. The comparison between the two groups is performed by q test. The rank sum test is used for the data that do not conform to the two tests. The test benchmark is α=0.05. Some changes occur to the mucous membrane of the rats in the MCAO group, such as hyperaemia, edema, spotty or patchy hemorrhage and partial mucous exfoliation. Under light microscope, the epithelial cells of mucous membrane are obviously damaged, there are necrosis and exfoliation, and inflammatory cells such as neutrophils and monocytes are infiltrated, and the glands are arranged in disorder. The similar changes occur to the mucous membrane of the rats of GI-R group, such as hyperaemia, edema, spotty or patchy hemorrhage and partial mucous exfoliation[9]. In the two sham operation group, the epithelium of gastric mucosa of rats has an intact structure, with clear stratification and regular arrangement of glands, and no obvious pathological changes such as inflammatory cell infiltration are observed (Figure 2). Capillary dilatation and hyperemia can also be seen in the submucosa. The G cells of MCAO group are mainly distributed in the 1/3 deep of antral mucosa, which is usually round, oval, and spindle. There is no significant difference in the morphological structure and distribution of light microscopy of G cells between the GI-R group and the two sham groups compared with the MCAO group (fig. 3). There is no statistically significant difference in the number of G cells and gas expression between the MCAO group and the GI-R group, but it is significantly higher than that of the corresponding sham operation group, group[10]. There is no statistically significant difference between the number of G cells and the expression of Gas in the two sham operation groups (Table 1). The G cells of MCAO group are mainly distributed in the 1/3 deep of antral mucosa, which is usually round, oval, and spindle. Some cell protrusions extend to adjacent cells or reach glandular cavity. There is no significant difference in the morphological structure and distribution of D-cells in the GI-R group and the two sham operation groups compared with the MCAO group. The number of D cells and the expression of somatostatin-receptor (SST) in gastric mucosa of MCAO group are significantly lower than those of MCAO-sham group (Table 2) . The number of D cells and the expression of SST in gastric mucosa of GI-R group rats are lower than those of GI-R-sham group. There is no significant difference in the number of D cells and SST expression in gastric mucosa between MCAO group and GI-R group. There is no significant difference in the number of D cells and SST expression between the two sham operation groups. After the establishment of focal cerebral ischemia model, the gastric mucosal blood flow is observed to be increased to a certain extent with different doses of CGRP, and the gastric mucosal blood flow is not increased significantly in low-dose group. Compared with NS group, there is no statistical significance. There is no significant statistical difference between middle and high dose group (p>0.05) (Table 3). The decrease of gastric mucosal perfusion is the primary factor of gastric mucosal injury induced by acute cerebral ischemia-reperfusion in rats. Microcirculatory disturbance is the central link in gastric mucosal membrane stress injury. One of the important mechanisms of CGRP protecting ischemic gastric mucosal injury is to increase gastric mucosal blood perfusion and improve microcirculation.
| Group | Gas average grey value | number of G cells |
|---|---|---|
| MCAO | 155.06 + 5.39 | 18.24 + 2. 11 |
| MCAO Sham | 167.09±4.64 | 13.72±1.89 |
| GI-R | 155.66±8. 61 | 17.92±2.28 |
| GI-R Sham | 165. 95 ±4. 97 | 11. 97 ±1.93 |
Table 1: Results of Gas Immunohistochemical Staining in Each Group
| Group | STT average grey value | Number of G cells |
|---|---|---|
| MCAO Sham | 141.08+8. 13 | 16. 36±2. 55 |
| MCAO | 158. 34±7. 29 | 13. 03±1. 45 |
| GI-R Sham | 144.07±3.80 | 14.09±0.98 |
| GI-R | 158. 08±7. 05 | 12. 17±1.55 |
Table 2: Sst Immunohistochemical Staining Results of Rats in Each Group
| Group | Mean gastric blood flow (unit :pu) |
|---|---|
| NS group | 147.42±13.12 |
| Low dose group | 138.32±14.54 |
| Dose group | 217.28±8.57 |
| High dose group | 239.15±11.45 |
| The control group | 227.07±9.14 |
Table 3: Gastric Blood Flow of Rats Injected with Different Doses of Cgrp in Cerebral Ischemia Reperfusion
References
- Turner RJ, Vink R. NK1 tachykinin receptor treatment is superior to capsaicin pre-treatment in improving functional outcome following acute ischemic stroke. Neuropeptides 2014;48(5):267-72.
- Cao G, Ye X, Xu Y, Yin M, Chen H, Kou J, et al. YiQi FuMai powder injection ameliorates blood-brain barrier dysfunction and brain edema after focal cerebral ischemia-reperfusion injury in mice. Drug Des Dev Ther 2016;10(1):315-25.
- ElAli A, Doeppner TR, Zechariah A, Hermann DM. Increased blood-brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia: role of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9, and RhoA overactivation. Stroke 2011;42(11):3238-44.
- Cai H, Xu X, Liu Z, Wang Q, Feng G, Li Y, et al. The effects of calcitonin gene-related peptide on bFGF and AQP4 expression after focal cerebral ischemia. reperfusion in rats. Pharmazie 2010;65(4):274-8.
- Wu J, Chen J, Guo H, Peng F. Effects of High-Pressure Oxygen Therapy on Brain Tissue Water Content and AQP4 Expression in Rabbits with Cerebral Hemorrhage. Cell Biochem Biophys 2014;70(3):1579-84.
- Liu P, Wang J, Liu W, Zhang X, X Li, Yang G, et al. Protective Effects of Sodium Ferulate on Focal Brain Ischemia/Reperfusion Injury by Regulating ET/CGRP of Brain Tissue in Rats. Chin Pharm J 2007;42(24):1763-1859.
- Feng R, Zhang F. The Neuroprotective Effect of Electro-Acupuncture against Ischemic Stroke in Animal Model: A Review. Afr J Tradit Complement Altern Med 2014;11(3):25.
- Zhang JY, Yan GT, Liao J, Deng ZH, Xue H, Wang LH, et al. Leptin attenuates cerebral ischemia/reperfusion injury partially by CGRP expression. Eur J Pharmacol 2011;671(1-30):61-9.
- Feng G, Xu X, Wang Q, Liu Z, Li Z, Liu G. The protective effects of calcitonin gene-related peptide on gastric mucosa injury after cerebral ischemia reperfusion in rats. Immuno Pharmacol Immunotoxicol 2011;33(1):84-9.
- Yang S, Yuan Y, Jiao S, Luo Q, Yu J. Calcitonin gene-related peptide protects rats from cerebral ischemia/reperfusion injury via a mechanism of action in the MAPK pathway. Biomed Rep 2016;4(6):699-703.