- *Corresponding Author:
- Yanjuan Huang
Department of Anesthesiology, the Third Affiliated Hospital of Guangxi Medical University, Nanning 530031, China
E-mail: huangyanjuan66@163.com
| This article was originally published in a special issue, “Clinical Advancements in Life Sciences and Pharmaceutical Research” |
| Indian J Pharm Sci 2024:86(5) Spl Issue “167-174” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Remimazolam is a new short-acting gamma aminobutyric acid type A receptor agonist with minimal impact on cardiorespiratory suppression. However, the potential benefits of remimazolam . propofol in elderly patients undergoing colonoscopy are unknown. This was a multicentre, double-blinded randomized controlled trial. Elderly individuals, (65-85) y of age scheduled to undergo elective colonoscopy were randomized at a 1:1 ratio to receive sedation with either remimazolam at a rate of 5 mg/min or propofol at a rate of 50 mg/min at 1 min after a bolus injection of 0.05 mg fentanyl. When the Modified Observer’s Assessment of Alertness/ Sedation score reached ≤1, infusion was stopped and colonoscopy started. Modified Observer’s Assessment of Alertness/Sedation was maintained at ≤3 throughout the procedure. The primary outcome was the rate of hypotension (defined as systolic blood pressure at ≤90 mmHg or ≤70 % of the baseline) anytime during the procedure, as calculated in the intent-to-treat population. A total of 400 (201 women and 199 men, (71.0±5.0) y of age) were enrolled (200 per group). The rate of hypotension was 41.5 % (83/200) in the remimazolam group and 64.5 % (129/200) in the propofol group (p<0.001). The remimazolam group also had lower rate of bradycardia, as defined by heart rate ≤50 per minute (21.0 % . 42.5%, p<0.001) and respiratory depression, as defined by respiratory rate <8 per minute and/or saturation of peripheral oxygen <90 % (2.0 % . 6.0 %, p=0.029). For use to maintain adequate level of sedation in elderly patients undergoing colonoscopy, remimazolam infusion was associated with lower rate of hypotension as well as other measures indicative of cardiorespiratory inhibition than propofol.
Keywords
Colonoscopy, sedation, remimazolam, propofol, endoscopy
Propofol is the most commonly used sedative in patients undergoing gastrointestinal endoscopy[1-3], but is associated with circulatory and respiratory suppression[4-6], particularly in elderly patients[7-10]. Remimazolam is a short-acting Gamma- Aminobutyric Acid type A (GABAA) receptor agonist with faster onset of action and recovery than currently available short-acting sedatives[11]. The metabolism of remimazolam is independent of liver and kidney function[12], and thus is not prone to accumulation and respiratory and circulatory inhibition.
Remimazolam has shown to be safe and effective for procedural sedation in several clinical trials[13-16], including for colonoscopy[17]. Remimazolam tosilate (HR7056) was recently approved by China National Medical Products Administration for anesthesia and sedation[18]. Elderly patients are particularly prone to cardiovascular and respiratory inhibition upon sedation. We therefore conducted a multicentre, randomized trial to compare remimazolam tosilate . propofol in elderly patients undergoing colonoscopy.
Materials and Methods
This multicentre, randomized controlled trial was conducted during a period from September 2020 to September 2022 at the Third Affiliated Hospital of Guangxi Medical University (The Second Nanning People’s Hospital), Hechi Third People’s Hospital and Liuzhou Municipal Liutie Central Hospital. Ethical approvals for this study (Identification No: Y2020059, K2021001 and 2021037) were provided by the Ethical Committee of The Second Nanning People’s Hospital, Nanning, China on 31st August 2020, Hechi Third People’s Hospital, Hechi, China on 15th March 2021, and Liuzhou Municipal Liutie Central Hospital, Liuzhou, China on 30th May 2021, respectively. The trial protocol was registered at the China Clinical Trial Registry (No: ChiCTR2000035824) and performed in accordance with the Declaration of Helsinki. The study design adhered to the 2010 Consolidated Standards of Reporting Trials (CONSORT) statement. Written informed consent was obtained from all patients prior to enrolment.
General information:
Elderly individuals (65-85) y of age, scheduled to undergo colonoscopy either diagnostic or therapeutic were eligible.
American Society of Anesthesiologists (ASA) physical status IV or higher; a Body Mass Index (BMI) <18 or >30 kg/m2; requirement for tracheal intubation or expected difficult airways (Mallampati score of 3 or 4); estimated procedure time exceeding 30 min; acute respiratory infection, asthma attack, unstable angina, severe arrhythmia, uncontrolled hypertension (Systolic Blood Pressures (SBP) ≥160 mmHg or Diastolic Blood Pressure (DBP) ≥100 mmHg despite medical treatment) or hypotension (SBP ≤90 mmHg or DBP ≤60 mmHg); hemoglobin <80 g/l) a history of drug abuse and/or alcoholism within 2 y before screening; long-term use of hypnotic/sedative agents or opioid agents; known allergy or contraindication to either benzodiazepines or propofol were excluded from this study.
Randomization, masking and blinding:
Randomization (1:1 ratio to receive either remimazolam or propofol) and concealment were conducted using an interactive web service (www. medresman.org.cn). Both the patients and outcome assessors were blinded to the group assignment, and covered with opaque bags to achieve blinding. The attending anaesthesiologists were aware of the group assignment.
Intervention:
Patients were fasted for 6 h prior to the procedure; water intake was restricted for 2 h. Gastrointestinal tract preparation was conducted using a routine protocol. Oxygen was supplemented at a rate of 2 l/min via a nasal tube, starting at 5 min prior to induction and until complete recovery after the procedure.
At 1 min after fentanyl injection (0.05 mg, IV), patients started to receive intravenous infusion of designated intervention, remimazolam (remimazolam tosilate for injection, 1 mg/ml, HengRui Medicine Co., Ltd., China, Approval No: 2011148K) at 5 mg/min or propofol (propofol injection emulsion, 10 mg/ml, Aspen, Approval No: X19038B) at 50 mg/min. The rate of infusion for both propofol and remimazolam was adjusted to maintain a level of sedation sufficient to allow colonoscopy to proceed smoothly. Sedation level was assessed using MOAA/S[19] score once every 30 s during the first 3 min and once every 1 min thereafter. Colonoscopy started when MOAA/S reached ≤1. The level of sedation was maintained at MOAA/S score at ≤3 with bolus injection of either remimazolam (2.5 mg) or propofol (0.5 mg/kg) with at least 1 min interval and one assessment of sedation level with MOAA/S between the boluses; there was no limitation on the total dosage. Upon the completion of colonoscopy, patients were transferred to a Post- Anesthesia Care Unit (PACU) for observation for at least 30 min. The criteria for discharge (or transfer back to ward) was total Post Anesthetic Discharge Scoring System (PADSS) score of 9 or 10[20]. A telephone interview was conducted next day.
Hypotension was managed with rapid fluid infusion and/or phenylephrine 40 μg one time, as deemed appropriate by the attending anesthesiologist. Hypoxemia (Saturation of peripheral Oxygen (SpO2) <90 %) was managed by jaw thrust manoeuvre and/or increase of oxygen flow, as appropriate. Bradycardia was managed with atropine 0.5 mg one time if necessary. Severe nausea and vomiting was managed with tropisetron (5 mg).
Trial outcomes:
The primary outcome was hypotension, defined as SBP ≤90 mmHg or a >30 % decline from the baseline (immediately prior to fentanyl injection). Secondary outcomes included respiratory depression (respiratory rate <8/min), hypoxemia (SpO2 <90 % at any time), bradycardia (heart rate reduction by >20 % relative to the baseline or to ≤50/min), time to and dosage of the test drug required for adequate sedation (MOAA/S score ≤1), nausea and vomiting, procedure time (from the start of the procedure to endoscope removal), recovery time (from discontinuation of sedative use to the first of three consecutive MOAA/S scores of 5), and sedation time (from the start of intravenous infusion of sedative agent to fully alert). Blood pressure, heart rate and respiratory rate were assessed every 3 min during the trial. All outcomes were assessed by an anaesthesiologist otherwise not involved in this trial. Sedation success was defined as with a sedative agent other than the assigned treatment to maintain MOAA/S ≤3 throughout the procedure. Procedural success was defined as completion of the scheduled endoscopy.
Safety:
Adverse Events (AEs) were evaluated using the National Cancer Institute Common Terminology Criteria for AEs version 4.0, and included hypotension, bradycardia, respiratory inhibition, hypoxemia (SBP ≤90 mmHg or >30 % decline from the baseline), delayed recovery (recovery time ≥2 h), nausea and vomiting, pain at the site of infusion, headache, and dizziness[21].
Sample size and statistical analysis:
Sample size requirement was estimated using the following assumptions: Hypotension in 8/20 (40 %) of the patients receiving remimazolam . 13/20 (65 %) of the patients receiving propofol (based on our preliminary study of 40 patients undergoing gastroscopy); 2-sided alpha of 0.05 and a power of 0.8; a dropout rate of 20 %. The calculation yielded 200 subjects in each group. The induction regimen was identical between gastroscopy and colonoscopy and hypotension occurred in induction phase in majority of the cases.
Continuous variables with normal distribution are presented as mean±Standard Deviation (SD) and analysed using Student’s t-test; variables not following normal distribution are expressed as median (interquartile range) and analysed using Mann-Whitney U test. Continuous variables with repeated measures (e.g., systolic blood pressure) were analysed using a generalized linear model. Categorical variables were analysed using Chisquare test. Analysis of the primary outcome was conducted in the intent-to-treat population. p<0.05 (2-sided) was considered statistically significant. All statistical analyses were conducted using Statistical Package for the Social Sciences (SPSS) version 22.0.
Results and Discussion
A total of 400 patients (201 women and 199 men, (71.0±5.0) y of age) were enrolled (n=200 for each group). Patient flow through the trial was shown in fig. 1. Demographic and baseline characteristics of the patients in the 2 groups are shown in Table 1. All patients received assigned intervention and colonoscopy as planned.
| Remimazolam (n=200) | Propofol (n=200) | ||
|---|---|---|---|
| Female gender, n (%) | 98 (49) | 103 (51.5) | |
| Age (years), mean±SD | 71.7 (5.2) | 71.0 (4.9) | |
| Weight (kg), mean±SD | 57.7 (9.5) | 57.8 (9.5) | |
| Body mass index (kg/m2), mean±SD | 22.7 (2.5) | 22.6 (2.6) | |
| ASA classification, n (%) | |||
| I | 4 (2) | 8 (4) | |
| II | 175 (87.5) | 172 (86) | |
| III | 21 (10.5) | 20 (10) | |
| Major comorbidities, n (%) | |||
| Hypertension | 73 (36.5) | 63 (31.5) | |
| Coronary artery disease | 12 (6) | 16 (8) | |
| Stroke | 4 (2) | 7 (3.5) | |
| Lung infection | 3 (1.5) | 5 (2.5) | |
| Diabetes | 14 (7) | 17 (8.5) | |
| Systolic blood pressure (mmHg), mean±SD | 133.0 (13.2) | 134.5 (13.8) | |
| Heart rate (beats/min), mean±SD | 74.9 (8.5) | 75.6 (9.1) | |
| Respiratory rate (breaths/min), mean±SD | 19.8 (0.6) | 19.9 (0.6) | |
| SpO2 (%), mean±SD | 99.1 (1.3) | 99.2 (1.2) | |
Table 1: Demographic and Baseline Characteristics of the Patients
Both sedation success rate and procedural success rate were 100 % in both groups (Table 2). The median time to induction was 2.0 min (1.5 and 2.0) in the remimazolam group . 2.0 min (1.5 and 2.0) in the propofol group (p=0.786). The median procedural time was 18.0 min (13.0 and 24.8) in the remimazolam group . 17.0 min (12.0 and 22.0) in the propofol group (p=0.057). The median induction dosage was 9.7 mg (8.5 and 10.6; 0.17 mg/kg) in the remimazolam group . 97.5 mg (83.3 and 104.1; 1.70 mg/kg) in the propofol group.
| Remimazolam (n=200) | Propofol (n=200) |
p | |
|---|---|---|---|
| Sedation success, n (%) | 200 (100) | 200 (100) | >0.999 |
| Procedural success, n (%) | 200 (100) | 200 (100) | >0.999 |
| Time to induction (min), median (IQR) | 2.0 (1.5-2.0) | 2.0 (1.5-2.0) | 0.786 |
| Procedure time (min), median (IQR) | 18.0 (13.0-24.8) | 17.0 (12.0-22.0) | 0.057 |
| Recovery time (min), median (IQR) | 12.5 (9.1-16.0) | 12.0 (10-15.5) | 0.341 |
| Induction dosage (mg), median (IQR) | 9.7 (8.5-10.6) | 97.5 (83.3-104.1) | NA |
| Total dosage (mg), median (IQR) | 13.1 (10.8-17.1) | 124.6 (100.3-146.5) | NA |
Note: (IQR): Interquartile Range and (NA): Not Applicable
Table 2: Procedural Characteristics
The rate of hypotension was 41.5 % (83/200) in the remimazolam group . 64.5 % (129/200) in the propofol group (p<0.001) (Table 3). The rate of norepinephrine use was 22.0 % (44/200) in the remimazolam group . 43.0 % (86/200) in the propofol group (p<0.001). The remimazolam group also had lower rate of bradycardia (21.0 % . 42.5 %, p<0.001) and respiratory inhibition (2.0 % . 6.0 %, p=0.029). No postoperative headache, nausea and vomiting were reported.
| AEs, n (%) | Remimazolam (n=200) | Propofol (n=200) | p |
|---|---|---|---|
| All AEs | 84 | 180 | / |
| Patients with AEs | 76 (38.0) | 132 (66.0) | <0.001 |
| Specific AEs | / | ||
| Hypotension | 83 (41.5) | 129 (64.5) | <0.001 |
| Bradycardia | 42 (21.0) | 85 (42.5) | <0.001 |
| Respiratory depression | 4 (2.0) | 12 (6.0) | 0.029 |
| Hypoxemia | 1 (0.5) | 1 (0.5) | / |
| Pain at injection site | 0 | 24 (12.0) | <0.001 |
| Delayed recovery | 0 | 0 | / |
| Nausea | 0 | 0 | / |
| Vomiting | 0 | 0 | / |
| Headache | 0 | 0 | / |
| Inability to ambulate | 0 | 0 | / |
| Vasoactive drug use | 44 (22.0) | 86 (43.0) | <0.001 |
Table 3: Summary of AEs
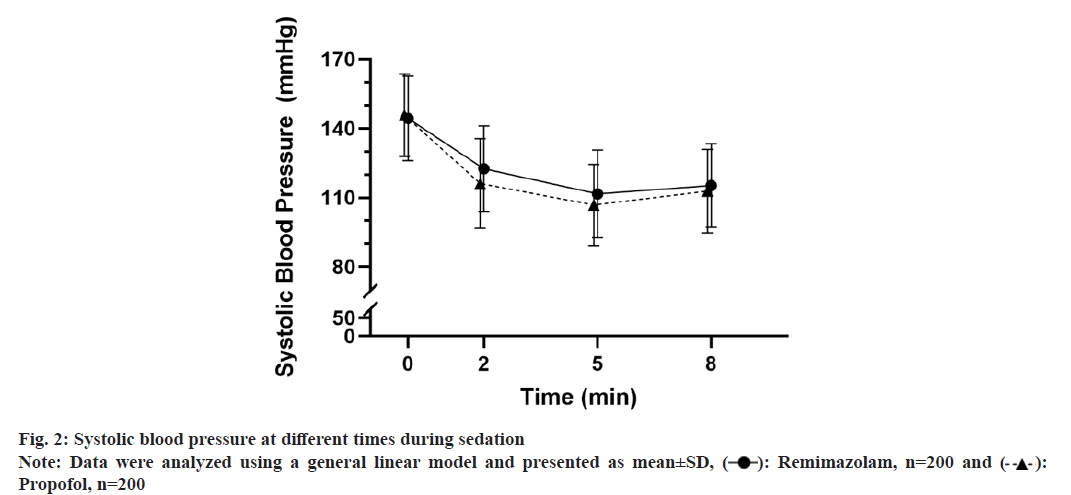
SBP at 2, 5 and 8 min were significantly lower than the baseline in both groups (fig. 2). Analysis using a generalized linear model revealed lower SBP in the propofol group (p=0.038 . the remimazolam group). There was a significant interaction between treatment and time (p<0.001).
The current trial showed significantly lower rate of hypotension in the remimazolam group (41.5 %) . the propofol group (64.5 %), primarily during the induction period. The lower rate of hypotension in the remimazolam group was supported by the higher SBP at 2 and 5 min. In comparison to the propofol control, remimazolam group also had lower rate of bradycardia (21.0 % . 42.5 %) and respiratory inhibition (2.0 % . 6.0 %). The lower rate of hypotension and respiratory inhibition was consistent with a trial in patients undergoing bronchoscopy[22], and extended the efficacy and safety profiles of remimazolam from elderly patients undergoing gastroscopy [16,23] to procedures that require deeper level of sedation.
The rate of hypotension in this trial (64.5 %) was higher than that reported for gastrointestinal endoscopy in previous studies (generally at about 50 %)[6,13-15,17,24,25]. Several factors may have contributed to such a discrepancy. First, all participants were at least 65 y of age in the current trial. Second, adequate sedation was started at a deeper level (MOAA/S score of 1 or 0) in the current trial due to the requirement for colonoscopy, whereas previous studies generally used MOAA/S score of 3 or less. Nevertheless, the higher rate of hypotension and respiratory inhibition in the current trial highlighted the risk of these complications and the potential benefit with remimazolam as an alternative sedative agent in elderly patients undergoing colonoscopy.
The sedation success rate with remimazolam in combination with fentanyl in the current trial was 100 % at a loading dose of 0.17 mg/kg. The findings were consistent with a previous trial in elderly patients undergoing gastroscopy from this group of investigators[16]. The induction dose in the current trial was also similar to that required to maintain MOAA/S score at 1 or 0 in a previous trial of remimazolam in combination with 0.1 μg/kg sufentanyl in elderly patients undergoing gastrointestinal endoscopy (ED95 of 0.164 mg/kg)[26]. Notably, the induction dose in this trial was significantly higher than the recommended dosage for sedation in adult patients who undergo gastroscopy or colonoscopy (5 mg, or 0.083 mg/kg for a person with 60 kg body weight) [27], again likely due to different sedation levels (MOAA/S score at 1 or 0 in this trial . 3 or less in phase 3 trials used for recommendation) and successful sedation rate (100 % in this trial . 91 %-97 % in previous trials)[14,27,28,24]. At an intermediate dosage of 7 mg, successful sedation rate in patients undergoing gastrointestinal endoscopy was reported to be 98.9 %[17,26]. A phase 2b trial investigated the use of 3 distinct regimens (loading and maintenance dosage at 5.0/3.0, 7.0/2.0, and 8.0/3.0 mg) in patients undergoing colonoscopy; MOAA/S at 4 or less was achieved in all patients in the 3 dosage groups, but the procedural success rate was less than optimal (92.5 %-97.5 %)[13].
A large retrospective analysis using 5 y data from 165 527 endoscopy procedures showed lower rate of cardiopulmonary complications (including cardiac arrest) in patients sedated with midazolam . propofol[6]. In a trial in adult patients undergoing endoscopic submucosal dissection, the remimazolam had more stable hemodynamics and lower rate of hypotension[29]. Less impact of remimazolam . propofol has also been shown in women undergoing endoscopic hysterectomy[30], and in obese patients undergoing gastroscopy[31], suggesting better safety profile with remimazolam across different patient characteristics (age, gender and BMI). However, a recent study by Sekiguchi et al.[32] showed no difference in mean arterial blood pressure, heart rate, cardiac output and stroke volume when remimazolam and propofol were administered using target-controlled infusion.
At anesthetic dosage, propofol produces dual inhibitory cardiovascular effects by directly inhibiting the heart and causing peripheral vasodilatation. When injected intravenously at 2-2.5 mg/kg, SBP decreases by 25 %-40 %. When injected at induction dosage of propofol, the rate of temporary respiratory arrest is 25 %-40 %[33]. These inhibitory effects were even more pronounced in elderly patients[34-36]. Remimazolam is a novel benzodiapine agent that produces sedative effects by activating the GABAA receptor[12]. Previously studies have demonstrated a variety of benefits, including rapid onset of action, rapid recovery upon discontinuation, minimal impact on cardiovascular function and rapid drug clearance independent of liver and kidney function[15,17,24,27]. We did not notice dizziness in any of the patients undergoing colonoscopy in either group. Although achieving post-anaesthesia discharge criteria after outpatient procedures does not mean that the patient has regained all his or her faculties[37], the absence of dizziness after awakening is really one of the most basic requirements for sedatives.
This trial has several limitations. First, remimazolam and propofol were infused at constant rate. Whether lower rate of infusion is sufficient requires further investigation. Second, the level of sedation was not monitored using an objective measure (e.g., bispectral index).
For use to maintain adequate level of sedation in elderly patients who underwent colonoscopy, remimazolam infusion was associated with lower rate of hypotension and respiratory inhibition than propofol.
Ethical approval:
This study was approved by the IRB of the Third Affiliated Hospital of Guangxi Medical University (No: Y2020059), Hechi Third People’s Hospital (No: K2021001), Liuzhou Municipal Liutie Central Hospital (No: 2021037), and registered at http://www.chictr.org.cn (18/08/2020, No: ChiCTR-2000035824). The study protocol followed the CONSORT guidelines. The trial was performed in compliance with all relevant guidelines. Written informed consent was obtained from all patients.
Funding:
This study was supported in part by the Guangxi Health Commission Self-Funded Scientific Research Projects Foundation (No: Z20210001 and Z-A20221153).
Authors’ contributions:
Xuelian Ran, Shanshan Wei and Wenwen Ling have contributed equally to this wor
Conflict of interests:
The authors declared no conflict of interests.
References
- Lin YJ, Wang YC, Huang HH, Huang CH, Liao MX, Lin PL. Target-controlled propofol infusion with or without bispectral index monitoring of sedation during advanced gastrointestinal endoscopy. J Gastroenterol Hepatol 2020;35(7):1189-95.
[Crossref] [Google Scholar] [PubMed]
- Riphaus A, Wehrmann T, Weber B, Arnold J, Beilenhoff U, Bitter H, et al. S3 Guideline: Sedation for gastrointestinal endoscopy 2008. Endoscopy 2009;41(9):787-815.
[Crossref] [Google Scholar] [PubMed]
- Heuss LT, Inauen W. The dawning of a new sedative: Propofol in gastrointestinal endoscopy. Digestion 2004;69(1):20-6.
[Crossref] [Google Scholar] [PubMed]
- Goudra B, Gouda G, Mohinder P. Recent developments in drugs for GI endoscopy sedation. Dig Dis Sci 2020;65(10):2781-8.
[Crossref] [Google Scholar] [PubMed]
- Uzman S, Gurbulak B, Gurbulak EK, Donmez T, Hut A, Yildirim D. A comparison of propofol and midazolam/meperidine sedation in upper gastrointestinal endoscopy. Wideochir Inne Tech Maloinwazyjne 2016;11(3):178-85.
[Crossref] [Google Scholar] [PubMed]
- Goudra B, Nuzat A, Singh PM, Borle A, Carlin A, Gouda G. Association between type of sedation and the adverse events associated with gastrointestinal endoscopy: An analysis of 5 years’ data from a tertiary center in the USA. Clin Endosc 2017;50(2):161-9.
[Crossref] [Google Scholar] [PubMed]
- Amornyotin S, Leelakusolvong S, Chalayonnawin W, Kongphlay S. Age-dependent safety analysis of propofol-based deep sedation for ERCP and EUS procedures at an endoscopy training center in a developing country. Clin Exp Gastroenterol 2012;5:123-8.
[Crossref] [Google Scholar] [PubMed]
- Heuss LT, Schnieper P, Drewe J, Pflimlin E, Beglinger C. Conscious sedation with propofol in elderly patients: A prospective evaluation. Aliment Pharmacol Ther 2003;17(12):1493-501.
[Crossref] [Google Scholar] [PubMed]
- Friedrich K, Stremmel W, Sieg A. Endoscopist-administered propofol sedation is safe-A prospective evaluation of 10 000 patients in an outpatient practice. J Gastrointestin Liver Dis 2012;21(3):259-63.
[Google Scholar] [PubMed]
- Rex DK, Deenadayalu VP, Eid E, Imperiale TF, Walker JA, Sandhu K, et al. Endoscopist-directed administration of propofol: A worldwide safety experience. Gastroenterology 2009;137(4):1229-37.
[Crossref] [Google Scholar] [PubMed]
- Rogers WK, McDowell TS. Remimazolam, a short-acting GABA (A) receptor agonist for intravenous sedation and/or anesthesia in day-case surgical and non-surgical procedures. IDrugs 2010;13(12):929-37.
[Google Scholar] [PubMed]
- Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, et al. CNS 7056: A novel ultra-short-acting benzodiazepine. Anesthesiology 2007;107(1):60-6.
[Crossref] [Google Scholar] [PubMed]
- Pambianco DJ, Borkett KM, Riff DS, Winkle PJ, Schwartz HI, Melson TI, et al. A phase IIb study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc 2016;83(5):984-92.
[Crossref] [Google Scholar] [PubMed]
- Rex DK, Bhandari R, Desta T, deMicco MP, Schaeffer C, Etzkorn K, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc 2018;88(3):427-37.
[Crossref] [Google Scholar] [PubMed]
- Borkett KM, Riff DS, Schwartz HI, Winkle PJ, Pambianco DJ, Lees JP, et al. A Phase IIa, randomized, double-blind study of remimazolam (CNS 7056) . midazolam for sedation in upper gastrointestinal endoscopy. Anesthesia Analgesia 2015;120(4):771-80.
[Crossref] [Google Scholar] [PubMed]
- Lu K, Wei S, Ling W, Wei Y, Ran X, Huang H, et al. Remimazolam . propofol for deep sedation/anaesthesia in upper gastrointestinal endoscopy in elderly patients: A multicenter, randomized controlled trial. J Clin Pharm Ther 2022;47(12):2230-6.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Hu X, Bai N, Li L, Zhang M, Cheng Z, et al. Safety and efficacy of remimazolam besylate in patients undergoing colonoscopy: A multicentre, single-blind, randomized, controlled, phase Ⅲ trial. Front Pharmacol 2022;13:900723.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Hu P, Huang Y, Sang N, Song K, Wang H, et al. Population pharmacokinetic/pharmacodynamic model-guided dosing optimization of a novel sedative HR7056 in Chinese healthy subjects. Front Pharmacol 2018;9:1316.
[Crossref] [Google Scholar] [PubMed]
- Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, et al. Validity and reliability of the observer's: Assessment of alertness/sedation scale: Study with: Intravenous midazolam. J Clin Psychopharmacol 1990;10(4):244-51.
[Google Scholar] [PubMed]
- Ead H. From Aldrete to PADSS: Reviewing discharge criteria after ambulatory surgery. J Perianesth Nurs 2006;21(4):259-67.
[Crossref] [Google Scholar] [PubMed]
- Misal US, Joshi SA, Shaikh MM. Delayed recovery from anesthesia: A postgraduate educational review. Anesth Essays Res 2016;10(2):164-72.
[Crossref] [Google Scholar] [PubMed]
- Pastis NJ, Yarmus LB, Schippers F, Ostroff R, Chen A, Akulian J, et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest 2019;155(1):137-46.
[Crossref] [Google Scholar] [PubMed]
- Li Z, Deng X, Sun T, Du Y, Li J. Chinese experts consensus on the diagnosis and treatment of sedation and anaesthesia in digestive endoscopy. Chin J Pract Intern Med 2014;34(8):756-64.
- Chen SH, Yuan TM, Zhang J, Bai H, Tian M, Pan CX, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: A multicenter, randomized, non-inferiority, phase III trial. J Gastroenterol Hepatol 2021;36(2):474-81.
[Crossref] [Google Scholar] [PubMed]
- Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA class III): Results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesthesia 2020;34:491-501.
[Crossref] [Google Scholar] [PubMed]
- Guo J, Qian Y, Zhang X, Han S, Shi Q, Xu J. Remimazolam tosilate compared with propofol for gastrointestinal endoscopy in elderly patients: A prospective, randomized and controlled study. BMC Anesthesiol 2022;22(1):180.
[Crossref] [Google Scholar] [PubMed]
- Chen S, Wang J, Xu X, Huang Y, Xue S, Wu A, et al. The efficacy and safety of remimazolam tosylate . propofol in patients undergoing colonoscopy: A multicentered, randomized, positive-controlled, phase III clinical trial. Am J Transl Res 2020;12(8):4594.
[Google Scholar] [PubMed]
- Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam . propofol for general anesthesia: A multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesthesia 2020;34:543-53.
[Crossref] [Google Scholar] [PubMed]
- Qiu Y, Gu W, Zhao M, Zhang Y, Wu J. The hemodynamic stability of remimazolam compared with propofol in patients undergoing endoscopic submucosal dissection: A randomized trial. Front Med 2022;9:938940.
[Crossref] [Google Scholar] [PubMed]
- Zhang S, Wang J, Ran R, Peng Y, Xiao Y. Efficacy and safety of remimazolam tosylate in hysteroscopy: A randomized, single-blind, parallel controlled trial. J Clin Pharm Ther 2022;47(1):55-60.
[Crossref] [Google Scholar] [PubMed]
- Zhang K, Bao Y, Han X, Zhai W, Yang Y, Luo M, et al. Effects of opioid-free propofol or remimazolam balanced anesthesia on hypoxemia incidence in patients with obesity during gastrointestinal endoscopy: A prospective, randomized clinical trial. Front Med 2023;10:1124743.
[Crossref] [Google Scholar] [PubMed]
- Sekiguchi R, Kinoshita M, Kawanishi R, Kakuta N, Sakai Y, Tanaka K. Comparison of hemodynamics during induction of general anesthesia with remimazolam and target-controlled propofol in middle-aged and elderly patients: A single-center, randomized, controlled trial. BMC Anesthesiol 2023;23(1):14.
[Crossref] [Google Scholar] [PubMed]
- Rd M, Li E, La F. Miller's Anesthesia: 8th ed; Elsevier Health Sciences; 2014. p. 827-9.
- Wadhwa V, Issa D, Garg S, Lopez R, Sanaka MR, Vargo JJ. Similar risk of cardiopulmonary adverse events between propofol and traditional anesthesia for gastrointestinal endoscopy: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2017;15(2):194-206.
[Crossref] [Google Scholar] [PubMed]
- Yoo YC, Park CH, Shin S, Park Y, Lee S, Min KT. A comparison of sedation protocols for gastric endoscopic submucosal dissection: Moderate sedation with analgesic supplementation . analgesia targeted light sedation. Br J Anaesth 2015;115(1):84-8.
[Crossref] [Google Scholar] [PubMed]
- Dinis-Oliveira RJ. Metabolic profiles of propofol and fospropofol: Clinical and forensic interpretative aspects. Biomed Res Int 2018;2018(1):6852857.
[Crossref] [Google Scholar] [PubMed]
- Lois F, Massart Q, Warner DO, Malengreaux C, Knops M, Nyssen AS, et al. Driving performance of outpatients achieving discharge criteria after deep sedation is worse than these of their escort-driver: A prospective observational study on simulator. Acta Gastroenterol Belg 2023;86(1):11-6.
[Crossref] [Google Scholar] [PubMed]

 ): Remimazolam, n=200 and (
): Remimazolam, n=200 and ( ):
Propofol, n=200
):
Propofol, n=200



